Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.30 no.1 Brasília 2021 Epub 31-Mar-2021
http://dx.doi.org/10.1590/s1679-49742021000100006
Original article
Different methods for assessing gestational weight gain and its association with birth weight *
1Fundação Instituto Oswaldo Cruz, Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro, RJ, Brazil
2Universidade Federal do Rio de Janeiro, Instituto de Nutrição Josué de Castro, Rio de Janeiro, RJ, Brazil
Objective
To analyze association of different methods of gestational weight gain assessment with live births small for gestational age (SGA) and large for gestational age (LGA).
Methods
This was a cross-sectional study with adult women, normal prepregnancy BMI, single pregnancy and gestational age at delivery ≥28 weeks, from the “Birth in Brazil” study, between 2011 and 2012.
Results
Among the 11,000 women participating in the study, prevalence of excessive weight gain was 33.1% according to the Brandão et al. and Institute of Medicine (IOM) methods, and 37.9% according to the Intergrowth method. The chance of being born SGA in the case of insufficient weight gain was OR=1.52 (95%CI 1.06;2.19), OR=1.52 (95%CI 1.05;2.20) and OR=1.56 (95%CI 1.06;2.30) for the Brandão et al., IOM and Intergrowth methods, respectively. Likelihood of excessive weight gain using the same methods was OR=1.53 (95%CI 1.28;1.82), OR=1.57 (95%CI 1.31;1.87) and OR=1.65 (95%CI 1.40;1.96), for LGA respectively.
Conclusion
Compared to the IOM recommendations, the Intergrowth and Brandão et al. methods show themselves to be alternatives for identifying SGA and LGA.
Keywords: Pregnancy; Weight Gain; Birth Weight; Cross-Sectional Studies
Introduction
Gestational weight gain (GWG) reflects different changes arising from gestation, including accumulation of body fat in women, liquid expansion, fetal and placental development, growth in breast tissue and the uterus.1 Insufficient gestational weight gain may increase the chance of spontaneous preterm delivery, and, as a consequence, birth of newborns small for gestational age (SGA).2-4 However, excessive gestational weight gain in women raises the chances of cesarean delivery, postpartum weight retention, and newborns large for gestational age (LGA).2-4
Newborns classified as SGA may present neurological development deficits, school performance below expected, short stature arising from growth hormone deficiency, and, in adult life, greater chances of metabolic syndrome.5 In LGA newborns a decrease in sensitivity to insulin can be seen, which can lead to increase in fat accumulation, and, in adult life, greater chance of developing excess weight, cardiometabolic diseases and type 2 diabetes mellitus.6,7
Considering the role of maternal weight gain in children’s wellbeing, the Institute of Medicine (IOM) (1990)8 developed guidelines for total GWG for the North-American population.1,8 In 2009, the IOM recommended new ranges for GPG, with estimates for total weight gain, GPG in the first quarter and a GPG rate, based on the World Health Organization (WHO)1 classification, for the pre- gestational age (BMI).1,2
New approaches have emerged in recent years, among them the International Fetal and Newborn Growth Consortium for the 21st (Intergrowth)9, which, in 2016, launched GWG standards according to gestational age, for women with normal BMI, in percentiles and z scores. That study was based on a multiethnic cohort (China, Italy, Oman, India, United States, United Kingdom, Kenya and Brazil).9 Brandão et al.,10 when analyzing data from the “Birth in Brazil” national survey on deliveries and births, proposed total GWG percentiles for all prepregnancy BMI categories, including classes I, II and III of obesity.10 The Brazilian Ministry of Health,11 in its publication entitled Low Risk Prenatal Care, corroborates the IOM recommendations (2009)2 for scheduling gestational weight gain, despite their not having been implemented universally.2,11
Although some analyses of gestational weight gain based on IOM recommendations2 have been performed SGA and LGA outcomes have still not been tested according to Intergrowth standards,9 nor according to the proposal put forward by Brandão et al.,10 which inclues in their sample a considerable diversity of data on the Brazilian population.
The objective of this study was to analyze association of different methods of gestational weight gain assessment with live births small for gestational age (SGA) or large for gestational age (LGA).
Methods
This is a cross-sectional study developed with data from the “Birth in Brazil” survey, conducted with postpartum women from February 2011 to October 2012. A complex sample was selected, that envolved 266 hospitals and 90 women in each hospital, totaling 23,940 interviewees. This sample was weighted by inverse probability of inclusion of each postpartum woman, and calibrated so as to distribute these women in a similar way to that observed in 2011. Additional details on the “Birth in Brazil” sample design are contained in other publications.12,13
The “Birth in Brazil” survey used standardized instruments for collecting information, face-to-face interviews with postpartum women in the period of hospitalization, data from prenatal cards (pictures taken, later typed), and examining medical records of the women and newborns, regarding “current pregnancy”.12,13 Demographic, socioeconomic, health and prenatal care data were collected.12,13
The study population was comprised of adult women (≥20 years), with single fetus gestation, live birth, gestational age at birth from 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2). Prepregnancy BMI (kg/m2) was calculated based on prepregnancy weight, divided by height in meters squared.1 Women for whom there was no information on weight at the end of pregnancy were excluded, as this measurement is fundamental for calculating total gestational weight gain.
For the composition of “prepregnancy weight” (kg), we considered the value described in the pregnant woman’s prenatal card and/or notebook. If prepregnancy weight was unknown, we adopted the weight measured up to the 13th week of pregnancy or the weight reported by the woman during the interview.10 For height, we considered the data recorded or reported by the woman in the interview.
