Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.14 Ananindeua 2023 Epub Dec 21, 2023
http://dx.doi.org/10.5123/s2176-6223202301437
ORIGINAL ARTICLE
Trends of leprosy detection rates in a Brazilian Northern Region State
1Centro Universitário do Estado do Pará, Belém, Pará, Brasil
2Universidade Federal do Pará, Belém, Pará, Brasil
OBJECTIVE:
To describe the trend of leprosy detection rates in 13 Regional Health Centers (RHC) of Pará State, Brazil.
MATERIALS AND METHODS:
Ecological exploratory study including new leprosy cases recorded in the Notifiable Diseases Information System from 2000 to 2019 within cities grouped by RHC in Pará. Variables included age, year of diagnosis, and city of residence. The classification of hyperendemicity followed parameters established by the Brazilian Ministry of Health.
RESULTS:
During the analyzed period, 98,342 new leprosy cases were notified. From these, 87,425 (88.9%) met the inclusion criteria, with an annual average of 4,371 (± 1,201) cases. Of all the notified cases, 10.57% (9,240) occurred in individuals under 15 years old. Hyperendemicity persisted in the general population in the 5th, 8th, 10th, 11th, and 12th RHC from 2000 to 2019. The same centers exhibited hyperendemicity in individuals under 15 years old, except for the 5th RHC.
CONCLUSION:
The heterogeneity of endemicity levels throughout the historical series and in different territories suggests that the analysis of an infectious disease in vast geographical areas with cultural diversities and social vulnerability should be particularized by specific territories. This approach helps identify populations contributing to the maintenance of elevated endemicity levels.
Keywords: Leprosy; Endemic Diseases; Primary Health Care; Historical Series
INTRODUCTION
In most so-called developing countries, leprosy is still a serious public health problem. It has been included in the list of neglected diseases because it is a chronic infection with a high number of cases, a higher prevalence in vulnerable populations, and a high cost of treatment1,2. Moreover, among these diseases, leprosy was the first to have a worldwide plan aimed at its elimination due to its high occurrence, its potential to cause physical disabilities, and because it affects the economically active population2. According to the World Health Organization (WHO)3, the high global rates are influenced by the number of new cases coming mainly from countries like India and Brazil, where there are pockets of disease high incidence.
Neglected diseases are commonly prevalent in poverty conditions. They are considered both a cause and a consequence of this situation, contributing to the perpetuation of socio-economic inequality2, given that when illness occurs, it becomes more challenging to get out of poverty4,5,6.
These facts may explain why reducing leprosy prevalence rates does not impact the reduction of transmission since social vulnerability is a critical factor in the heightened risk of contracting the disease6,7.
In 2022, 174,059 new cases of leprosy were reported to the WHO. From these, 19,635 (11.3%) occurred in Brazil, which led the country to remain in second place in the world ranking of cases. Although there was a reduction in the detection rate from 17.17 to 8.59 cases per 100,000 inhabitants between 2012 and 2021, the country remained at a level of medium endemicity. The North, Northeast, and Midwest Regions contributed to this endemic by maintaining the disease transmission3,8,9.
In this scenario, Pará State was considered hyperendemic for leprosy in 2010 (46.93/100,000 inhab.), with an annual detection rate higher than the Brazilian average. In 2020, although it was still considered to have very high endemicity (29.82/100,000 inhab.), it showed a significant decline in rates8,9.
These data point to the need for more effective early diagnosis and treatment, especially in the regions with the highest concentration of cases, as well as intensified monitoring of the epidemiological situation, using information systems to do so, to contribute to the goal of eliminating leprosy as a public health problem9,10.
According to the Brazilian Ministry of Health, health services must be structured to carry out the Leprosy Control Plan (LCP) actions so trained professionals can deal with the essential aspects of controlling the disease11. Thus, among the neglected diseases, eliminating leprosy as a United Nations (UN) target for 2030 requires that patients have guaranteed access to essential health services and adequate treatment12.
However, what hinders early diagnosis and treatment is the discrimination suffered by leprosy patients and their families, which leads to the disease progressing with the presence of deformities and disabilities, and it is therefore considered one of the main obstacles in the development of epidemiological surveillance actions. The presence of deformities leads to negative impacts on patients' daily lives, as well as psychosocial prejudice13.
The LCP actions were decentralized to Primary Health Care (PHC) services to reduce inequalities in access to health services and promote access to curative treatment. This is expected to make the impact of early case detection more transparent. Likewise, epidemiological surveillance actions must be repeatedly refined and systematized to direct people to seek health services when signs and symptoms are perceived14.
In this situation, health information has become an essential instrument for decision-making as a tool for epidemiological surveillance14. For this reason, one of the Health Information Systems (HIS) objectives in the conception of the Unified Health System (Sistema Único de Saúde - SUS) is to make it possible to analyze the health situation at the local level, taking territories as a reference and necessarily considering the population's living conditions in determining the health-disease process15. The local level is responsible not only for feeding the HIS but also for its organization and management16.
However, the way the LCP actions are carried out, due to the fragility of the services structure for the program operationalization, leprosy can remain at endemic levels even after the elimination target has been reached16. This is why it is important to constantly evaluate the program to support leprosy planning control actions16.
In this context, the policies formulation in equity search, aimed at the control/elimination of neglected diseases, faces two significant challenges: the public policies establishment of a transversal nature and the actions incorporation for vulnerable groups, often marked by overlapping vulnerabilities within sectoral policies. In other words, the development of such policies must incorporate greater decision-making power on the part of these population groups17. Thus, among the commitments made in 2017 by the UN to promote well-being and ensure a sustainable life is eradicating neglected tropical diseases such as leprosy, an action included in Sustainable Development Goal 318.
