Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.4 Brasília oct./dic. 2016
http://dx.doi.org/10.5123/S1679-49742016000400007
ORIGINAL ARTICLE
Incidence of dengue cases (2007-2013) and seasonal distribution of mosquitoes (Diptera: Culicidae) (2012-2013) in Barreiras, Bahia, Brazil*
1Universidade Federal do Oeste da Bahia, Programa de Pós-Graduação em Ciências Ambientais, Barreiras-BA, Brasil
OBJECTIVE:
to describe seasonal mosquito distribution and the incidence of dengue cases in Barreiras, Bahia, Brazil.
METHODS:
this is a descriptive study using primary data on mosquito distribution in ovitraps from April 2012 to March 2013, as well as secondary data from the Notifiable Diseases Information System, Epidemiological Surveillance and the Zoonosis Control Center about dengue cases and Aedes aegypti infestation rates from January 2007 to March 2013.
RESULTS:
16,512 mosquito specimens were collected, 62.0% were Culex quinquefasciatus (most frequent in the dry season) and 38.0% were Aedes aegypti (most frequent in the rainy season); 8,373 dengue cases were recorded, with highest incidence per 100,000 inhabitants in 2009 (n=704.5), 2011 (n=429.3) and 2013 (n=247.2), between January and June.
CONCLUSION:
Culex quinquefasciatus and Aedes aegypti occurred in all months; dengue incidence was higher in the rainy season and in alternating years.
Key words: Culicidae; Aedes; Dengue; Epidemiology, Descriptive
Introduction
Dengue is one of the main infectious diseases in Brazil, and represents a serious problem to the country's and world's Public Health, especially in tropical and subtropical regions.1 Climate, disordered population growth, rural-urban migration and inadequacy of basic structures in cities are among the favorable conditions for the development of the vector Aedes aegypti, and, consequently, for the viral transmission of dengue.2
The urban distribution of the disease is limited by the distribution of the vector, although its sole presence is not sufficient for the spread of the disease. The pattern of dengue's transmission depends on the interaction of many parameters, including the dynamic of the virus' multiplication, the ecology and behavior of these vectors, in addition to the ecology, behavior and immunity of its human hosts.3
In this context, regional studies are highly important. It is in this geographical scale that the process of the disease transmission occurs, allowing an observation of variables and indicators that, in other levels of analysis, would not be detectable.4
It is important to highlight that Aedes aegypti has the ability to carry the dengue virus serotypes (DENV - 1 to DENV - 4), varying according to the culicidae' population, the females' nutritional status, the vector's infection status and the vertical or transovarial transmission.5
In Brazil, dengue presents a seasonal pattern, with highest rates of cases appearing in the first five months of the year, which is the most humid and hot period, typical in tropical climates.6 In Barreiras, Bahia, there were records of dengue epidemics in 2009, 2011 and 2013, with the occurrence of one death due to the disease in 2009.7
Although there are articles related to the study of the vector and the occurrence of cases of the disease in the country,8,9 little attention is given to the cities located in Cerrado biome, where the distribution of rainfall is different. Moreover, Aedes aegypti represents an important vector of arboviruses, with direct impacts on the population's health. For the western region of Bahia, only one study was carried out in 2014, through the collection of adult culicidae in an urban area during the night,10 with no knowledge of Aedes aegypti's dynamics during the long rainy and dry periods observed in Bahia's Cerrado.
The objective of the present study was to describe seasonal culicidae distribution and the incidence of dengue cases in Barreiras, Bahia, Brazil.
Methods
This is a descriptive epidemiological study, conducted using primary data of the harvest of culicidae and secondary data, which were obtained from the Information System for Notifiable Diseases (Sinan), the 25th Regional Health Administration, Barreiras' Epidemiological Surveillance and Zoonosis Control Center.
Barreiras, which is within Cerrado biome, is located in the outermost part of Western Bahia, between the coordinates 11°37' and 12º25' S and 44°34' and 46º23' W. In 2010, Barreiras had a population of approximately 137,427 inhabitants, of which 90% lived in the urban area, distributed in 7,859 km2 of territorial area with a well-developed commercial and agro industrial center.11
This study started with the installation of ovitraps in 50 points of the city, for a period of 12 months (from April 2012 to March 2013), aiming to collect culicidae in their immature state and assess their distribution throughout the year. The data of dengue cases per neighborhood were used, in order to choose the places for installing the traps; these data were provided by Barreiras' Epidemiological Surveillance, and corresponded to the period from 2003 to 2011.