Reported measurements were validated in a previous study, conducted with the same population. On that occasion, good correlation between the information held on prenatal cards (gold standard) and information reported by the postpartum women could be seen: an intraclass correlation coefficient (ICC) of 0.96 for prepregnancy weight and 0.89 for height.14
The data imputation method was used to treat missing data on prepregnancy weight and height. In the initial sample (n=23.940), 17.5% of the women had one piece of their data imputed (15.5% maternal height data and 4.4% prepregnancy weight data), by means of the chained equations method (MICE).15 The model for predicting multiple imputation of BMI included the following variables: national macroregion; source of payment for the delivery of the child; education level (years of schooling); race/skin color; age; parity; presence of diabetes mellitus or hypertension (chronic or gestational); prepregnancy weight; weight by the end of pregnancy; and height.
We chose the Fully Conditional Specification (FCS) method from the IBM SPSS version 21.0 software to obtain ten sets of imputed data. Following this, the models were set up based on those multiple sets of imputed data, adopting Rubin’s rules for combining estimates of effect and estimates of standard error.15,16 The results presented in this article were obtained following imputation of prepregnancy weight and height. The same imputation resource was used by Bodnar et al.17 to estimate association between gestational weight gain using z scores and maternal and neonatal outcomes, in women with obesity class I, II and III.17
Gestational weight gain (kg) - GWG, the exposure variable - was built from the difference between final gestation weight and prepregnancy weight.18 The following methods were used for GWG assessment: (i) total GWG in percentiles as proposed by Brandão et al.;10 (ii) Intergrowth percentiles9 - standardized GWG -; and (iii) IOM recommendations on total GWG.2
Brandão et al.10 recommended percentiles (P) between P30 and P70 for normal BMI. The same interval was adopted with the aim of achieving correspondence with the Intergowth percentiles.9 Gestational weight gain, in this study, was classified as insufficient GWG (<P30), adequate GWG (P30 to P70) and excessive GWG (>P70).
For Intergrowth,9 the percentiles for classifying women with normal BMI were estimated by typing total gestational weight gain and gestational age in weeks and days as at delivery. Given the large size of the database, this procedure used an Excel spreadsheet available on the Intergrowth study webpage.9
For IOM,12 the total GWG variable was classified according to the reference interval for women with normal BMI: insufficient GWG (<11.5kg); adequate GWG (11.5 to 16.0kg); and excessive GWG (>16.0kg). In the method proposed by Brandão et al.,10 the selected percentiles presented values for P30 and P70 corresponding to 11.0kg and 16.0kg respectively.10 The selected P30 and P70 intervals for Intergrowth9 did not present ranges of fixed weight values, unlike the other two methods. The <P10, P10-P20, P20-P30, P30-P70, P70-P80, P80-P90 and >P90 intervals were also considered for comparison with the method proposed by Brandão et al.,10 and with the Intergrowth method.9
Birth weight for gestational age is the outcome variable for this study. We used the SGA definition as birth weight for gestational age below P10 and LGA as birth weight for gestational age above P90, both compared with adequate weight for gestational age - the reference category, according to the child’s sex. The percentiles were standardized with reference to Intergrowth.9,19
The variables used as potential confounders in the relation between gestational weight gain and SGA and LGA outcomes were: national macroregion (North; Northeast; Southeast; South; Midwest); source of payment for the delivery of the child (public; private); mother’s age (in years: 20 to 24; 25 to 29; 30 to 34; 35 to 39; 40 or older); race/skin color (white; black; brown; Asian; Indigenous); education level (in years of schooling: up to 7; 8 to 11; 12 to 14; 15 or more); number of prenatal care medical appointments (no prenatal care; 1 to 3; 4 to 6; 7 or more); gestational age (in weeks: 28 to 31; 32 to 36; 37 to 41; 42 or more); type of delivery (normal; cesarean delivery); parity (nulliparity; 1 to 2 previous deliveries; 3 or more previous deliveries); smoking during pregnancy (yes; no).
Average gestational weight gain was calculated using univariate logistic regression analysis for complex samples, using the Bonferroni test to assess statistical significance. The differences between the SGA outcome and the LGA outcome prevalence rates were assessed according to 95% confidence intervals (95% CI) and Pearson’s chi-squared test. A 5% significance level was adopted. Sample weight and design were considered in all analyses.
Logistic regression analysis was conducted for each of the outcomes - SGA and LGA -, with GWG as the main exposure variable; crude and adjusted odds ratio (OR) were estimated, with a 95% CI for all outcomes, using all participants presenting adequate GWG (P30 to P70) as a reference. The models were adjusted for potential confounders. Only the IOM recommendations model2 and the model proposed by Brandão et al.10 were adjusted according to the variable “gestational age”, since the Intergrowth9 classification adjusts for gestational age via the software made available by the authors. All analyses were conducted using the IBM SPSS, version 21.0, statistics software.
The study was based on data from the “Birth in Brazil” survey, the sample design of which was submitted to and approved by the Research Ethics Committee of the Sergio Arouca National School of Public Health, Fundação Instituto Oswaldo Cruz (ENSP/Fiocruz): Certificate of Submission for Ethical Appraisal No. 92182418.9.0000.5240, issued on July 08, 2018. The “Birth in Brazil” survey provided a Free and Informed Consent form to be signed by each respondent before carrying out the interview.
