Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude vol.14 Ananindeua 2023 Epub 27-Set-2023
http://dx.doi.org/10.5123/s2176-6223202301427
ORIGINAL ARTICLE
List of sand fly (Diptera: Psychodidae) and spatial distribution of leishmaniasis main vector species in Mato Grosso State, Brazil
1 Secretaria de Estado de Saúde de Mato Grosso, Escritório Regional de Saúde de Sinop, Sinop, Mato Grosso, Brasil
2 Secretaria de Estado de Saúde de Mato Grosso, Laboratório de Entomologia, Cuiabá, Mato Grosso, Brasil
3 Secretaria de Estado de Saúde de Mato Grosso, Escritório Regional de Saúde de Barra do Graças, Barra do Garças, Mato Grosso, Brasil
OBJECTIVES:
To update the sand fly species list (Diptera: Psychodidae) in Mato Grosso State, Brazil, and to demonstrate the spatial distribution of leishmaniasis main vector species in the state.
MATERIALS AND METHODS:
Data were obtained from academic publications on Mato Grosso phlebotomine fauna and entomological reports from the Mato Grosso State Health Department database from 2006 to 2021. The taxonomic nomenclature of the specimens followed recent updates by leading researchers in the field.
RESULTS:
Twenty-six new species were detected in the Mato Grosso phlebotomine fauna, with 132 species total: six of the genus Brumptomyia and 126 distributed among 16 other genera. The main vectors of American cutaneous leishmaniasis (Nyssomyia whitmani and Bichromomyia flaviscutellata) and visceral leishmaniasis (Lutzomyia (Lutzomyia) longipalpis and Lutzomyia (Lutzomyia) cruzi) were recorded in all three existing biomes in the state, especially in Cerrado.
CONCLUSION:
The phlebotomine fauna of Mato Grosso is one of the richest and most diversified in the country, with important species in leishmaniasis epidemiology distributed across all three biomes, a fact that reinforces the significance and necessity of entomological surveys to monitor the occurrence and distribution of leishmaniasis vectors in the region.
Keywords: Phlebotomus; Disease Vectors; Leishmaniasis; Entomology; Biomes
INTRODUCTION
Leishmaniases are infectious diseases of public health importance due to their high detection rate and ability to produce deformities1. They belong to the neglected tropical diseases group and are caused by protozoa of the genus Leishmania Ross, 1903 (Kinetoplastida: Trypanosomatidae) transmitted to humans by female hematophagous sand flies of the subfamily Phlebotominae (Diptera: Psychodidae)2,3,4. They comprise a spectrum of diseases classified as American tegumentary leishmaniasis (ATL) or cutaneous leishmaniasis (more common) and visceral leishmaniasis (VL), known as kala-azar, both widespread in tropical and subtropical regions of the world1.
In Brazil, ATL is considered a serious public health problem and has been recorded in all Brazilian states3,5. Between 2010 and 2019, Mato Grosso State recorded 23,980 disease cases, with autochthonous cases detected in all its cities5.
VL is a severe and potentially fatal disease that resurfaced in several Brazilian locations during the 1980s and has since spread to new areas, including the Midwest Region6. In Mato Grosso, VL is reported in urban and rural areas of the state's northern, central-southern, and southeastern regions, distributed in 34 cities, prevailing in the central-southern and southeastern regions6. Between 2010 and 2019, 258 disease cases were registered in the state5.
The transmission of etiological agents involves different phlebotomines in specific associations with the parasites and their hosts, with transmission cycles occurring throughout the Brazilian territory7,8. More than a thousand species are described worldwide, of which more than 530 come from the Neotropical region9. In the Americas, 40 species of sand flies are involved in transmitting the Leishmania sp. parasite10. In Brazil, the main vectors associated with ATL transmission are Bichromomyia flaviscutellata (Mangabeira, 1942), Nyssomyia umbratilis (Ward & Fraiha, 1977), Nyssomyia intermedia (Lutz & Neiva, 1912), Psychodopygus wellcomei Fraiha, Shaw & Lainson, 1971, Migonemyia (Migonemyia) migonei (France, 1920), and Nyssomyia whitmani (Antunes & Coutinho, 1939), the latter being the main species associated with ATL transmission and widely distributed in Brazil, as well as in Mato Grosso11,12,13,14,15.