The ovitraps were composed by large, black, plastic containers, with a maximum capacity of 1,000 mL, filled with tap water and a solution of grass (hay) at 10%. These traps were installed once a month, and stayed in field for seven days. The immature that were captured were put into transport containers and sent to the laboratory; they were fed with fish ration until the achievement of 3rd/4th larval instar, of the pupa or adult phase. The traps that were taken from the field were also sent to the laboratory to verify the presence of eggs, that is why the traps were filled with water and fish ration: to favor the hatching of the eggs.
The Pearson correlation coefficient (with a 5% significance level) was used to verify the possible correlations between the data of the culicidae distribution, the average temperature (maximum, average and minimum) and the accumulated value of rainfall. The worksheets with daily meteorological data were provided by the National Institute of Meteorology (INMET), from Barreiras' automatic weather station (12°09'S, 45°01'W). Concerning this data, the retrospective average of the maximum average and minimum temperatures were calculated for each period, including the 15 days that preceded the harvesting and the days in which the traps remained in field.
For the calculation of the accumulated rainfall, we used the sum of the data related to the 15 days that preceded the harvesting and the days in which the traps remained in field. Not only did the comparisons of the results allow identifying the level, but also the direction of the correlation (positive or negative) between the meteorological variables and data of the culicidae distribution in each harvesting.
The data on dengue cases referred to the period from 2007 to 2013. The following variables were analyzed:
absolute frequency of dengue cases per month and year of notification;
classification of dengue cases (classic dengue, dengue with complications, dengue hemorrhagic fever, dengue shock syndrome, discarded and inconclusive) per year of notification. Since 2014, Brazil has adopted the classification suggested by the World Health Organization: dengue without warning sings, dengue with warning signs and severe dengue;25
evolution of dengue cases (ignored/blank, cure, death due to dengue, and death by another cause) per year of notification; and
dengue serotypes per year of notification.
The coefficients of dengue incidence rates were calculated as follows: number of new cases in the period, divided by the population exposed in the period, multiplied by 100 thousand. The population used was based on estimates by the Brazilian Institute of Geography and Statistics (IBGE).11
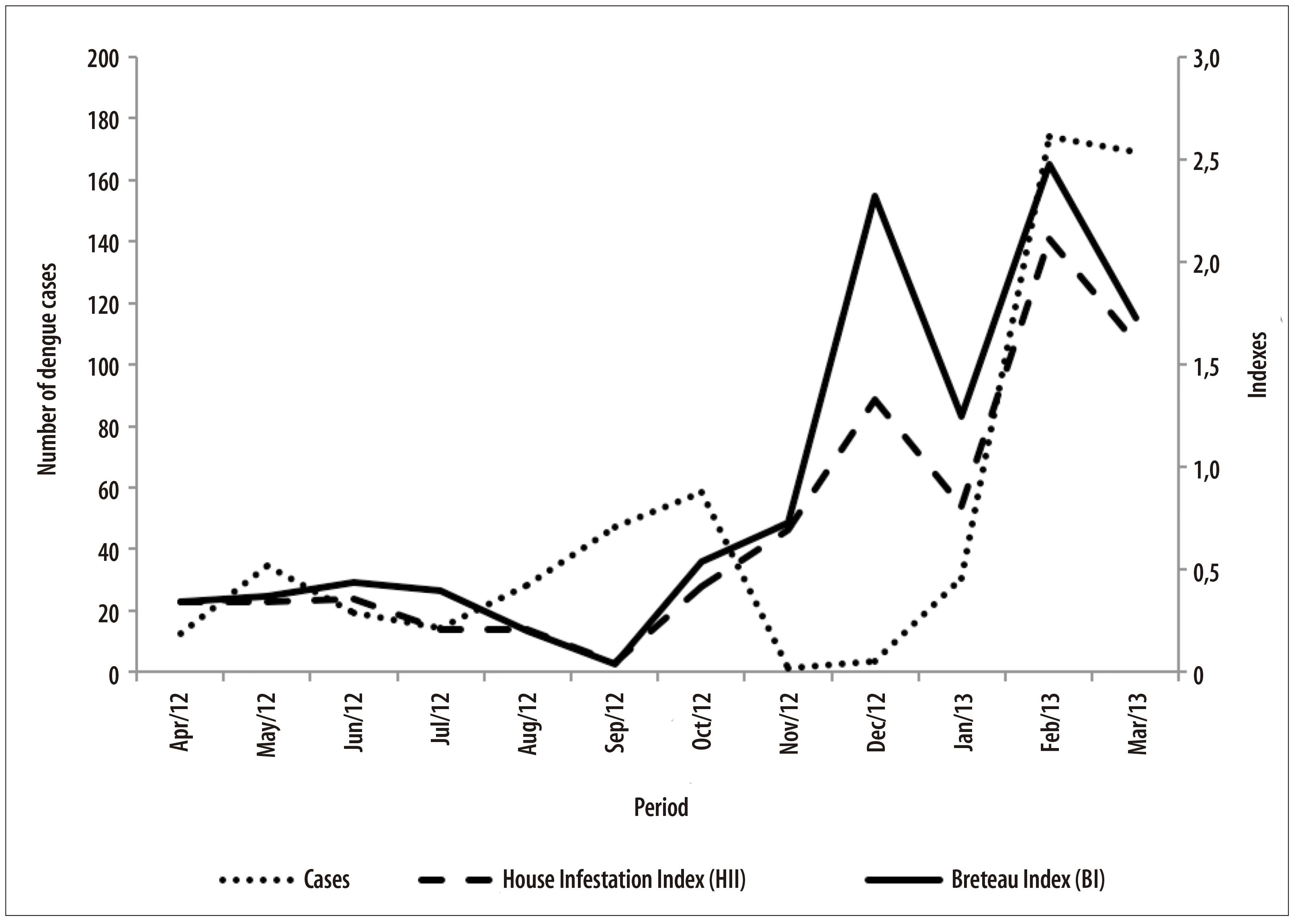