Results
Among the 23,940 women, 11,034 fulfilled the criteria for inclusion in the sample; 34 of them did not have information on final gestational weight, which is fundamental for calculating total gestational weight gain, and this variable could not be imputed for these women. For this reason, the final study sample had exactly 11,000 participants, whose characteristics are described in Table 1. It can be seen that 42.7% of the interviewed women lived in Southeastern region, 75.9% had their deliveries in public health facilities, 38.0% were between 20 and 24 years old, 55.1% had brown skin color, 45.1% had 12 to 14 years of schooling, 60.8% attended 7 or more prenatal care medical appointments, and 53.2% had cesarean delivery.
Table 1 Distribution of maternal characteristics among the 11,000 womena taking part in the “Birth in Brazil” survey, 2011-2012
| Variables | Sample description | 95%CIb | Average gestational weight gain (kg) | p-valuec | |
|---|---|---|---|---|---|
| n | % | ||||
| Total | 11,000 | 100.0 | 14.30 | ||
| National macroregion | |||||
| Southeast | 4,699 | 42.7 | 40.0;45.5 | 14.41 | 0.015 |
| Northeast | 3,253 | 29.6 | 27.5;31.7 | 14.05 | |
| South | 1,391 | 12.6 | 11.5;13.8 | 14.29 | |
| North | 944 | 8.6 | 7.8;9.4 | 14.28 | |
| Midwest | 713 | 6.5 | 5.7;7.4 | 14.77 | |
| Delivery payment source | |||||
| Public | 8,344 | 75.9 | 74.2;77.5 | 14.22 | 0.059 |
| Private | 2,656 | 24.1 | 22.5;25.8 | 14.57 | |
| Age group (years) | |||||
| 20-24 | 4,182 | 38.0 | 36.7;39.4 | 14.29 | 0.300 |
| 25-29 | 3,275 | 29.8 | 28.8;30.8 | 14.29 | |
| 30-34 | 2,325 | 21.1 | 20.1;22.2 | 14.65 | |
| 35-39 | 999 | 9.1 | 8.4;9.8 | 13.82 | |
| ≥40 | 218 | 2.0 | 1.7;2.3 | 13.25 | |
| Race/skin color | |||||
| Brown | 6,066 | 55.1 | 53.0;57.2 | 14.33 | 1.000 |
| White | 3,953 | 35.9 | 33.8;38.2 | 14.26 | |
| Black | 806 | 7.3 | 6.5;8.3 | 14.19 | |
| Asian | 134 | 1.2 | 0.9;1.6 | 14.96 | |
| Indigenous | 40 | 0.4 | 0.2;0.5 | 14.93 | |
| Maternal education level (years of study) | |||||
| ≤7 | 2,386 | 21.7 | 20.2;23.2 | 13.49 | <0.001 |
| 8-11 | 2,329 | 21.2 | 19.9;22.4 | 14.29 | |
| 12-14 | 4,963 | 45.1 | 43.0;47.3 | 14.65 | |
| ≥15 | 1,322 | 12.0 | 10.4;13.8 | 14.46 | |
| Parity | |||||
| First birth | 4,618 | 42.0 | 40.6;43.3 | 14.71 | <0.001 |
| 1 previous birth | 3,583 | 32.6 | 31.5;33.7 | 14.13 | |
| 2 previous births | 1,620 | 14.7 | 13.8;15.7 | 14.01 | |
| 3 previous births | 609 | 5.5 | 5.0;6.2 | 13.76 | |
| 4 or more previous births | 569 | 5.2 | 4.5;6.0 | 13.48 | |
| Number of prenatal care medical appointments | |||||
| No prenatal care | 107 | 1.0 | 0.7;1.3 | 13.21 | <0.001 |
| 1 to 3 | 901 | 8.2 | 7.3;9.6 | 13.49 | |
| 4 to 6 | 3,081 | 28.0 | 27.5;30.1 | 13.98 | |
| 7 or more | 6,684 | 60.8 | 60.1;63.6 | 13.98 | |
| Type of delivery | |||||
| Vaginal | 5,145 | 46.8 | 44.3;49.4 | 13.60 | <0.001 |
| Caesarean | 5,855 | 53.2 | 50.7;55.7 | 14.92 | |
Notes: a) Women taking part in the study: adults (≥20 years), with single fetus gestation, live birth, gestational age at birth 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2). b) 95% CI: 95% confidence interval; c) Bonferroni test. Sample weight and design were considered in all analyses.
The method proposed by Brandão et al.,10 the IOM2 recommendations, and the Intergrowth9 standard classified 39.2%, 36.5% and 35.9% of the women, respectively, as having adequate gestational weight gain. Intergrowth9 presented the lowest average for excessive gestational weight gain, classifying more women under excessive gain, 37.9%, in comparison with Brandão et al.10 and IOM,2 each with 33.1% (Table 2). Among the three methods, IOM was the method that classified most women as having insufficient gestational weight gain, 30.4% (Table 2).