The main species associated with VL transmission in Brazil is Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912). However, studies highlight the relevance of Lutzomyia (Lutzomyia) cruzi (Mangabeira, 1938) in the transmission process of VL3,11,16,17. In urban areas, dogs are considered important domestic reservoirs of Leishmania infantum due to their close contact with humans18,19.
Mato Grosso State has three distinct biomes: Cerrado, Pantanal, and Amazon Rainforest, with much of its territory in the Legal Amazon20. More than 50% of Mato Grosso cities have Amazon Rainforest territory, followed by Cerrado20,21. It shares borders with Amazonas and Pará States to the north, Tocantins and Goiás to the east, Mato Grosso do Sul to the south, and Rondônia and Bolivia to the west20.
The phlebotomine fauna diversity in Mato Grosso was first reported by Missawa and Maciel22, listing 106 relevant species to the ATL and VL epidemiology, such as Lu. (Lut.) cruzi, Lu. (Lut.) longipalpis, Bi. flaviscutellata, Ny. intermedia, Mg. (Mig.) migonei, and Ny. whitmani. Ribeiro et al.23 presented these vectors' distribution in the state's biomes (Amazon Rainforest, Cerrado, and Pantanal), reporting Lu. (Lut.) cruzi, Lu. (Lut.) longipalpis, and Ny. whitmani in all three biomes; and Bi. flaviscutellata is not found only in the Pantanal biome.
Environmental transformations resulting from rural migration to the outskirts of cities increase the irregular housing construction, resulting in a lack of basic sanitation and promoting social inequalities24. Thus, changes related to urbanization and the growing use of monoculture have avored phlebotomines in disease transmission25. The disease's spread to new frontiers likely follows deforestation between large cities, aiming to implement development projects26,27, crossed by an extensive network of access roads, promoting human-vector contact.
Knowledge of phlebotomine fauna and its geographical distribution according to the biome contributes to a better understanding of the leishmaniasis transmission dynamics. Therefore, this study aims to update the list of phlebotomine species found in Mato Grosso and show the spatial distribution of leishmaniasis's four main vector species in the state, according to the biome: Lu. (Lut.) longipalpis and Lu. (Lut.) cruzi, vectors of VL, and Bi. flaviscutellata and Ny. whitmani, vectors of ATL, to identify areas at risk of transmission and prone to entomological and epidemiological surveillance.
MATERIALS AND METHODS
STUDY AREA
Mato Grosso State is located in Brazil's Center-West Region, covering an area of 903,358 km², spread across 141 cities, with an estimated population of 3,658,813 inhabitants28. The state's geography is characterized by three biomes: the Amazon Rainforest, the Cerrado, and the Pantanal20.
The climate is hot tropical, with approximately five months of drought (May to September). The average annual rainfall is 1,750 mm, peaking from December to February. Summer months experience maximum temperatures around 40 °C, with an average of around 27 °C21.
DATA COLLECTION
Data on the vector species distribution in Mato Grosso was obtained using Google Scholar and the Scientific Electronic Library Online (SciELO - Brazil) platform for academic content, along with entomological reports from the Mato Grosso State Health Department (SES-MT) database from 2006 to 2021. The taxonomic nomenclature of the specimens adhered to recent updates by leading researchers in the field.
CAPTURE PROCEDURES AND TAXONOMIC IDENTIFICATION OF SPECIMENS
Capture techniques involved using CDC light traps29 and Shannon tents30, placed in intra-domicile, peridomicile, and extra-domicile areas. CDC traps were deployed at dusk and retrieved at dawn, lasting approximately 12 h, for three consecutive nights, while the Shannon tents were set up for one night at dusk and monitored for 4 h.
Captured specimens were sorted by city, date, and environment, duly labeled, preserved in 70% alcohol, and sent to the SES-MT Entomology Laboratory for clarification, assembly, identification, and quantification, according to Young and Duncan31, with a period of approximately eight days elapsed between capture and processing. The dichotomous keys of Young and Duncan31 and Galati32 were used for identification, following the taxonomic classification proposed by Galati32.