The house infestation index (HII) and the Breteau index (BI) were provided by the Zoonosis Control Center and were calculated as follows:
HII - number of infested houses divided by the number of inspected houses, multiplied by 100; and
BI - number of containers with larva divided by the number of inspected houses, multiplied by 100.12
The data referring to dengue cases were obtained exclusively from secondary sources (official data and public domain), with no identification of the patients, so the approval of the study project by the Ethics Committee in Research was exempted, in conformity with the Resolution of the National Health Council (CNS) No. 466, dated 12 December, 2012.
Results
From April 2012 to March 2013, 16,512 specimens of culicidae were captured in the ovitraps located in the urban area of Barreiras. From this total, 6,197 (38.0%) were Aedes aegypti and 10,315 (62.0%), Culex quinquefasciatus. Most of the Culex quinquefasciatus was collected in the months that corresponded to the dry season, and a smaller amount was collected between December 2012 and March 2013, period with high rainfall incidence in the region. The month with higher distribution of Aedes aegypti was March 2013, with 1,065 specimens, and the month with lower distribution was May 2012, with 127 specimens (Figure 1A).

Figure 1A .- Number of Aedes aegypti and Culex quinquefasciatus captured in ovitraps and B. Temperatures (maximum, average and minimum per month) and accumulated rainfall in Barreiras, Bahia, from April 2012 to March 2013
In Figure 1B, the values of temperature and rainfall in Barreiras are presented, for the period that this study was conducted. Although the first months of the rainy season (November and December) had the greatest distribution of immature forms of Aedes aegypti, the analysis of the correlation between the number of culicidae and the values of meteorological variables were not significant. For this species, the values of Pearson's correlation coefficient that were obtained with data related to the 15 days that preceded the harvest were: maximum temperature (r=0.000), average temperature (r=0.125), minimum temperature (r=0.147) and accumulated rainfall (r=0.015). For Culex quinquefasciatus, the values obtained with Pearson's correlation coefficient were: maximum temperature (r=0.587), average temperature (r=0.061), minimum temperature (r= -0.447) and accumulated rainfall (r= -0.465) (the data were not represented in the table).
With regard to the monthly distribution of dengue cases in Barreiras and its comparison with rainfall data, it was observed that from January to May, months that correspond to rain period in Cerrado, there was an increase in the number of dengue cases, and a reduction of cases between June and November, i.e., the dry period (Figure 2).