Table 2 Average gestational weight gain and prevalence of SGAa and LGAb related to different intervals of gestational weight gain, according to different methods, among the 11,000 womenc taking part in the “Birth in Brazil” survey, 2011-2012
| Variable | Sample description | Average gestational weight gain (kg) | p-valued | SGAd (n=732) | LGAb (n=1,468) | p-valuee | |
|---|---|---|---|---|---|---|---|
| n | % | ||||||
| Total | 11,000 | 100.0 | 6.7 | 13.4 | |||
| Total gestational weight gain - Brandão et al. (2020)10 | |||||||
| Insufficient weight gain | 3,050 | 27.7 | 8.00 | <0.001 | 10.2 | 8.8 | <0.001 |
| Adequate weight gain | 4,313 | 39.2 | 13.52 | 6.5 | 12.2 | ||
| Excessive weight gain | 3,636 | 33.1 | 20.51 | 3.9 | 18.5 | ||
| Total gestational weight gain - IOMf (2009)2 | |||||||
| Insufficient weight gain | 3,345 | 30.4 | 8.28 | <0.001 | 9.9 | 9.4 | <0.001 |
| Adequate weight gain | 4,019 | 36.5 | 13.53 | 6.4 | 12.0 | ||
| Excessive weight gain | 3,636 | 33.1 | 20.23 | 3.9 | 18.5 | ||
| Total gestational weight gain - Intergrowth (2016)9 | |||||||
| Insufficient weight gain | 2,885 | 26.2 | 7.92 | <0.001 | 10.4 | 8.9 | <0.001 |
| Adequate weight gain | 3,951 | 35.9 | 13.16 | 6.6 | 11.4 | ||
| Excessive weight gain | 4,164 | 37.9 | 19.80 | 4.1 | 18.2 | ||
Notes: a) SGA: small for gestational age (n = absolute number); b) LGA: large for gestational age (n = absolute number); c) Women taking part in the study: adults (≥20 years), with single fetus gestation, live birth, gestational age at birth 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2); d) Bonferroni test; e) χ2 test; f) IOM: Institute of Medicine. Sample weight and design were considered in all analyses.
For all three methods, women with insufficient weight gain reported higher prevalence of children small for gestational age (SGA), in comparison with those with adequate weight gain. Excessive gestational weight gain, compared with adequate weight gain, revealed higher prevalence of LGA: 18.5% versus 12.2% for Brandão et al.;10 18.5% versus 12.0% for IOM;2 and 18.2% versus 11.4% for Intergrowth9 (Table 2).
In Table 3, after adjustment for confounding variables, women with insufficient weight gain according to the method proposed by Brandão et al.,10 by IOM2, and by Intergrowth standards,9 had higher chances of having a SGA child, with ORs of 1.52 (95% CI 1.06;2.19), 1.52 (95% CI 1.05;2.20) and 1,56 (95% CI 1.06;2.30), respectively.
Table 3 Association of gestational weight gain with “small for gestational age” (SGA) outcome, among the 11,000 womena taking part in the “Birth in Brazil” survey, 2011-2012
| Variable | Small for gestational age | |||
|---|---|---|---|---|
| ORb not adjusted | 95% CIc | ORd adjusted | 95% CIc | |
| Brandão et al. (2020)10 | ||||
| Insufficient weight gain | 1.58 | 1.11;2.26 | 1.52 | 1.06;2.19 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 0.63 | 0.48;0.83 | 0.61 | 0.46;0.81 |
| IOMe (2009)2 | ||||
| Insufficient weight gain | 1.57 | 1.09;2.24 | 1.52 | 1.05;2.20 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 0.64 | 0.48;0.84 | 0.62 | 0.47;0.83 |
| Intergrowth (2016)9 | ||||
| Insufficient weight gain | 1.61 | 1.12;2.33 | 1.56 | 1.06;2.30 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 0.66 | 0.51;0.86 | 0.65 | 0.50;0.84 |
Notes: a) Women taking part in the study: adults (≥20 years), with single fetus gestation, live birth, gestational age at birth 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2); b) OR not adjusted: odds ratio not adjusted; c) 95% CI: 95% confidence interval; d) OR adjusted: odds ratio adjusted, where Brandão et al.10 and IOM2 adjusted according to the following variables: national macroregion; mother’s age; race/skin color; parity, smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source, gestational age; Intergrowth9 adjusted according to the following variables: national macroregion; mother’s age; race/skin color; parity; smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source; e) IOM: Institute of Medicine. Sample weight and design were considered in all analyses.
Regarding excessive weight gain, both the estimate obtained using the method proposed by Brandão et al.10 and the estimate using the Intergrowth standards,9 presented a higher chance of the LGA outcome, in comparison with adequate gestational weight gain, with OR=1.53 (95% CI 1.28;1.82) and OR=1.65 (95% CI 1.40;1.96), respectively, after adjusting for possible confounding variables. In the case of IOM,2 excessive weight gain had a higher chance of LGA, with OR=1.57 (95% CI 1.31;1.87) (Table 4).