SPATIAL DISTRIBUTION OF LOCALITIES
For the phlebotomines spatial distribution in Mato Grosso, the studies by Missawa and Maciel22 and Mestre and Fontes6 were used as initial data sources, in addition to the studies by Missawa and Lima24, Ribeiro et al.23, Missawa et al.33, Zeilhofer et al.34, Missawa et al.35, Queiroz et al.36, Alves et al.37, Brito et al.17, Moraes38, and Thies et al.39. An unpublished database of species identified by the SES-MT Entomology Laboratory between 2006 and 2021 was used. This database provided a historical series of 16 years, serving as a faithful repository of specimen collections.
CLASSIFICATION OF CITIES ACCORDING TO BIOME
The 141 cities were classified according to biome using the ArcGIS program, considering the total area of each city (km²) included in the biome20,28. Coverage proportions were calculated for places encompassing multiple biomes, and the city was categorized into the biome with the greatest area coverage: Cerrado, Amazon Rainforest, or Pantanal.
RESULTS
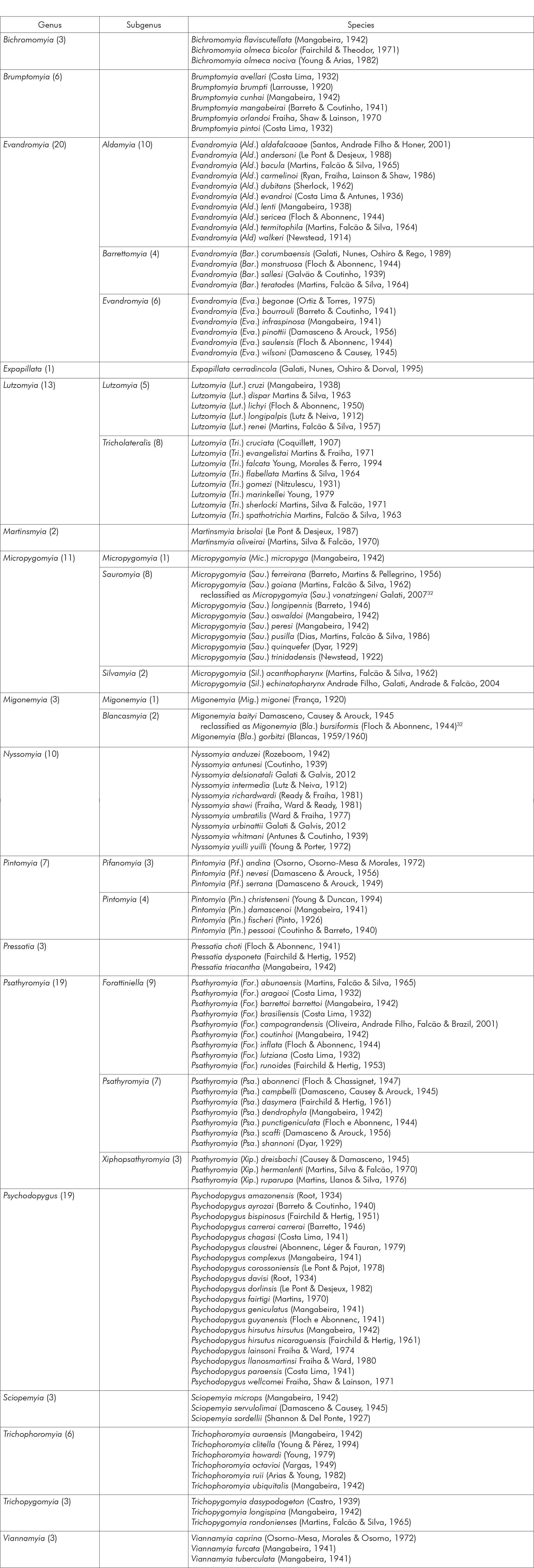
From 2006 to 2021, entomological surveys were conducted in 118/141 (83.69%) cities in Mato Grosso: Acorizal, Água Boa, Alta Floresta, Alto Araguaia, Alto Boa Vista, Alto Garças, Alto Paraguai, Apiacás, Araguaiana, Araguainha, Arenápolis, Aripuanã, Barão de Melgaço, Barra do Bugres, Barra do Garças, Brasnorte, Cáceres, Campinápolis, Campo Novo do Parecis, Campo Verde, Campos de Júlio, Canabrava do Norte, Canarana, Carlinda, Chapada dos Guimarães, Cláudia, Colíder, Colniza, Comodoro, Confresa, Cotriguaçu, Cuiabá, Denise, Diamantino, Dom Aquino, Feliz Natal, Gaúcha do Norte, General Carneiro, Guarantã do Norte, Guiratinga, Ipiranga do Norte, Itanhangá, Itiquira, Jaciara, Jangada, Juara, Juína, Juruena, Juscimeira, Lucas do Rio Verde, Luciara, Marcelândia, Matupá, Mirassol D'Oeste, Nobres, Nortelândia, Nossa Senhora do Livramento, Nova Bandeirantes, Nova Brasilândia, Nova Canaã do Norte, Nova Guarita, Nova Lacerda, Nova Marilândia, Nova Maringá, Nova Mutum, Nova Santa Helena, Nova Ubiratã, Nova Xavantina, Novo Horizonte do Norte, Novo Mundo, Novo Santo Antônio, Novo São Joaquim, Paranaíta, Paranatinga, Pedra Preta, Peixoto de