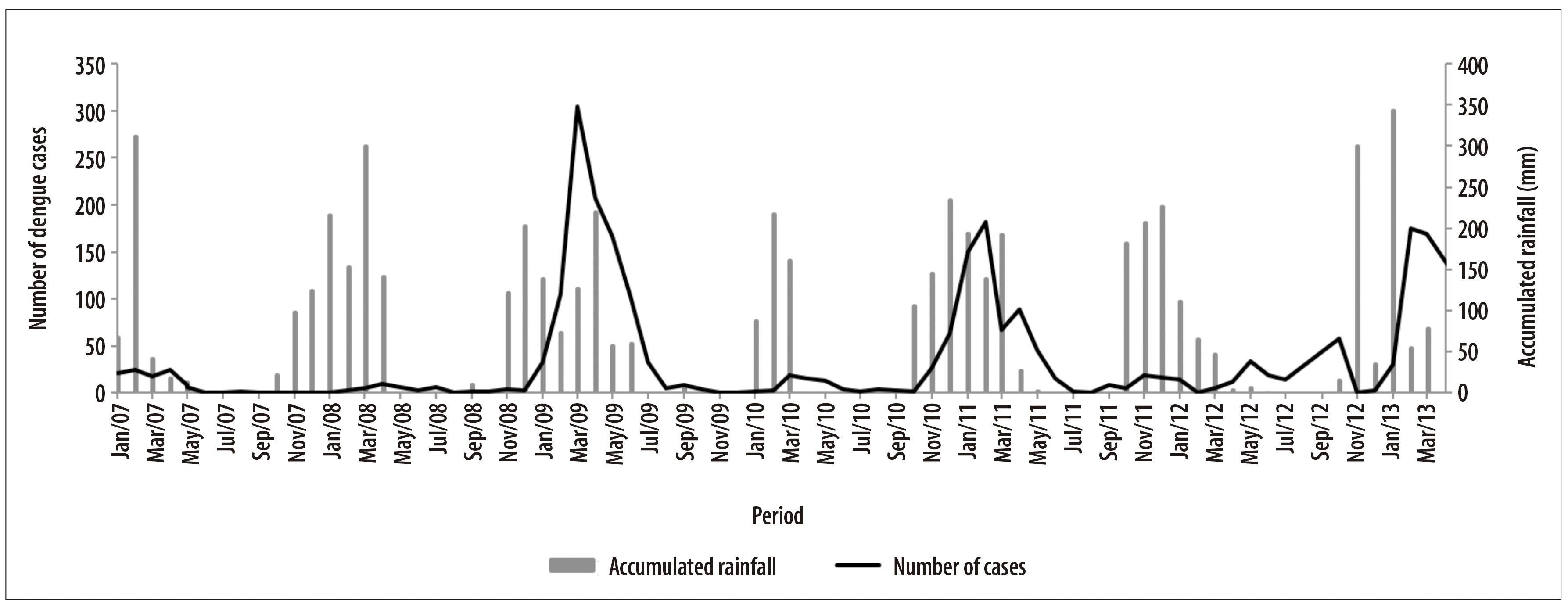
Figure 2 - Monthly number of dengue cases and accumulated rainfall in Barreiras, Bahia, from January 2007 to March 2013
Between January 2007 and March 2013, 8,373 dengue cases were notified, from which 68.8% (n=5,757) were probable cases of the disease. There were records of dengue epidemic in 2009, 2011 and 2013, whose incidence coefficients were of 704.5, 429.3 and 247.2 per 100 thousand inhabitants, respectively (Table 1).