Table 4 Association of gestational weight gain and “large for gestational age” (LGA) outcome, among the 11,000 womena taking part in the “Birth in Brazil” survey, 2011-2012
| Variable | Large for gestational age | |||
|---|---|---|---|---|
| ORb not adjusted | 95% CIc | ORd adjusted | 95% CIc | |
| Brandão et al. (2020)10 | ||||
| Insufficient weight gain | 0.73 | 0.61;0.87 | 0.68 | 0.57;0.83 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 1.59 | 1.34;1.88 | 1.53 | 1.28;1.82 |
| IOMe (2009)2 | ||||
| Insufficient weight gain | 0.80 | 0.65;0.98 | 0.76 | 0.61;0.93 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 1.63 | 1.36;1.94 | 1.57 | 1.31;1.87 |
| Intergrowth (2016)9 | ||||
| Insufficient weight gain | 0.80 | 0.63;1.01 | 0.76 | 0.59;0.97 |
| Adequate weight gain | 1.00 | - | 1.00 | - |
| Excessive weight gain | 1.69 | 1.43;2.00 | 1.65 | 1.40;1.96 |
Notes: a) Women taking part in the study: adults (≥20 years), with single fetus gestation, live birth, gestational age at birth 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2); b) OR not adjusted: odds ratio not adjusted; c) 95% CI: 95% confidence interval; d) OR adjusted: odds ratio adjusted, where Brandão et al.10 and IOM2 adjusted according to the following variables: national macroregion; mother’s age; race/skin color; parity, smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source, gestational age; Intergrowth9 adjusted according to the following variables: national macroregion; mother’s age; race/skin color; parity; smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source; e) IOM: Institute of Medicine. Sample weight and design were considered in all analyses
The chances of having SGA and LGA newborns according to gestational weight gain in percentiles, as per the Intergrowth standards9 and the method proposed by Brandão et al.10 can be seen in Figure 1. Gestational weight gain below P10 showed a higher chance of SGA according to the Intergrowth standard,9 while the method proposed by Brandão et al.10 did not identify a significant increase in the chance of SGA, for the same percentile. In comparison with the P30-P70 interval, Intergrowth9 and Brandão et al.10 showed higher chance of LGA with effect from the P70-P80 gestational weight gain interval: OR=1.35 (95% CI 1.02;1.78) and OR=1.24 (95% CI 0.99;1.54) respectively.
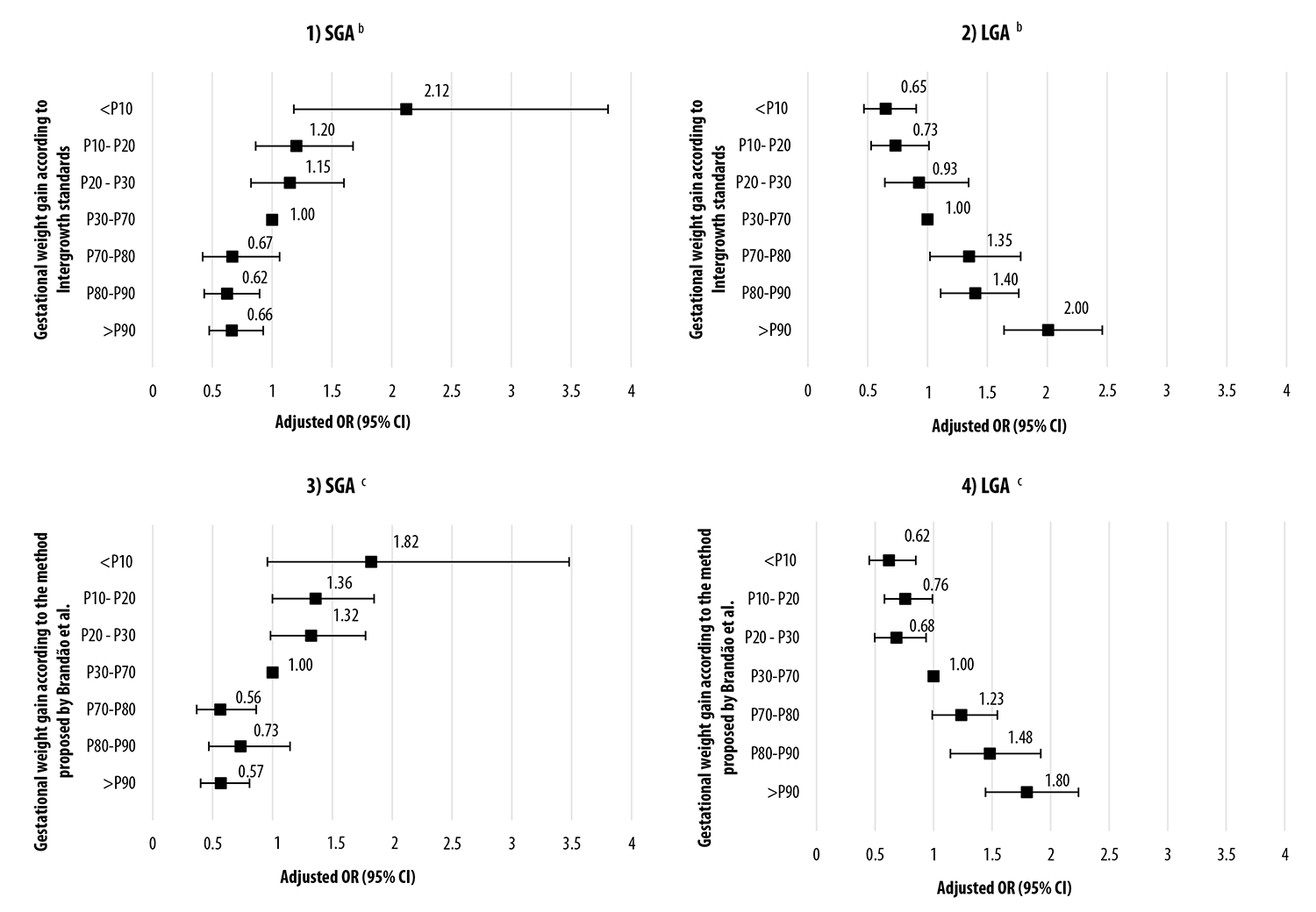
Notes: a) Women taking part in the study: adults (≥20 years), with single fetus gestation, live birth, gestational age at birth 28 weeks onwards and normal prepregnancy BMI (18.5 to 24.9kg/m2); b) Brandão et al. adjusted according to the following variables: national macroregion of residence; mother’s age; race/skin color; parity, smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source, gestational age; c) Intergrowth9 adjusted according to the following variables: national macroregion of residence; mother’s age; race/skin color; parity, smoking; education level (years of study); number of prenatal care medical appointments; type of delivery; delivery payment source.