Azevedo, Planalto da Serra, Poconé, Pontal da Araguaia, Ponte Branca, Pontes e Lacerda, Porto Alegre do Norte, Porto dos Gaúchos, Porto Esperidião, Porto Estrela, Poxoréo, Primavera do Leste, Querência, Reserva do Cabaçal, Ribeirão Cascalheira, Ribeirãozinho, Rondolândia, Rondonópolis, Rosário Oeste, Salto do Céu, Santa Carmem, Santa Rita do Trivelato, Santo Antônio do Leverger, São Félix do Araguaia, São José do Povo, São José do Rio Claro, São José do Xingu, São José dos Quatro Marcos, São Pedro da Cipa, Sapezal, Serra Nova Dourada, Sinop, Sorriso, Tabaporã, Tangará da Serra, Tapurah, Terra Nova do Norte, Torixoréu, União do Sul, Várzea Grande, Vera, Vila Bela da Santíssima Trindade, and Vila Rica (Figure 1).
List of cities: 1-Acorizal, 2-Água Boa, 3-Alta Floresta, 4-Alto Araguaia, 5-Alto Boa Vista, 6-Alto Garças, 7-Alto Paraguai, 8-Alto Taquari, 9-Apiacás, 10-Araguaiana, 11-Araguainha, 12-Araputanga, 13-Arenápolis, 14-Aripuanã, 15-Barão de Melgaço, 16-Barra do Bugres, 17-Barra do Garças, 18-Bom Jesus do Araguaia, 19-Brasnorte, 20-Cáceres, 21-Campinápolis, 22-Campo Novo do Parecis, 23-Campo Verde, 24-Campos de Júlio, 25-Canabrava do Norte, 26-Canarana, 27-Carlinda, 28-Castanheira, 29-Chapada dos Guimarães, 30-Cláudia, 31-Cocalinho, 32-Colíder, 33-Colniza, 34-Comodoro, 35-Confresa, 36-Conquista D'Oeste, 37-Cotriguaçu, 38-Cuiabá, 39-Curvelândia, 40-Denise, 41-Diamantino, 42-Dom Aquino, 43-Feliz Natal, 44-Figueirópolis D'Oeste, 45-Gaúcha do Norte, 46-General Carneiro, 47-Glória D'Oeste, 48-Guarantã do Norte, 49-Guiratinga, 50-Indiavaí, 51-Ipiranga do Norte, 52-Itanhangá, 53-Itaúba, 54-Itiquira, 55-Jaciara, 56-Jangada, 57-Jauru, 58-Juara, 59-Juína, 60-Juruena, 61-Juscimeira, 62-Lambari D'Oeste, 63-Lucas do Rio Verde, 64-Luciara, 65-Marcelândia, 66-Matupá, 67-Mirassol D'Oeste, 68-Nobres, 69-Nortelândia, 70-Nossa Senhora do Livramento, 71-Nova Bandeirantes, 72-Nova Brasilândia, 73-Nova Canaã do Norte, 74-Nova Guarita, 75-Nova Lacerda, 76-Nova Marilândia, 77-Nova Maringá, 78-Nova Monte Verde, 79-Nova Mutum, 80-Nova Nazaré, 81-Nova Olímpia, 82-Nova Santa Helena, 83-Nova Ubiratã, 84-Nova Xavantina, 85-Novo Horizonte do Norte, 86-Novo Mundo, 87-Novo Santo Antônio, 88-Novo São