Table 1 - Distribution of dengue cases according to the final classification and incidence (per 100 thousand inhabitants), per year of notification, in Barreiras, Bahia, from January 2007 to March 2013
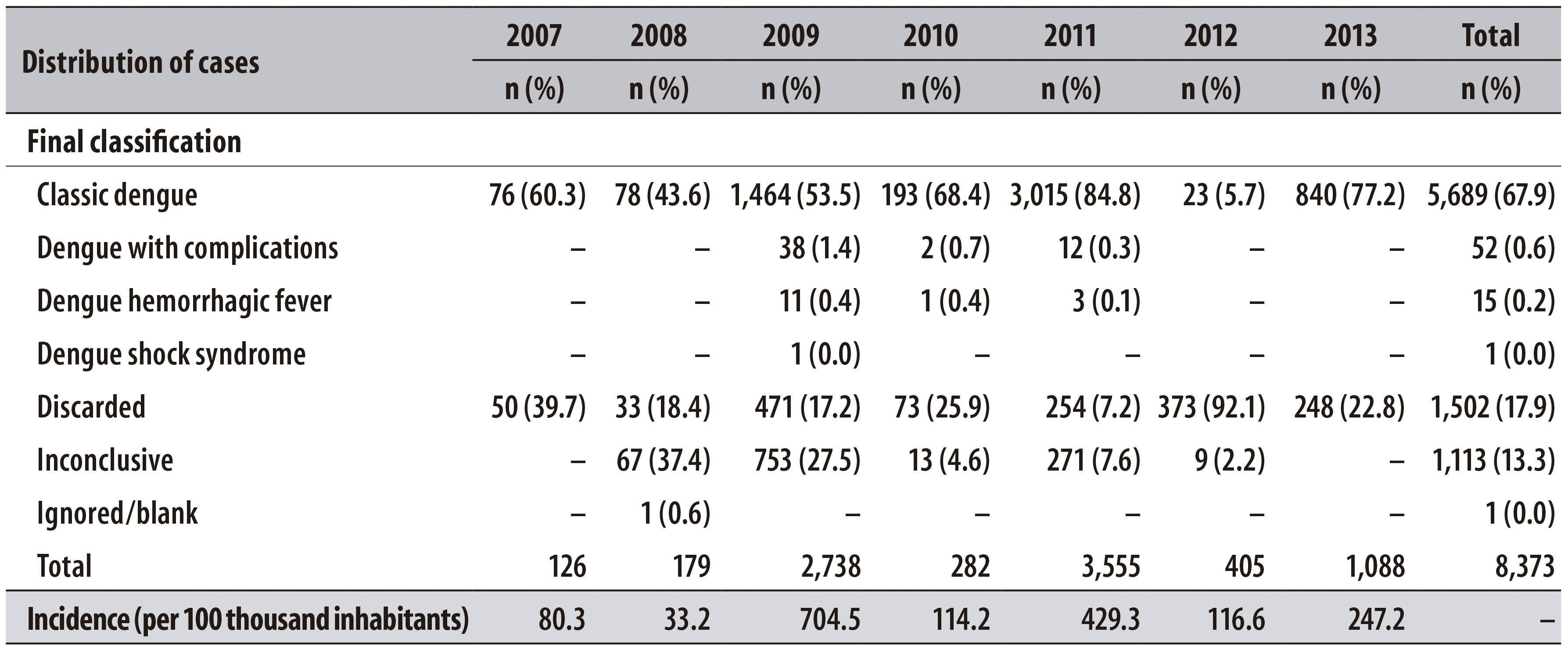
During the period of the study, most of the cases were classified as classic dengue (67.9%; n=5,689). From the total of cases, 31.2% were represented by discarded (17.9%; n=1,502) and inconclusive (13.3%; n=1,113) cases. The most severe cases represented 0.2% (n=15) of dengue hemorrhagic fever and there was one case of dengue shock syndrome (Table 1). Despite the elevated number of dengue cases, 77.9% (n=6,525) evolved to cure. Three deaths due to dengue and three due to other causes were registered (Table 2).
Table 2 - Distribution of cases according to the evolution of dengue virus serotype, per year of notification, in Barreiras, Bahia, from January 2007 to March 2013

From January 2007 to March 2013, 102 (1.2%) dengue cases were identified as viral serotype. For the years that the municipality had dengue epidemics, 298 samples for viral isolation were processed in 2009, 110 in 2010 and 245 up to March 2013: from this total (n=653), dengue viral serotypes were isolated in 13.6% (n=89) of the cases (Table 2). The virus DENV - 1 circulated from 2009 to 2012, reappearing in the first trimester of 2013, corresponding to 58.8% (n=60) of the total of identified cases. DENV - 2 circulated in the city in 2008 and 2009, and returned in 2011 (n=37; 36.3%), and the virus DENV - 3 was found from 2007 to 2010 (n=5; 4.9%) (Table 2). During the period of the study, there was no record of DENV - 4 circulation in Barreiras.
The data on the incidence of Aedes aegypti and the number of dengue cases obtained with the city's Epidemiological Surveillance (Figure 3) show an HII lower than 1% in the period from April to November 2012, despite the elevated number of dengue cases during the same period. Between December 2012 and February 2012, the HII raised to an alert status, with a percentage of 2.1%, accompanying the increasing trend in the number of dengue cases.