Despite this context, few studies have focused on estimating the detection rate target achievement in territories with specific populations. Thus, this study aims to describe the trend in leprosy case detection rates in 13 Regional Health Centers (RHC) of Pará State, Brazil, from 2000 to 2019. In addition to contributing to scientific knowledge, it provides subsidies for better-targeting leprosy surveillance and control actions in the cities that make up the RHC in Pará.
MATERIALS AND METHODS
This ecological and temporal exploratory study is based on the new leprosy case records in the Notifiable Diseases Information System (SINAN), made available by the Pará State Public Health Secretariat (SESPA). Based on these records, detection rates were calculated for the years selected for the study: 2000, 2005, 2010, 2015, and 2019.
Records of new cases diagnosed as leprosy in the years selected for the study, of any age, clinical form or operational classification, and with residence identified in one of the 144 cities of Pará State were included. Cases of transfers from another country or state were excluded, as well as relapses and other re-entries (modes of entry), unknown residence city, and misdiagnosis. New cases were allocated to their respective RHC according to their residence city19.
The grouping of cities into RHCs is justified because they are SESPA administrative units responsible for advising, following up, evaluating, and monitoring the performance of health programs in the cities within their coverage area, as well as organizing the services decentralization for better access by citizens19.
To calculate the detection rate in the total population and each age group, according to the year of the study, the population of the cities within each RHC was used, as provided in the 2000 and 2010 Censuses by the Brazilian Institute of Geography and Statistics (IBGE), along with population estimates for the intercensus years from 2001 to 200920 and 2011 to 201920,21,22.
According to the Ministry of Health, the groups of cities in the respective RHC were classified, according to the detection rate and age group, into hyperendemic, very high endemicity, high endemicity, medium endemicity, and low endemicity9.
The choropleth maps were made using QGIS v.3.32.0 and later edited in CorelDRAW 2019 to improve layout and colorization. The cartographic database of Brazil provided by the IBGE was used at a 1:250,000 scale and updated in 2019 with the SIRGAS 2000 coordinate system.