Figure 1 Odds ratio for small for gestational age (SGA) and large for gestational age (LGA) births, according to the Intergrowth standards (Graphs 1 and 2) and the method proposed by Brandão et al., (Graphs 3 and 4) among the 11,000 women taking part in the “Birth in Brazil” survey, 2011-2012
Discussion
This was the first study to compare the methods proposed by Brandão et al.,10 IOM2 and Intergrowth standards9 for gestational weight gain, to identify women with higher chances of having SGA and LGA newborns, describing gestational weight gain in Brazilian women with normal BMI. LGA prevalence was higher than SGA prevalence in this study. IOM2 classified more women as having insufficient weight gain, the method proposed by Brandão et al.10 classified more women as having adequate weight gain, while the Intergrowth standards9 classified more women as having excessive weight gain. The cut-off points used for insufficient weight gain and excessive weight gain were associated with SGA and LGA, respectively, for all three methods.
The maternal characteristics identified are in keeping with the profile of women who use Brazilian National Health System (SUS) Primary Care services. Most of the deliveries took place in public facilities. However, more than half of the pregnant women had cesarean delivery, this proportion being much higher than that recommended by the WHO, i.e. between 10 and 15% (1985).20
The women analyzed showed a trend of excessive gestational weight gain. Over the years, some studies on postpartum women with normal BMI, using the gestational weight gain interval recommended by IOM,2 have also identified excessive weight gain.21-23 In their studies, Kominiarek & Peaceman,21 Marano et al.22 and Yeo et al.23 found prevalence rates of 37.3%, 30.0% and 40.3% for excessive GWG, respectively.
Excessive weight gain among women interviewed for this study had an impact on the LGA newborn outcome, the prevalence of which was high. Some authors already consider that overweight at birth is a Public Health problem.24,25 A systematic review conducted by Czarnobay et al.25 found LGA prevalence rates of between 4.0 and 30.0%, drawing attention to taking care with risk factors that are possible to change, such as maternal nutrition and weight gain during pregnancy.25
There was no difference in the magnitude of association between the methods applied with regard to SGA identification. However, strong and negative association was found between insufficient gestational weight gain and the SGA outcome. In the case of excessive weight gain and the LGA outcome, association was strong and positive, and considering the three methods, the chances of LGA newborns were higher when gestational weight gain was excessive according to the Intergrowth9 method.
Within the clinical follow-up scenario, defining cut-off points for gestational weight gain may help to establish better procedures, meeting the needs of pregnant women and minimizing possible impacts on health.2,11,26 Intergrowth9 did not establish cut-off points for adequate weight gain, because the study population was comprised of healthy pregnant women free from significant health and sociodemographic risks, so that all the percentiles were set to favor favorable outcomes in pregnancy.9 Brandão et. al.10, who also included women with lower health risk and favorable outcomes, proposed cut-off points between P30 and P70 for women with normal BMI. In turn, IOM2 set ideal intervals for gestational weight gain, based on studies presenting lower risk for adverse outcomes, including postpartum weight retention, hence why this method may seem more restrictive regarding its GWG ranges.
With regard to extreme percentiles, it is expected that there are greater chances of developing adverse results. Notwithstanding, as Intergrowth9 is a standard, and therefore has a prescriptive character, it is crucial to be cautious when using this reference due to gestational weight gain higher than P70 (excessive) already being associated with LGA. In the cohort study conducted by Hutcheon et al.,26 the authors assessed the risk of excessive maternal weight retention in the postpartum period involving weight change due to successive pregnancies, and found that between P51 and P84, risk of excessive weight retention also increased significantly. Jin et al.27 compared the ability to identify gestational diabetes in women with excessive GWG using three different methods (IOM,2 Intergrowth9 and a Chinese reference), and concluded that GWG higher than P84 according to Intergrowth9 and the local Chinese reference, presented higher risk of gestational diabetes.27
In this study, we found that Intergrowth9 was more restrictive regarding gestational weight gain, average weight among the women was lower, and many of them were classified as having excessive weight gain. However, Brandão et al.10 and IOM2 presented greater prevalence of women with adequate gestational weight gain. The advantage of the Intergrowth9 standards is that it provides follow-up of gestational weight gain throughout pregnancy and does not consider weight gain as constant throughout the entire period. However, this proposal needs to be extended to other categories of prepregnancy BMI, and validation studies are also needed to estimate the performance of GWG in predicting birth weight adequacy in different populations.
Most epidemiological studies assessing gestational weight gain have information on prepregnancy weight and weight at the end of pregnancy, so that it is possible to calculate only total gestational weight gain. Therefore, both the method proposed by Brandão et al.10 and Intergrowth9 standards could be used in large epidemiological studies, to assess association between inadequate GWG and different factors, besides clinical practice. It should be noted that the method proposed by Brandão et al.10 refers to three classes of obesity that still need to be validated.
Regarding the information on prepregnancy weight and height reported by the women, having data validation14 increases the reliability of the findings of this study; and multiple data imputation of these same variables, considered one of the most adequate strategies in treating missing data, minimizes possible impacts on prevalence rates and measurements of association.16,28
The theme of gestational weight gain continues to be relevant, in view of the dynamics of weight change. It is a factor that is both critical for obstetric results and can also cause changes in them. Furthermore, as at the publication of this paper, we have no knowledge of other studies using the three methods presented for LGA and SGA outcomes.