Discussion
Two important disease vectors that attack humans (Culex quinquefasciatus and Aedes aegypti) were identified by collecting immatures in Barreiras' urban area, during all months of the year - including during the long drought period that is a characteristic of Cerrado (April to October). The incidence of dengue and the occurrence of the vector Aedes aegypti during the period of the study were higher in the rainy period, which are the months from January to May 2013. In this study, despite the rainfall and the temperature having not directly influenced Aedes aegypti infestation index, they have allowed ideal conditions for the reproduction of the vector and the consequent proliferation of dengue cases.
Culex quinquefasciatus is efficient in the transmission of the virus bancroftian filariasis, endemic disease present in tropical and subtropical regions, particularly in the metropolitan area of Recife (Pernambuco, Brazil), besides transmitting West Nile fever, St. Louis encephalitis and Oropouche virus.13,14,15,16 The elevated occurrence of Culex quinquefasciatus during the dry season has already been verified by the harvest of adult insects in an urban area in western Bahia, in 2014;10 however, the factors that regulate this species' populations are still unknown. It is possible that the lack of sanitation enables the existence of many breeding grounds, even in long dry periods.17 In other urban centers, Culex quinquefasciatus is also abundant in dry season, with the rain being a factor of species control since it alters the physicochemical conditions of breeding or even carries larvae, pupae and rafts of eggs from one point of the breeding to another.18
Aedes aegypti has vectorial competence for transmitting the four dengue serotypes, Zika virus, chikungunya and yellow fever.19-21 The data resulting from the harvesting of immature specimens were similar to the ones supplied by the Municipal Surveillance Service and show the same trends regarding the occurrence of the species throughout the 12 months. However, the number of dengue cases notified in Barreiras and the values of HII and BI do not follow the same trends in those 12 months. Thus, although there significant correlation between the meteorological variables and the occurrence of immatures in the traps, it was possible to identify that the populations of Aedes aegypti are more abundant in the rainy period, especially in January, February and March.
The patterns for the occurrence of both species may be related with the interaction of many elements: competition between both species, way of life of human populations, favorable climate conditions to the development of each insect's species, actions to control the vectors and environmental management, among other factors. The absence of rain in certain months of the year with the presence of the vector, indicates that humans keep reservoirs with the necessary conditions to oviposition and development of immatures.2,22 Researches suggest that there is a good relationship between the density of an egg per ovitrap and the incidence of the disease.22 In this regard, the epidemic relations with the social-urban structure can and should be studied, which, in a determined historical and political moment, interact with the transmission of the disease.
In Barreiras, the incidence of dengue also seems to be higher in the rainy period, i.e., between January and May 2013, similar to the populations of the vector Aedes aegypti. Although there are no data on the mosquito's life course in this region, a study conducted in 2012 points out that the temperature influences the time of larval development, the survival rate and the period of incubation of dengue virus in Aedes aegypti, thus, compromising its capacity for viral transmission.3 The low humidity may affect negatively the survival of adults, and diminish the vector population.3 Although there is a great decrease in the adult population of Aedes aegypti in drier months, the eggs may still be found in ovitraps during these periods in different regions of the country.2,23 These data confirmed the information obtained in this present study and highlight the need for a constant monitoring of populations, due to the risk of increasing in the distribution of vectors and transmission of arboviruses.2,24
In Brazil, dengue presents a seasonal pattern, with most cases appearing during the first five months of the year, which is a more humid and hot period, typical in tropical climates.6 Regardless of the fact that Cerrado has a different climate from the other regions of the country, it was verified that December and the first three months of the year are the ones with highest occurrence of the vector in this biome. The monthly records of dengue cases informed by Barreiras' Epidemiological Surveillance show a growth in the number of cases in the first semester of the year, the city's rainy period. The disease seems to manifest in cycles, possibly due to the increase or decrease of the actions to control the vector and the environmental education to the population, as an example of the reduction observed in 2008, 2010 and 2012.
In the case of Barreiras, the information regarding the clinical profile of the patients revealed a great number of discarded and inconclusive cases, compromising the development of epidemiological studies. However, situations where the disease worsens or evolves to death - due to dengue itself, or to other causes - represented a small percentage in the city.