The trend in detection rates was analyzed based on the mapping of the state, with the cities grouped into RHCs. With the aid of the BioEstat v.5.3 program, the chi-square trend test was performed, and the α = 5% level (p-value ≤ 0.05) was considered a statistically significant difference.
This study was approved by the Human Research Ethics Committee of the Centro Universitário do Estado do Pará, under opinion no. 4.100.909, on June 21, 2020.
RESULTS
Between 2000 and 2019, 98,342 new leprosy cases were reported in Pará. From this total, 87,425 (88.9%) met the inclusion criteria, with an annual average of 4,371 (± 1,201) cases. Based on the total notification number, 10.57% (9,240) corresponded to children under 15 years old.
In 2000, there was a hyperendemicity predominance in the RHC, except the medium endemicity in the cities of Marajó archipelago, which make up the 7th RHC (Afuá, Chaves, Cachoeira do Arari, Muaná, Ponta de Pedras, Santa Cruz do Arari, Soure, Salvaterra, and São Sebastião da Boa Vista). However, in 2019, only the 5th, 8th, 10th, 11th, and 12th RHC had detection rates ≥ 40/100,000 inhab. (hyperendemic), while the 2nd and 13th had detection rates of 34.3 and 29.1/100,000 inhab. (very high endemicity), respectively, and the remaining RHC were considered to have high endemicity (Figure 1).
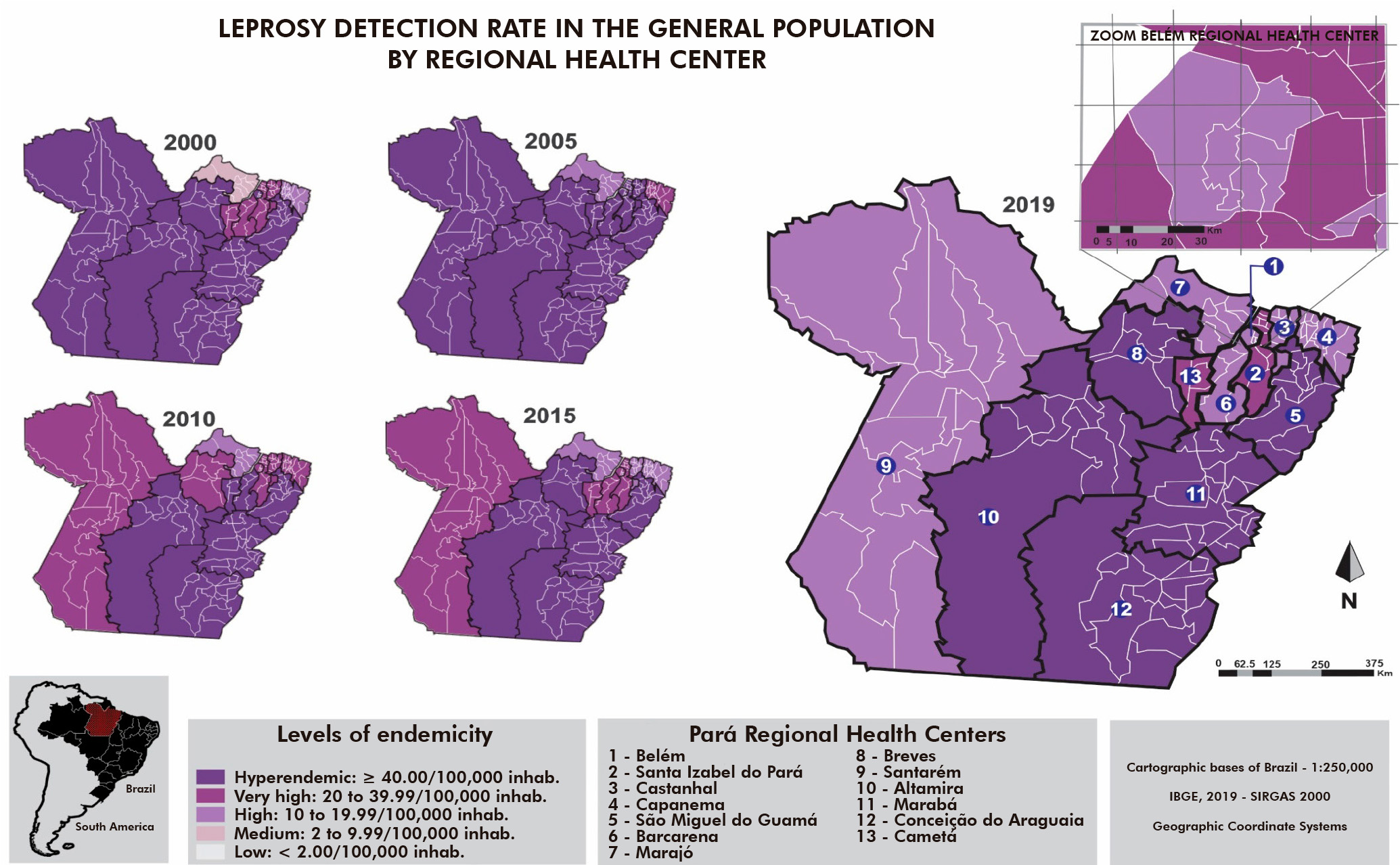
Figure 1 - Comparison of the leprosy detection rate (per 100,000 inhab.) in the general population, according to the Regional Health Centers, Pará State, Brazil, in 2000, 2005, 2010, 2015, and 2019
When comparing detection rates in children under 15 years old (Figure 2), there was also a hyperendemicity predominance in the state, except for the endemicity average recorded in the 4th RHC. The 7th RHC oscillated between hyperendemicity in 2000, medium endemicity in 2010 and 2015, and high endemicity in 2019. In the 8th, 10th, 11th, and 12th RHC, leprosy was hyperendemic in the under-15 population in 2019.
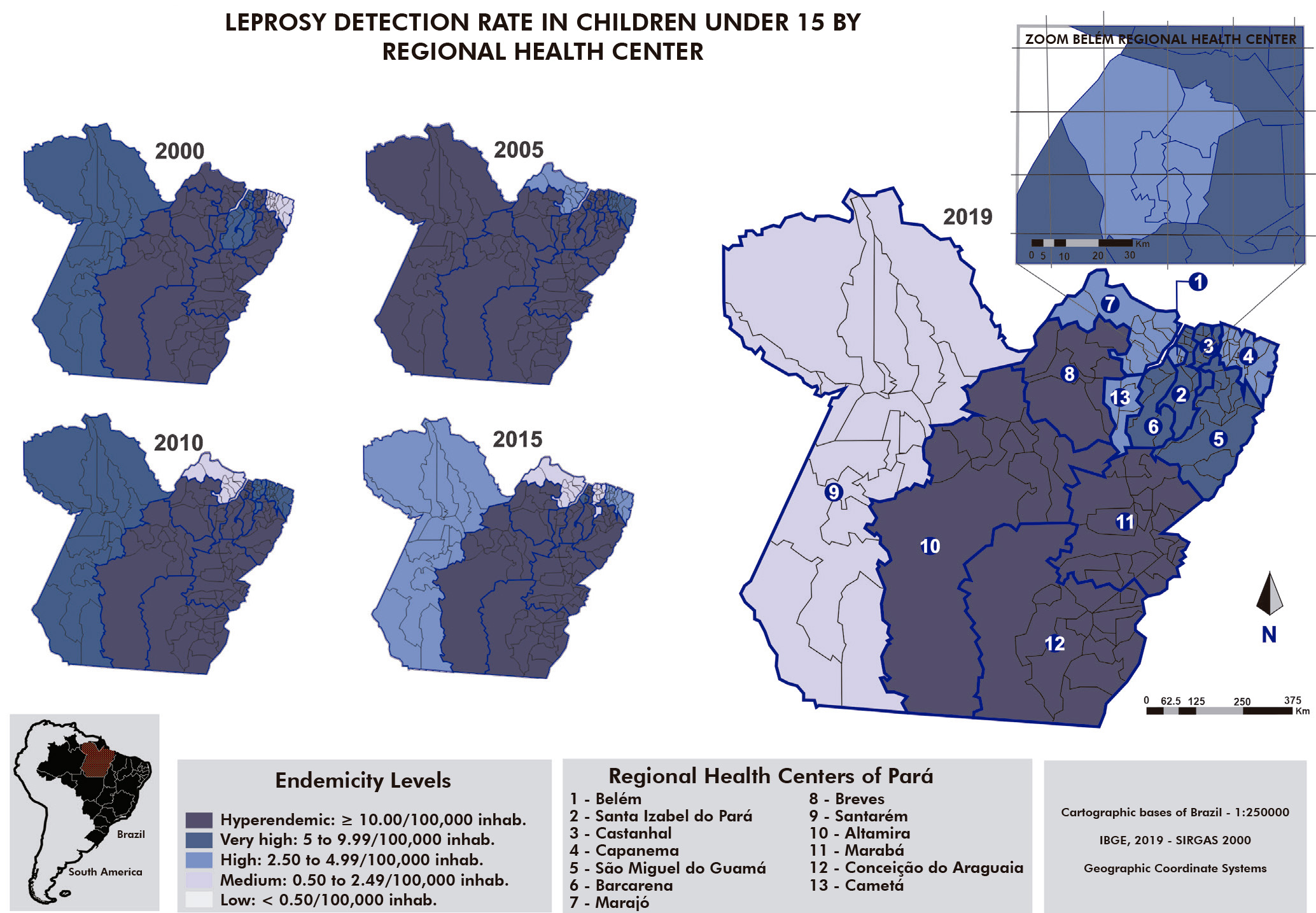
Figure 2 - Comparison of the leprosy detection rate (per 100,000 inhab.) in the population under 15 years old, according to the Regional Health Centers, Pará State, Brazil, in 2000, 2005, 2010, 2015, and 2019
Only in the 7th RHC was a non-significant increasing trend (p = 0.0542) in the leprosy detection rate in the general population (Table 1). In the population under 15 years old (Table 2), there was also a non-significant increase in the case detection rate of 7th, 8th, and 13th RHC. Although the detection rate was reduced in ten out of the 13 RHCs between 2000 and 2019, they remained hyperendemic (Table 2).