In comparison with the results obtained by applying the IOM method, insufficient and excessive gestational weight gain found using the Intergrowth method9 and the method proposed by Brandão et al.10 are more associated with the increase in the chances of LGA and SGA, in the population studied. The method proposed by Brandão et al.10 and the Intergrowth standards are presented as alternatives for identifying women with higher chances of SGA and LGA outcomes, rather than the IOM method, and can help with regard to the quality of prenatal care of Brazilian women with characteristics similar to those observed in this study. Notwithstanding, prospective studies are still needed for assessing other health outcomes.
REFERENCES
1. World Health Organization - WHO. Physical status: the use and interpretation of anthropometry: report of a WHO expert committee [Internet]. Genebra: World Health Organization; 1995 [cited 2020 Oct 5]. 452 p. Available from: https://www.who.int/childgrowth/publications/physical_status/en / [ Links ]
2. Institute of Medicine - IOM; National Research Council (USA) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Rasmussen KM, Yaktine AL, editor. Washington, D.C.: National Academies Press; 2009 [cited 2020 Oct 5]. 868 p. Available from: https://doi.org/10.17226/12584 [ Links ]
3. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Helen Black M, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. Jama [Internet]. 2017 Jun [cited 2018 Mar 12];317(21):2207-25. Available from: https://doi.org/10.1001/jama.2017.3635 [ Links ]
4. Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across c4ontinents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med [Internet]. 2018 Aug [cited 2020 Mar 19];16(1):153. Available from: https://doi.org/10.1186/s12916-018-1128-1 [ Links ]
5. Lee P, Chernausek S, Hokken-Koelega A, Czernichow P. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics [Internet]. 2003 Jun [cited 2020 Oct 5];111(6 Pt 1):1253-61. Available from: https://doi.org/10.1542/peds.111.6.1253 [ Links ]
6. Lei X, Zhao D, Huang L, Luo Z, Zhang J, Yu X, et al. Childhood health outcomes in term, large-for-gestational-age babies with different postnatal growth patterns. Am J Epidemiol [Internet]. 2018 Mar [cited 2020 Oct 5];187(3):507-14. Available from: https://doi.org/10.1093/aje/kwx271 [ Links ]
7. Chiavaroli V, Marcovecchio ML, De Giorgis T, Diesse L, Chiarelli F, Mohn A. Progression of cardio-metabolic risk factors in subjects born small and large for gestational age. PLoS One [Internet]. 2014 Aug [cited 2020 Oct 5];9(8):e104278. Availble from: https://doi.org/10.1371/journal.pone.0104278 [ Links ]
8. Institute of Medicine - IOM; Committee on Nutritional Status During Pregnancy and Lactation. Perspectives on nutrition during pregnancy: part i, weight gain; part ii, nutrient supplements [Internet]. Washington, D. C.: The National Academies Press; 1990 [cited 2020 Oct 5]. Available from: https://doi.org/10.17226/1451 [ Links ]
9. Cheikh Ismail L, Bishop DC, Pang R, Ohuma EO, Kac G, Abrams B, et al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ [Internet]. 2016 Feb [cited 2020 Oct 5];352:i555. Available from: https://doi.org/10.1136/bmj.i555 [ Links ]
10. Brandão T, Padilha PC, Gama SGN, Leal MC, Araújo RGPS, Barros DC, et al. Gestational weight gain and adverse maternal outcomes in Brazilian women according to body mass index categories: an analysis of data from the Birth in Brazil survey. Clin Nutr ESPEN [Internet]. 2020 Jun [cited 2020 Oct 5];37:114-20. Available from: https://doi.org/10.1016/j.clnesp.2020.03.009 [ Links ]
11. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Cadernos e atenção básica: atenção ao pré-natal de baixo risco [Internet]. Brasília: Ministério da Saúde; 2013 [citado 2020 out 5]. 316 p. Disponível em: http://bvs.saude.gov.br/bvs/publicacoes/atencao_pre_natal_baixo_risco.pdf [ Links ]
12. Vasconcellos MTL, Silva PLN, Pereira APE, Schilithz AOC, Souza Junior PRB, Szwarcwald CL. Sampling design for the birth in Brazil: national survey into labor and birth. Cad Saúde Pública [Internet]. 2014 [cited 2020 Oct 5];30(Suppl 1):S49-58. Available from: https://doi.org/10.1590/0102-311X00176013 [ Links ]
13. Viellas EF, Domingues RMSM, Dias MAB, Gama SGN, Theme Filha MM, Costa JV, et al. Assistência pré-natal no Brasil. Cad Saúde Pública [Internet]. 2014 [citado 2020 out 5];30(suppl 1):S85-100. Disponível em: https://doi.org/10.1590/0102-311X00126013 [ Links ]
14. Araújo RGPS, Gama SGN, Barros DCCS, Mattos IE. Validity of self-reported weight, height, and BMI in mothers of the research Birth in Brazil. Rev Saúde Pública [Internet]. 2017 [cited 2020 Oct 5];51(115):1-11. Available from: https://doi.org/10.11606/s1518-8787.2017051006775 [ Links ]