It is important to highlight that the classification of dengue cases has changed since 2014. According to the World Health Organization (WHO), only the nomenclatures 'dengue', 'dengue with warning signs' and 'severe dengue' should be used.25
In Barreiras, the majority of dengue cases did not have identification of viral serotypes, which points to the need of improving timely serological investigation. According to the local Epidemiological Surveillance, the samples for viral isolation are sent to the Central Public Health Laboratory (Lacen) in Salvador, the state's capital. In 2012, no viral serotype was identified, despite of the 405 cases registered. Up to March 2013, serotype DENV - 4 was not found among the samples for isolation of dengue virus. According to the National Program for Dengue Control (PNCD) of the Ministry of Health, the objective of laboratorial diagnosis of cases is the early detection of viral circulation and the monitoring of circulating serotypes. Therefore, the laboratorial surveillance must be employed to answer to demands of epidemiological surveillance, not being its objective to diagnose all suspect cases, when there is an epidemic situation.26
This is the first study on seasonal distribution of culicidae in artificial breeding in western Bahia, with an epidemiological importance. Moreover, recent introductions to other arboviruses in the state, caused by Zika27 and Chikungunya28 viruses, make the efficiency of the diagnosis system for the region and the constant monitoring of vectors even more urgent.
It is worth to highlight that Barreiras is the biggest urban center in Western Bahia, and is responsible for the hospital care of people who live in other nearby cities, what points to the urgent need of having reference laboratories for the detection of the disease's etiological agents also in the countryside. Considering that the vector remains in activity in Bahia's Cerrado during all months of the year, continuous actions of surveillance and for the insect's control are necessary. Future studies on the variables that contributed for the maintenance of arboviruses in urban areas should be conducted, with the aim of monitoring vectors and the circulating arbovirus, reducing the risks of epidemics in the region.
REFERENCES
1. Costa AG, Santos JD, Conceição JKT, Alecrim PH, Casseb AA, Batista WC, et al. Aspectos epidemiológicos do surto de Dengue em Coari-AM, 2008 a 2009. Rev Soc Bras Med Trop. 2011 jul-ago;44(4):471-4. [ Links ]
2. Costa FS, Silva JJ, Souza CM, Mendes J. Dinâmica populacional de Aedes aegypti (L) em área urbana de alta incidência de dengue. Rev Soc Bras Med Trop. 2008 maio-jun;41(3):309-12. [ Links ]
3. Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010 Jan;12(4):272-9. [ Links ]
4. Flauzino RF, Souza-Santos R, Oliveira RM. Indicadores socioambientais para vigilância da dengue em nível local. Saude Soc. 2011 jan-mar;20(1):225-40. [ Links ]
5. Forattini OP. Culicidologia médica: identificação, biologia e epidemiologia. Vol 2. São Paulo: Editora da Universidade de São Paulo; 2002. [ Links ]
6. Braga IA, Valle D. Aedes aegypti: histórico do controle no Brasil. Epidemiol Serv Saude. 2007 abr-jun;16(2):113-8. [ Links ]
7. Secretaria Municipal de Saúde de Barreiras (BA). Situação epidemiológica da Dengue no município de Barreiras. Boletim Epidemiológico. 2014;(1):1-2. [ Links ]
8. Arduino MB, Marques GRAM, Serpa LLN. Registro de larvas e pupas de Aedes aegypti e Aedes albopictus em recipientes com água salina em condições naturais. BEPA, Bol Epidemiol Paulista. 2010 nov;7(83):22-8. [ Links ]
9. Nunes LS, Trindade RBR, Souto RNP. Avaliação da atratividade de ovitrampas a Aedes (Stegomyia) aegypti Linneus (Diptera: Culicidae) no bairro Hospitalidade, Santana, Amapá. Biota Amazônia. 2011;1(1):26-31. [ Links ]
10. Santos IM, Calado D. Captura de mosquitos antropofílicos (Diptera, Culicidae) em uma área urbana da região oeste da Bahia, Brasil. Iheringia, Ser Zool. 2014 mar; 104(1):32-8. [ Links ]