Table 1 - Evolution of detection rates of new leprosy cases in the general population, according to the Regional Health Center and year of diagnosis, Pará State, Brazil, 2000, 2005, 2010, 2015, and 2019
| Regional Health Center | Year | p-value* (trend) | ||||
|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2019 | ||
| 1st | 42.2 | 46.0 | 32.9 | 27.8 | 19.8 | < 0.0001 (A < 0) |
| 2nd | 31.4 | 70.8 | 39.8 | 29.1 | 34.3 | 0.0206 (A < 0) |
| 3rd | 39.7 | 55.3 | 27.6 | 17.5 | 19.4 | < 0.0001 (A < 0) |
| 4th | 19.2 | 33.5 | 28.5 | 19.5 | 18.8 | 0.2971 (A < 0) |
| 5th | 86.7 | 81.1 | 75.4 | 56.0 | 44.7 | < 0.0001 (A < 0) |
| 6th | 31.6 | 96.1 | 67.4 | 38.3 | 28.7 | < 0.0001 (A < 0) |
| 7th | 4.8 | 16.6 | 14.7 | 15.8 | 15.7 | 0.0542 (A > 0) |
| 8th | 45.2 | 61.6 | 39.5 | 43.8 | 44.5 | 0.2541 (A < 0) |
| 9th | 46.5 | 56.5 | 35.0 | 28.5 | 19.9 | < 0.0001 (A < 0) |
| 10th | 64.0 | 135.7 | 77.0 | 54.7 | 54.8 | < 0.0001 (A < 0) |
| 11th | 190.8 | 187.6 | 93.4 | 77.3 | 53.9 | < 0.0001 (A < 0) |
| 12th | 244.1 | 189.5 | 100.0 | 69.8 | 71.3 | < 0.0001 (A < 0) |
| 13th | 30.7 | 69.2 | 55.0 | 38.6 | 29.1 | 0.0305 (A < 0) |
| Pará State | 71.1 | 81.7 | 52.3 | 40.2 | 34.1 | < 0.0001 (A < 0) |
* Chi-Square test for trend; Increasing trend (A > 0); Decreasing trend (A < 0).
Table 2 - Evolution of detection rates of new leprosy cases in children under 15 years old, according to Regional Health Center and year of diagnosis, Pará State, Brazil, 2000, 2005, 2010, 2015, and 2019
| Regional Health Center | Year | p-value* (trends) | ||||
|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2019 | ||
| 1st | 16.0 | 16.6 | 13.6 | 8.4 | 5.9 | 0.0051 (A < 0) |
| 2nd | 8.4 | 25.8 | 11.3 | 15.1 | 10.3 | 0.5867 (A < 0) |
| 3rd | 11.4 | 21.8 | 9.0 | 1.7 | 8.2 | 0.0071 (A < 0) |
| 4th | 3.6 | 9.9 | 8.6 | 3.5 | 5.1 | 0.6053 (A < 0) |
| 5th | 26.4 | 14.1 | 23.7 | 22.1 | 8.8 | 0.0361 (A < 0) |
| 6th | 7.5 | 21.3 | 22.2 | 14.1 | 9.2 | 0.6561 (A < 0) |
| 7th | 2.9 | 3.9 | 1.3 | 2.5 | 3.5 | 0.8529 (A > 0) |
| 8th | 16.6 | 20.1 | 11.9 | 24.5 | 17.3 | 0.6820 (A > 0) |
| 9th | 7.8 | 12.0 | 10.1 | 3.8 | 1.7 | 0.0144 (A < 0) |
| 10th | 12.2 | 51.5 | 20.3 | 11.5 | 16.5 | 0.0120 (A < 0) |
| 11th | 73.2 | 64.1 | 37.8 | 30.4 | 15.6 | 0.0001 (A < 0) |
| 12th | 82.6 | 67.3 | 32.9 | 26.7 | 25.0 | 0.0001 (A < 0) |
| 13th | 2.5 | 13.9 | 18.3 | 13.7 | 7.0 | 0.4224 (A > 0) |
| Pará State | 22.0 | 24.6 | 17.4 | 12.7 | 8.4 | 0.0008 (A < 0) |
* Chi-Square test for trend; Increasing trend (A > 0); Decreasing trend (A < 0).