Joaquim, 89-Paranaíta, 90-Paranatinga, 91-Pedra Preta, 92-Peixoto de Azevedo, 93-Planalto da Serra, 94-Poconé, 95-Pontal do Araguaia, 96-Ponte Branca, 97-Pontes e Lacerda, 98-Porto Alegre do Norte, 99-Porto dos Gaúchos, 100-Porto Esperidião, 101-Porto Estrela, 102-Poxoréu, 103-Primavera do Leste, 104-Querência, 105-Reserva do Cabaçal, 106-Ribeirão Cascalheira, 107-Ribeirãozinho, 108-Rio Branco, 109-Rondolândia, 110-Rondonópolis, 111-Rosário Oeste, 112-Salto do Céu, 113-Santa Carmem, 114-Santa Cruz do Xingu, 115-Santa Rita do Trivelato, 116-Santa Terezinha, 117-Santo Afonso, 118-Santo Antônio de Leverger, 119-Santo Antônio do Leste, 120-São Félix do Araguaia, 121-São José do Povo, 122-São José do Rio Claro, 123-São José do Xingu, 124-São José dos Quatro Marcos, 125-São Pedro da Cipa, 126-Sapezal, 127-Serra Nova Dourada, 128-Sinop, 129-Sorriso, 130-Tabaporã, 131-Tangará da Serra, 132-Tapurah, 133-Terra Nova do Norte, 134-Tesouro, 135-Torixoréu, 136-União do Sul, 137-Vale de São Domingos, 138-Várzea Grande, 139-Vera, 140-Vila Bela da Santíssima Trindade, 141-Vila Rica.
Figure 1 - Distribution of phlebotomine entomological surveys conducted in Mato Grosso cities, Brazil, from 2006 to 2021
As for the cities' distribution by biome, the adopted classification revealed a predominance of the Amazon Rainforest biome, covering 53.19% of the geographical area and present in 75 cities; followed by the Cerrado, with coverage of 43.97%, reaching 62 cities; and the Pantanal, present in four cities, occupying 2.84% of Mato Grosso's territory.
Entomological collections took place in 100% (4/4) of cities in the Pantanal biome, in 77.33% (58/75) of those belonging to the Amazon Rainforest biome, and in 90.32% (56/62) of cities in the Cerrado biome.
A total of 132 phlebotomine species were recorded (26 had not yet been described for Mato Grosso State), distributed in 17 genera: Evandromyia (20); Psathyromyia (19); Psychodopygus (19); Lutzomyia (13); Micropygomyia (11); Nyssomyia (10); Pintomyia (7); Brumptomyia (6); Trichophoromyia (6); Bichromomyia (3); Migonemyia (3); Pressatia (3); Sciopemyia (3); Trichopygomyia (3); Viannamyia (3); Martinsmyia (2); and Expapillata (1) (Table 1).