11. Instituto Brasileiro de Geografia e Estatística. População residente, por situação do domicílio e localização da área, segundo as Grandes Regiões, as Unidades da Federação e o sexo: 2010 [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2010 [citado 2013 abr 13]. Disponível em: Disponível em: http://www.censo2010.ibge.gov.br/sinopse/index.php?uf=29&dados=1 . [ Links ]
12. Gomes AC. Medidas dos níveis de infestação urbana para Aedes (stegomyia) aegypti e Aedes (stegomyia) albopictus em Programa de Vigilância Entomológica. Inf Epidemiol SUS. 1998 set;2(3):49-57. [ Links ]
13. Henriques DA. Caracterização molecular de arbovírus isolados da fauna díptera nematocera do Estado de Rondônia (Amazônia Ocidental Brasileira) [tese]. São Paulo: Universidade de São Paulo, Instituto de Ciências Biomédicas; 2008. [ Links ]
14. Kuwabara EF. Fauna de Culicidae (Diptera: culicidae) em área litorânea do Estado do Paraná, Brasil [dissertação]. Curitiba: Universidade do Paraná. Curitiba; 2004. [ Links ]
15. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Guia de vigilância do Culex quinquefasciatus. Brasília: Ministério da Saúde; 2011. (Série A. Normas e manuais técnicos). [ Links ]
16. Amorim LB, Helvecio E, Oliveira CM, Ayres CF. Susceptibility status of Culex quinquefasciatus (Diptera: Culicidae) populations to the chemical insecticide temephos in Pernambuco, Brazil. Pest Manag Sci. 2013 Dec;69(12):1307-14. [ Links ]
17. Medeiros Z, Oliveira C, Quaresma J, Barbosa E, Aguiar-Santos AM, Bonfim C, et al. A filariose bancroftiana no município de Moreno - Pernambuco, Brasil. Rev Bras Epidemiol. 2004 mar;7(1):73-9. [ Links ]
18. Prefeitura Municipal (BA). Plano setorial de abastecimento de água e esgotamento sanitário de Barreiras. Barreiras: Prefeitura Municipal; 2010 [citado 2013 ago 14]. Disponível em: Disponível em: http://barreiras.ba.gov.br/pdf/rel_pssb_barreiras.pdf . [ Links ]
19. Dibo MR, Menezes RMT, Ghirardelli CP, Mendonça AL, Chiaravalloti Neto F. Presença de culicídeos em município de porte médio do Estado de São Paulo e risco de ocorrência de febre do Nilo Ocidental e outras arboviroses. Rev Soc Bras Med Trop. 2011 jul-ago;44(4):496-503. [ Links ]
20. Pinto Júnior VL, Luz K, Parreira R, Ferrinho P. Vírus Zika: revisão para clínicos. Acta Med Port. 2015 nov-dec;28(6);760-5. [ Links ]
21. Vansconcelos PFC. Doença pelo vírus Zika: um problema emergente nas Américas? Rev Pan-Amaz Saude. 2015 abr-jun;6(2):9-10. [ Links ]
22. Cohnstaedt LW, Rochon K, Duehl AJ, Anderson JF, Barrera R, Nan-Yao Su, et al. Arthropod surveillance programs: basic, components, strategies and analysis. Ann Entomol Soc Am. 2012 Mar;105(2):135-49. [ Links ]
23. Braga IA, Gomes AC, Nelson M, Mello RCG, Bergamaschi DP, Souza JMP. Comparação entre pesquisa larvária e armadilha de oviposição, para detecção de Aedes aegypti. Rev Soc Bras Med Trop. 2000 jul-ago;33(4):347-53. [ Links ]
24. Leandro RS. Competição e dispersão de Aedes (Stegomyia) aegypti (Linnaeus, 1762) e Aedes (Stegomyia) albopictus (Skuse, 1894) (Diptera: culicidae) em áreas de ocorrência no município de João Pessoa - PB [dissertação]. Campina Grande: Universidade Estadual da Paraíba, Centro de Ciência e Tecnologia; 2012. [ Links ]
25. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Dengue: diagnóstico e manejo clínico: adulto e criança [Internet]. 5 ed. Brasília: Ministério da Saúde ; 2016. [ Links ]
26. Ministério da Saúde (BR); Fundação Nacional de Saúde. Programa Nacional de Controle da Dengue (PNCD). Brasília: Ministério da Saúde ; 2002. [ Links ]
27. Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015 Oct;21(10):1885-6. [ Links ]
28. Donalísio MR, Freitas ARR. Chikungunya no Brasil: um desafio emergente. Rev Bras Epidemiol. 2015 jan-mar;18(1):283-5. [ Links ]
*This article resulted from Isabelle Matos Master's thesis, presented to the Post-graduate program in Environmental Sciences of the Universidade Federal do Oeste da Bahia, in 2014. The study had the financial support of the Foundation of Support for Research of the State of Bahia (Fapesb): Protocol No. PPP0072/2010.
Received: February 06, 2016; Accepted: April 22, 2016











 texto en
texto en