DISCUSSION
Despite the reduction in detection rates of leprosy cases in Pará, areas with hyperendemic levels were still found (Figure 1). Similar results were observed in other studies23,24, in which the authors found a higher proportion of cases in the population over 15 years old, with a predominance in the 20-49 age group, considered economically productive. However, a study conducted in Belém, the capital of Pará State25, identified the predominance of cases in the elderly compared to those aged 15 or younger, as did another study analyzing SINAN data nationwide in the 2016-2018 triennium26.
Social and historical determinants associated with the Legal Amazon occupation and the maintenance of social inequalities help to explain the accumulation of infected people when dealing with a disease with a long incubation period. The intensification of epidemiological surveillance in the most endemic areas and the maintenance of practical actions in those with endemic stability depend on significant social mobilization, including the political will of all managers, the commitment and motivation of technicians, and social control5.
However, such efforts are still insufficient to reduce the presence of factors that increase the risk of leprosy transmission. These factors include close human contact, uncontrolled population growth, high urban density, marginalization of population groups, migration, among others, reinforcing low overall health levels and increased exposure to the risk of acquiring communicable diseases27.
The prevalence coefficient, established by the WHO as a priority indicator for achieving leprosy control (< 1/10,000 inhab.), allows for emphasizing the frequency with which cases occur, helping to explain the levels, patterns, and illness trends in the collective and subsidizing the health services actions28. Therefore, studying the occurrence of leprosy in a 20-year historical series enables the analysis of this trend, which is essential for evaluating the development of LCP in health services according to the characteristics of historically constructed populations and territories.
Some authors studying leprosy cases in the elderly of Pará State concluded that the reduction in the detection rate in the general population is accompanied by an increase in this rate, possibly due to the higher susceptibility of this age group29. Although the Ministry of Health9 emphasizes the importance of monitoring the leprosy epidemiological situation, some authors30 consider that the involvement of adults is beyond the scope of health services.
In a study using leprosy data in Pará, from 2004 to 2006, it was observed that, although the disease occurs throughout the state, it is concentrated in the cities that make up the 11th and 12th RHC, as well as the Metropolitan Region30, which does not differ from the findings found in this study regarding these regions. In the analysis of a ten-year historical series of new leprosy cases in Marabá City, in the 11th RHC region, the authors observed a 41.12% reduction in detection rates between 2005 and 2014. However, such reduction did not change the endemicity level, with the city remaining hyperendemic24.
When stratifying detection rates by RHC, different levels of hyperendemicity were identified. Thus, it is possible to minimize the biased view of leprosy elimination when analyzing a state or country with large territorial dimensions, cultural diversities, and social vulnerabilities. The regional analysis also more accurately reflects that the distribution of the disease is becoming more localized in territories with social characteristics and health care services that perpetuate high endemicity31.
In this context, attention to early diagnosis is essential to minimize disabilities and the costs they generate for the health system and the patient32. However, even after reaching the elimination target, leprosy may remain at endemic levels, probably due to the sluggish responses given by the strategies currently developed to tackle the disease. Thus, integrating the health sector and others is necessary to strengthen the capacity to use resources and establish clearer responsibilities among stakeholders in developing countries to achieve the goals by 20307.
When analyzing the detection rate in children under 15 years old, hyperendemicity remained in the 8th, 10th, 11th, and 12th RHC (Figure 2). A study conducted on schoolchildren from eight different cities in Pará, located in the 1st, 3rd, 5th, 8th, 9th, 10th, 11th, and 12th RHC, diagnosed leprosy in 3.9% of those examined33,34. These findings highlight intradomiciliary transmission of the infection and reinforce the need to intensify actions aimed at early detection through active community search, thereby interrupting the transmission chain24,33,34,35. A study conducted in six cities in the 11th RHC coverage area diagnosed leprosy cases in children, indicating that transmission has not been reduced, in addition to the presence of some degree of disability at diagnosis, representing the existence of hidden prevalence36.
Overall, this study found that detection rates in individuals under 15 years old have a downward trend in most RHCs (Table 2), but with no significant impact on detection rates in the general population (Table 1). These observations reinforce the need for investments in training health professionals working in PHC services to establish an early diagnosis, which can be difficult due to the wide variety of clinical aspects of skin lesions and the difficulty of clinical evaluation of peripheral nerves37.
In a study conducted by Sousa et al.16 in a city of the 11th RHC, although the locality was highly oriented towards the development of LCP actions, it was identified that, among the PHC essential attributes, access to the service was rated below the cutoff point established by researchers. The authors concluded, therefore, that the PHC service has fragility in providing necessary access to users and needs to be adequately oriented towards developing actions inherent to leprosy control16. This fact may reflect the inadequate preparation of Family Health Teams (eSF) in leprosy control actions, especially in detecting new cases. The findings highlight the importance of examining household contacts as an indicator of service quality assessment.
In addition to team preparation, the necessary infrastructure for adequate patient care must be considered so that the professional guidance attribute in PHC is properly conducted11. These points are necessary to achieve effective action to transform the work process of eSF38.