15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med [Internet]. 2011 Feb [cited 2017 Dec 4];30(4):377-99. Available from: https://doi.org/10.1002/sim.4067 [ Links ]
16. Rubin DB. Multiple imputation for nonresponse in surveys [Internet]. [S.l.]: John Wiley & Sons; 1987 [cited 2017 Dec 4]. Available from: http://doi.wiley.com/10.1002/9780470316696.fmatter [ Links ]
17. Bodnar LM, Pugh SJ, Lash TL, Hutcheon JA, Himes KP, Parisi SM, et al. Low gestational weight gain and risk of adverse perinatal outcomes in obese and severely obese women. Epidemiology [Internet]. 2017 Nov [cited 2018 Mar 12];27(6):894-902. Available from: https://dx.doi.org/10.1097%2FEDE.0000000000000535 [ Links ]
18. School T, Hediger M, Schall J, Ances I, Smith W. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstet Gynecol [Internet]. 1995 Sep [cited 2020 Oct 5];86(3):423-7. Available from: https://doi.org/10.1016/0029-7844(95)00190-3 [ Links ]
19. Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet [Internet]. 2014 Sep[cited 2020 Oct 5];384(9946):857-68. Available from: https://doi.org/10.1016/s0140-6736(14)60932-6 [ Links ]
20. World Health Organization - WHO. Appropriate technology for birth. Lancet [Internet]. 1985 Aug [cited 2020 Oct 5];326(8452):436-7. Available from: https://doi.org/10.1016/S0140-6736(85)92750-3 [ Links ]
21. Kominiarek MA, Peaceman AM. Gestational weight gain. Am J Obstet Gynecol [Internet]. 2017 Dec [cited 2020 Oct 5];217(6):642-51. Available from: https://dx.doi.org/10.1016%2Fj.ajog.2017.05.040 [ Links ]
22. Marano D, Gama SGN, Pereira APE, Souza-Júnior PRB. Adequação do ganho ponderal de gestantes em dois municípios do Estado do Rio de Janeiro (RJ), Brasil, 2008. Rev Bras Ginecol Obstet [Internet]. 2012 ago [citado 2017 set 7];34(8):386-93. Disponível em: https://doi.org/10.1590/S0100-72032012000800008 [ Links ]
23. Yeo S, Crandell JL, Jones-Vessey K. Adequacy of prenatal care and gestational weight gain. J Women Health [Internet]. 2016 Feb [cited 2017 Jul 15];25(2):117-23. Available from: https://dx.doi.org/10.1089%2Fjwh.2015.5468 [ Links ]
24. World Health Organization - WHO. Report of the Commission on Ending Childhood Obesity: implementation plan: executive summary [Internet]. Geneva: WHO; 2017 [cited 2020 Oct 5]. Available from: https://www.who.int/end-childhood-obesity/en / [ Links ]
25. Czarnobay SA, Kroll C, Schultz LF, Malinovski J, Mastroeni MF, Mastroeni MF. Predictors of excess birth weight in Brazil: a systematic review. J Pediatr [Internet]. 2019 Mar [cited 2020 Oct 5];95(2):128-54. Available from: https://doi.org/10.1016/j.jped.2018.04.006 [ Links ]
26. Hutcheon JA, Chapinal N, Bodnar LM, Lee L. The INTERGROWTH-21st gestational weight gain standard and interpregnancy weight increase: A population-based study of successive pregnancies. Obesity (Silver Spring) [Internet]. 2017 Jun [cited 2017 Nov 2];25(6):1122-7. Available from: https://dx.doi.org/10.1002%2Foby.21858 [ Links ]
27. Jin C, Lin L, Han N, Zhao Z, Liu Z, Luo S, et al. Excessive gestational weight gain and the risk of gestational diabetes: comparison of Intergrowth-21st standards, IOM recommendations and a local reference. Diabetes Res Clin Pract [Internet]. 2019 Dec [cited 2020 Oct 5];158:107912. Available from: https://doi.org/10.1016/j.diabres.2019.107912 [ Links ]
28. Razzaghi H, Tinker SC, Herring AH, Howards PP, Waller DK, Johnson CY, National Birth Defects Prevention Study. Impact of missing data for body mass index in an epidemiologic study. Matern Child Health J [Internet]. 2016 Jul [cited 2018 Feb 16];20(7):1497-505. Available from: https://doi.org/10.1007/s10995-016-1948-6 [ Links ]
*This article is part of the Ph.D. thesis entitled “Avaliação do ganho ponderal e construção de curvas para o ganho de peso na gestação, segundo índice de massa corporal pré-gestacional” [Assessment of weight gain and curve building for gestational weight gain, according to pre-gestational body mass index”, defended by Roberta Gabriela Pimenta da Silva Araújo at the Graduate Program in Epidemiology of the National School of Public Health/Fundação Instituto Oswaldo Cruz (ENSP/Fiocruz) in 2020. The study received support from the National Council for Scientific and Technological Development/Ministry of Science, Technology, Innovation and Communication, the Department of Science and Technology/Secretariat of Science, Technology and Strategic Inputs/Ministry of Health; the Sergio Arouca National School of Public Health/Fundação Instituto Oswaldo Cruz (Project Inova/ENSP – MCT/CNPq/CT-Saúde/MS/SCTID/DECIT No. 057/2009); as well as support from the Carlos Chagas Filho Research Support Foundation of the State of Rio de Janeiro (FAPERJ – File No. E-26/103.083/2011); and the Coordination for the Improvement of Higher Education Personnel/Ministry of Education (Capes/MEC – Funding Code 001).
Received: April 06, 2020; Accepted: September 02, 2020











 texto en
texto en 