One of the challenges for endemic control lies in ensuring the treatment and monitoring of cases in PHC, supported by a referral and counter-referral network, as well as timely health surveillance actions executed within the primary care service network39. Neves et al.40 showed stability in leprosy detection rates in the population aged 10 to 19 years old between 2010 and 2014, indicating the persistence of high endemicity, the lack of adequate health education actions for this population, and the low commitment of human resources in health to the goal of reducing the disease in Pará. For these authors, early diagnosis of leprosy in children and adolescents should be a priority, as the established diagnosis implies physical, emotional, and social impacts40.
Thus, the Ministry of Health considers that teaching-service integration should be incorporated into the operationalization of the National Strategy to Combat Leprosy 2019-2022 through a permanent education strategy for eSFs39.
In a systematic review of PHC attributes, Almeida et al.41 observed inequalities in access to and use of health services in most countries related to the type of service used. Therefore, to minimize these differences, it is necessary to expand health services coverage, which will reduce inequality in access generated by social inequities41.
eSFs tend to concentrate in metropolitan regions42. This heterogeneous distribution reinforces social inequalities and does not offer access to services to specific populations, such as indigenous, quilombolas, gypsies, rural and forest peoples, and riverside dwellers, among others43.
To improve the LCP actions, it is necessary not only to detect new cases but also to understand how these citizens access the service and how LCP actions are being developed to meet the patient's health needs. Services must be aware of the daily life activities of these patients to direct actions toward this clientele38. It is, therefore, essential to dispel prejudices and stigmas, which can help improve the service-patient relationship43.
In Brazil, health policies establish that PHC be integrated with other levels of care due to its importance in disseminating and providing ongoing guidance to the enrolled population about the disease's signs and symptoms. Moreover, timely diagnosis, especially in children, in the longitudinal treatment actions, and the prevention of physical disabilities aim to increase the proportion of cured cases38.
Nevertheless, information about the disease, examining household contacts of recently diagnosed cases, and integrating health services, especially primary care and leprosy referral services, constitute essential strategies for early disease diagnosis and endemic situation control39. In this sense, it is necessary to reorganize/change the actions carried out in the health services network inherent to leprosy control and, above all, based on preventive practices that promote patient identification and early diagnosis of the disease. The eSFs may be trained not only to diagnose leprosy but also to maintain ill patients from the beginning to the end of treatment. Thus, there is a need for a new approach in the technical training of health teams to deal with adverse situations that contribute to abandonment, especially among socio-economically disadvantaged and socially stigmatized segments. Primary care will be genuinely trained and engaged in effectively controlling and eradicating leprosy in Brazil43,44.
Some limitations need to be considered in this study, as the analysis was based on cases notified on SINAN; therefore, the validity of the results presented here depends on the reliability of these records. It is considered that the analyzed data underestimate the actual number of cases in the cities of each RHC, as they are undiagnosed and/or unreported.
CONCLUSION
The results show that leprosy remained at hyperendemic levels in five of the 13 RHC in Pará State. It can be inferred that the analysis of an infectious disease in states with large territorial dimensions, diverse populations and cultural characteristics, and vast social vulnerability should be particularized by specific territories, thus identifying the populations that contribute to maintaining high levels of endemicity.
REFERENCES
1 World Health Organization. Control of neglected tropical diseases [Internet]. Geneva: World Health Organization; 2017 [cited 2021 Jul 17]. Available from: Available from: http://www.who.int/neglected_diseases/diseases/en/ . [ Links ]
2 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Saúde Brasil 2017: uma análise da situação de saúde e os desafios para o alcance dos Objetivos de Desenvolvimento Sustentável [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2022 fev 23]. Disponível em: Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/saude_brasil_2017_analise_situacao_saude_desafios_objetivos_desenvolvimento_sustetantavel.pdf . [ Links ]
3 World Health Organization. The global health observatory: number of new leprosy cases [Internet]. Geneva: World Health Organization ; 2023 [cited 2023 Sep 25]. Available from: Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/leprosy-new-cases-detection-rate . [ Links ]
4 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Saúde Brasil 2013: uma análise da situação de saúde e das doenças transmissíveis relacionadas à pobreza. Brasília: Ministério da Saúde ; 2014. 384 p. [Link] [ Links ]
5 World Health Organization. Global leprosy update, 2017: reducing the disease burden due to leprosy. Wkly Epidemiol Rec. 2018 Aug;93(35):445-56. [Link] [ Links ]
6 World Health Organization. Report of the informal consultation on stopping discrimination and promoting inclusion of persons affected by leprosy: New Delhi, 14-16 november 2017 [Internet]. New Delhi: World Health Organization; 2017 [cited 2019 Nov 12]. Available from: Available from: https://www.who.int/publications/i/item/SEA-GLP-2018.1 . [ Links ]
7 World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030 [Internet]. Geneva: World Health Organization ; 2020 [cited 2021 Apr 3]. Available from: Available from: https://iris.who.int/handle/10665/338565 . [ Links ]
8 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Hanseníase 2023. Bol Epidemiol. 2023 jan;(n. esp):1-51. [Link] [ Links ]
9 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Diretrizes para vigilância, atenção e eliminação da hanseníase como problema de saúde pública: manual técnico-operacional. Brasília: Ministério da Saúde ; 2016. [Link] [ Links ]
10 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Plano integrado de ações estratégicas de eliminação da hanseníase, filariose, esquistossomose e oncocercose como problema de saúde pública, tracoma como causa de cegueira e controle das geohelmintíases: plano de ação 2011-2015. Brasília: Ministério da Saúde ; c2012. 104 p. [Link] [ Links ]
11 Sousa GS, Silva RLF, Xavier MB. Hanseníase e atenção primária à saúde: uma avaliação de estrutura do programa. Saude Debate. 2017 jan-mar;41(112):230-42. Doi: 10.1590/0103-1104201711219 [Link] [ Links ]
12 Pettigrew LM, De Maeseneer J, Anderson MIP, Essuman A, Kidd MR, Haines A. Primary health care and the Sustainable Development Goals. Lancet. 2015 Nov;386(10009):2119-21. Doi: 10.1016/S0140-6736(15)00949-6 [Link] [ Links ]
13 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Hanseníase: conhecendo estigma, discriminação e os direitos das pessoas acometidas pela hanseníase. Brasília: Ministério da Saúde ; 2020. 42 p. [Link] [ Links ]
14 Araújo KMFA, Lana FCF. Relação da hanseníase com a cobertura da estratégia saúde da família e condições socioeconômicas. Cienc Enferm. 2020 ene;26:1. Doi: 10.4067/s0717-95532020000100201 [Link] [ Links ]
15 Pinto LF, Freitas MPS, Figueiredo AWS. Sistemas Nacionais de Informação e levantamentos populacionais: algumas contribuições do Ministério da Saúde e do IBGE para a análise das capitais brasileiras nos últimos 30 anos. Cienc Saude Colet. 2018 jun;23(6):1859-70. Doi: 10.1590/1413-81232018236.05072018 [Link] [ Links ]
16 Sousa GS, Ferreira RLS, Xavier MB. Evaluation of the hanseniasis control program: a study by triangulation of methods. Online Braz J Nurs. 2016 Nov;15(Suppl):583-6. Doi: 10.17665/1676-4285.20165594 [Link] [ Links ]
17 Siqueira SAV, Hollanda E, Motta JIJ. Políticas de promoção de equidade em saúde para grupos vulneráveis: o papel do Ministério da Saúde. Cien Saude Colet. 2017 mai;22(5):1397-406. Doi: 10.1590/1413-81232017225.33552016 [Link] [ Links ]
18 Organização das Nações Unidas (BR). Objetivos de desenvolvimento sustentável no Brasil [Internet]. Brasília: Organização das Nações Unidas Brasil; 2017 [citado 2021 mar 25]. Disponível em: Disponível em: https://brasil.un.org/pt-br/sdgs/3 . [ Links ]
19 Secretaria de Saúde Pública do Estado do Pará (BR). Centros Regionais de Saúde [Internet]. Belém: Secretaria de Saúde Pública do Estado do Pará; 2019 [citado 2020 fev 15]. Disponível em: Disponível em: http://www.saude.pa.gov.br/institucional/centros-regionais-de-saude . [ Links ]
20 Instituto Brasileiro de Geografia e Estatística. Projeção da população das unidades da federação por sexo e idade: 2000-2030, 2019 [Internet]. Rio de Janeiro: IBGE; 2019 [citado 2020 fev 10]. Disponível em: Disponível em: https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html?=&t=downloads . [ Links ]
21 Fundação Amazônia de Amparo a Estudos e Pesquisas (PA). Anuário estatístico do Pará 2019: população por faixa etária, Pará e municípios - 2011 a 2015 [Internet]. Belém: FAPESPA; 2019 [citado 2020 fev 15]. Disponível em: Disponível em: https://www.fapespa.pa.gov.br/sistemas/anuario2019/tabelas/demografia/tab-1.2-populacao-por-faixa-etaria-2011-a-2015.htm . [ Links ]
22 Fundação Amazônia de Amparo a Estudos e Pesquisas (PA). Anuário estatístico do Pará 2019: população total e estimativas populacionais, Pará e municípios - 2014 a 2018. Belém: FAPESPA ; 2019 [citado 2020 fev 15]. Disponível em: Disponível em: https://www.fapespa.pa.gov.br/sistemas/anuario2019/tabelas/demografia/tab-1.1-populacao-total-e-estimativas-populacionais-2014-a-2018.htm . [ Links ]
23 Monteiro MJSD, Santos GM, Barreto MTS, Silva RVS, Jesus RLR, Silva HJN. Perfil epidemiológico de casos de hanseníase em um estado do Nordeste brasileiro. Rev Aten Saude. 2017 out-dez;15(54):21-8. Doi: 10.13037/ras.vol15n54.4766 [Link] [ Links ]
24 Sá SC, Silva DS. Perfil epidemiológico da hanseníase em um município da região Norte do Brasil. Braz J Dev. 2021 jan;7(1):8959-74. Doi: 10.34117/bjdv7n1-608 [Link] [ Links ]
25 Gonçalves NV, Alcântara RCC, Sousa Jr AS, Pereira ALRR, Miranda CSC, Oliveira JSS, et al. A hanseníase em um distrito administrativo de Belém, estado do Pará, Brasil: relações entre território, socioeconomia e política pública em saúde, 2007-2013. Rev Pan-Amaz Saude. 2018 jun;9(2):21-30. Doi: 10.5123/s2176-62232018000200003 [Link] [ Links ]
26 Rocha MCN, Nobre ML, Garcia LP. Características epidemiológicas da hanseníase nos idosos e comparação com outros grupos etários, Brasil (2016-2018). Cad Saude Publica. 2020;36(9):e00048019. Doi: 10.1590/0102/311X00048019 [Link] [ Links ]
27 World Health Organization. Global report for research on infectious diseases of poverty. Geneva: World Health Organization ; 2012. [Link] [ Links ]
28 World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. New Delhi: World Health Organization , Regional Office for South-East Asia; 2018. [Link] [ Links ]
29 Oliveira JSS, Reis ALM, Margalho LP, Lopes GL, Silva AR, Moraes NS, et al. Leprosy in elderly people and the profile of a retrospective cohort in an endemic region of the Brazilian Amazon. PLoS Negl Trop Dis. 2019 Sep;13(9):e0007709. Doi: 10.1371/journal.pntd.0007709 [] [ Links ]
30 Palácios VRCM, Dias RS, Neves DCO. Estudo da situação da hanseníase no estado do Pará. Rev Para Med. 2010 abr-jun;24(2):49-56. [Link] [ Links ]
31 Brook CE, Beauclair R, Ngwenya O, Worden L, Ndeffo-Mbah M, Lietman TM, et al. Spatial heterogeneity in projected leprosy trends in India. Parasit Vectors. 2015 Oct;8:542. Doi: 10.1186/s13071-015-1124-7 [Link] [ Links ]
32 World Health Organization. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases [Internet]. Geneva: World Health Organization ; 2010 [cited 2019 Apr 18]. Available from: Available from: https://iris.who.int/bitstream/handle/10665/44440/9789241564090_eng.pdf?sequence=1 . [ Links ]
33 Barreto JG, Guimarães LS, Frade MAC, Rosa PS, Salgado CG. High rates of undiagnosed leprosy and subclinical infection amongst school children in the Amazon Region. Mem Inst Oswaldo Cruz. 2012 Dec;107(Suppl 1):60-7. Doi: 10.1590/s0074-02762012000900011 [Link] [ Links ]
34 Barreto JG, Bisanzio D, Frade MAC, Moraes TMP, Gobbo AR, Guimarães LS, et al. Spatial epidemiology and serologic cohorts increase the early detection of leprosy. BMC Infect Dis. 2015 Nov;15:527. Doi: 10.1186/s12879-015-1254-8 [Link] [ Links ]
35 Sousa BRM, Moraes FHA, Andrade JS, Lobo ES, Macêdo EA, Pires CAA, et al. Educação em saúde e busca ativa de casos de hanseníase em uma escola pública em Ananindeua, Pará, Brasil. Rev Bras Med Fam Comunidade. 2013 abr-jun;8(27):143-9. Doi: 10.5712/rbmfc8(27)467 [Link] [ Links ]
36 Costa LA, Borba-Pinheiro CJ, Reis JH, Reis Jr SH. Análise epidemiológica da hanseníase na Microrregião de Tucuruí, Amazônia brasileira, com alto percentual de incapacidade física e de casos entre jovens. Rev Pan-Amaz Saude. 2017 set;8(3):9-17. Doi: 10.5123/s2176-62232017000300002 [Link] [ Links ]
37 Barreto JG, Frade MAC, Bernardes Filho F, Silva MB, Spencer JS, Salgado CG. Leprosy in Children. Curr Infect Dis Rep. 2017 Jun;19(6):23. Doi: 10.1007/s11908-017-0577-6 [Link] [ Links ]
38 Nascimento AG, Cordeiro JC. Núcleo ampliado de saúde da família e atenção básica: análise do processo de trabalho. Trab Educ Saude. 2019;17(2):e0019424. Doi: 10.1590/1981-7746-sol00194 [Link] [ Links ]
39 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Estratégia nacional para enfrentamento da hanseníase: 2019-2022 [Internet]. Brasília: Ministério da Saúde ; 2021 [citado 2020 set 22]. Disponível em: Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/estrategia_nacional_enfrentamento_hanseniase_2019.pdf . [ Links ]
40 Neves DCO, Ribeiro CDT, Santos LES, Lobato DC. Tendência das taxas de detecção de hanseníase em jovens de 10 a 19 anos de idade nas Regiões de Integração do estado do Pará, Brasil, no período de 2005 a 2014. Rev Pan-Amaz Saude. 2017 mar;8(1):29-37. Doi: 10.5123/S2176-62232017000100005 [Link] [ Links ]
41 Almeida APSC, Nunes BP, Duro SMS, Facchini LA. Determinantes socioeconômicos do acesso a serviços de saúde em idosos: revisão sistemática. Rev Saude Publica. 2017;51:50. Doi: 10.1590/S1518-8787.2017051006661 [Link] [ Links ]
42 Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Informática do Sistema Único de Saúde. Cadastro Nacional de Estabelecimentos de Saúde. Rede assistencial [Internet]. Brasília: Ministério da Saúde ; 2021 [citado 2021 mar 3]. Disponível em: Disponível em: http://www2.datasus.gov.br/DATASUS/index.php?area=0204&id=11672&VObj=http://tabnet.datasus.gov.br/cgi/deftohtm.exe?cnes/cnv/prid02 . [ Links ]
43 Ministério da Saúde (BR). Secretaria de Gestão Estratégica e Participativa. Políticas de promoção da equidade em saúde. Brasília: Ministério da Saúde ; 2013. 15 p. [Link] [ Links ]
44 Lira RM, Silva MVS, Gonçalves GB. Factors related to abandonment or interruption of leprosy treatment: an integrative literature review. Rev Enferm UFPI. 2017 Oct-Dec;6(4):53-8. Doi: 10.26694/2238-7234.6453-58 [Link] [ Links ]
Received: November 06, 2022; Accepted: August 29, 2023











 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI