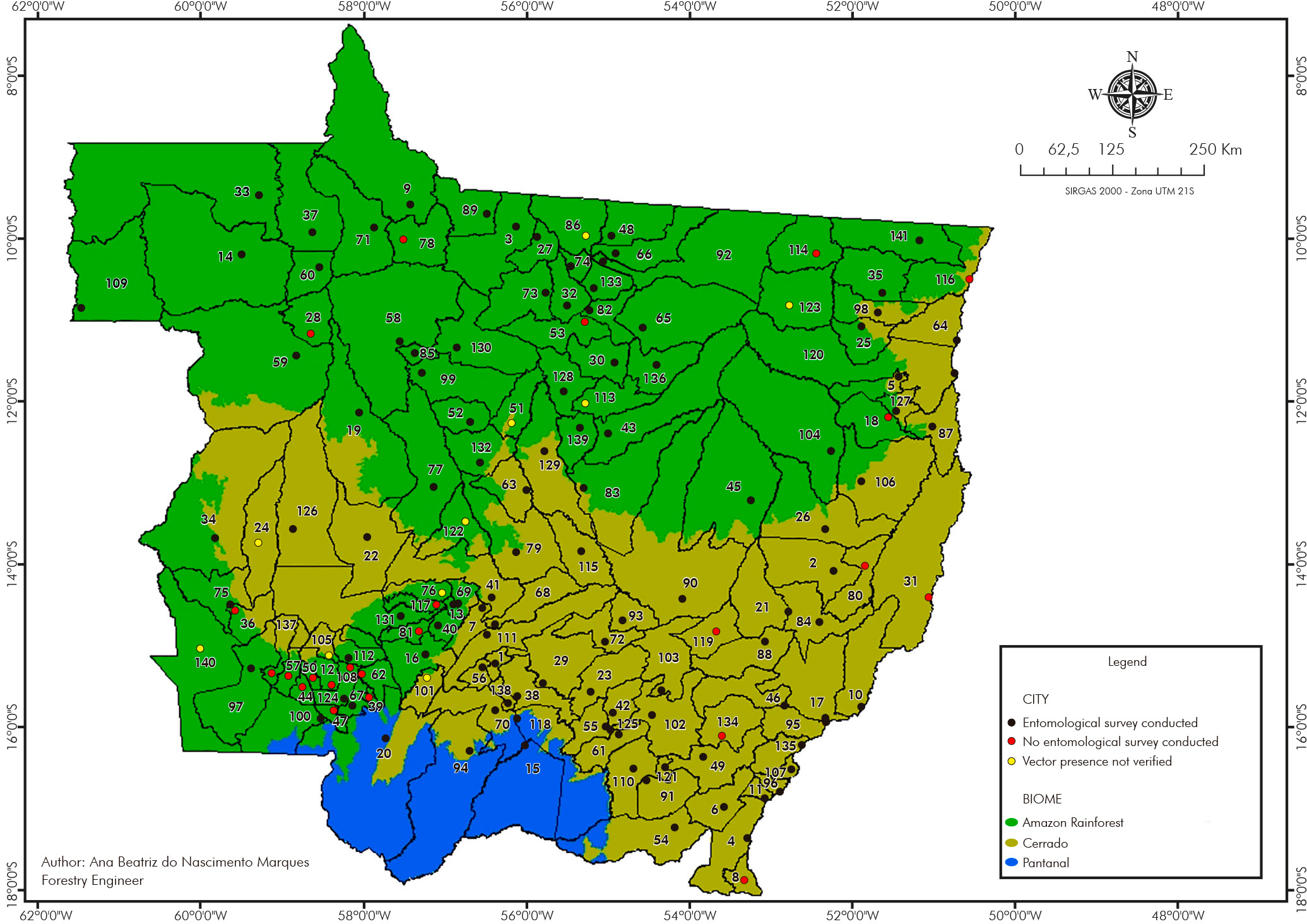
Ny. whitmani and Bi. flaviscutellata, considered the main ATL vectors in Brazil and Mato Grosso, were found in 105/118 (88.98%) and 54/118 (45.76%) cities in Mato Grosso, respectively. Sympatry between these species was recorded in 53/118 (44.91%) of them (Figure 2).
List of cities: 1-Acorizal, 2-Água Boa, 3-Alta Floresta, 4-Alto Araguaia, 5-Alto Boa Vista, 6-Alto Garças, 7-Alto Paraguai, 8-Alto Taquari, 9-Apiacás, 10-Araguaiana, 11-Araguainha, 12-Araputanga, 13-Arenápolis, 14-Aripuanã, 15-Barão de Melgaço, 16-Barra do Bugres, 17-Barra do Garças, 18-Bom Jesus do Araguaia, 19-Brasnorte, 20-Cáceres, 21-Campinápolis, 22-Campo Novo do Parecis, 23-Campo Verde, 24-Campos de Júlio, 25-Canabrava do Norte, 26-Canarana, 27-Carlinda, 28-Castanheira, 29-Chapada dos Guimarães, 30-Cláudia, 31-Cocalinho, 32-Colíder, 33-Colniza, 34-Comodoro, 35-Confresa, 36-Conquista D'Oeste, 37-Cotriguaçu, 38-Cuiabá, 39-Curvelândia, 40-Denise, 41-Diamantino, 42-Dom Aquino, 43-Feliz Natal, 44-Figueirópolis D'Oeste, 45-Gaúcha do Norte, 46-General Carneiro, 47-Glória D'Oeste, 48-Guarantã do Norte, 49-Guiratinga, 50-Indiavaí, 51-Ipiranga do Norte, 52-Itanhangá, 53-Itaúba, 54-Itiquira, 55-Jaciara, 56-Jangada, 57-Jauru, 58-Juara, 59-Juína, 60-Juruena, 61-Juscimeira, 62-Lambari D'Oeste, 63-Lucas do Rio Verde, 64-Luciara, 65-Marcelândia, 66-Matupá, 67-Mirassol D'Oeste, 68-Nobres, 69-Nortelândia, 70-Nossa Senhora do Livramento, 71-Nova Bandeirantes, 72-Nova Brasilândia, 73-Nova Canaã do Norte, 74-Nova Guarita, 75-Nova Lacerda, 76-Nova Marilândia, 77-Nova Maringá, 78-Nova Monte Verde, 79-Nova Mutum, 80-Nova Nazaré, 81-Nova Olímpia, 82-Nova Santa Helena, 83-Nova Ubiratã, 84-Nova Xavantina, 85-Novo Horizonte do Norte, 86-Novo Mundo, 87-Novo Santo Antônio, 88-Novo São Joaquim, 89-Paranaíta, 90-Paranatinga, 91-Pedra Preta, 92-Peixoto de Azevedo, 93-Planalto da Serra, 94-Poconé, 95-Pontal do Araguaia, 96-Ponte Branca, 97-Pontes e Lacerda, 98-Porto Alegre do Norte, 99-Porto dos Gaúchos, 100-Porto Esperidião, 101-Porto Estrela, 102-Poxoréu, 103-Primavera do Leste, 104-Querência, 105-Reserva do Cabaçal, 106-Ribeirão Cascalheira, 107-Ribeirãozinho, 108-Rio Branco, 109-Rondolândia, 110-Rondonópolis, 111-Rosário Oeste, 112-Salto do Céu, 113-Santa Carmem, 114-Santa Cruz do Xingu, 115-Santa Rita do Trivelato, 116-Santa Terezinha, 117-Santo Afonso, 118-Santo Antônio de Leverger, 119-Santo Antônio do Leste, 120-São Félix do Araguaia, 121-São José do Povo, 122-São José do Rio Claro, 123-São José do Xingu, 124-São José dos Quatro Marcos, 125-São Pedro da Cipa, 126-Sapezal, 127-Serra Nova Dourada, 128-Sinop, 129-Sorriso, 130-Tabaporã, 131-Tangará da Serra, 132-Tapurah, 133-Terra Nova do Norte, 134-Tesouro, 135-Torixoréu, 136-União do Sul, 137-Vale de São Domingos, 138-Várzea Grande, 139-Vera, 140-Vila Bela da Santíssima Trindade, 141-Vila Rica.
Figure 2 - Ny. whitmani and Bi. flaviscutellata spatial distribution in Mato Grosso State, Brazil, from 2006 to 2021
Ny. whitmani was the predominant species in all three biomes. Bi. flaviscutellata, although also detected in the same biomes, was less frequent in the Pantanal (1/4 cities), being found for the first time in Barão do Melgaço, corresponding to the first record of this species in this biome.
In the Amazon Rainforest biome, Ny. whitmani was recorded in 86.21% (50/58) of the cities, while Bi. flaviscutellata was present in 44.83% (26/58).
In cities with the Cerrado biome, Ny. whitmani occurred in 91.07% (51/56) and Bi. flaviscutellata in 50.00% (28/56).
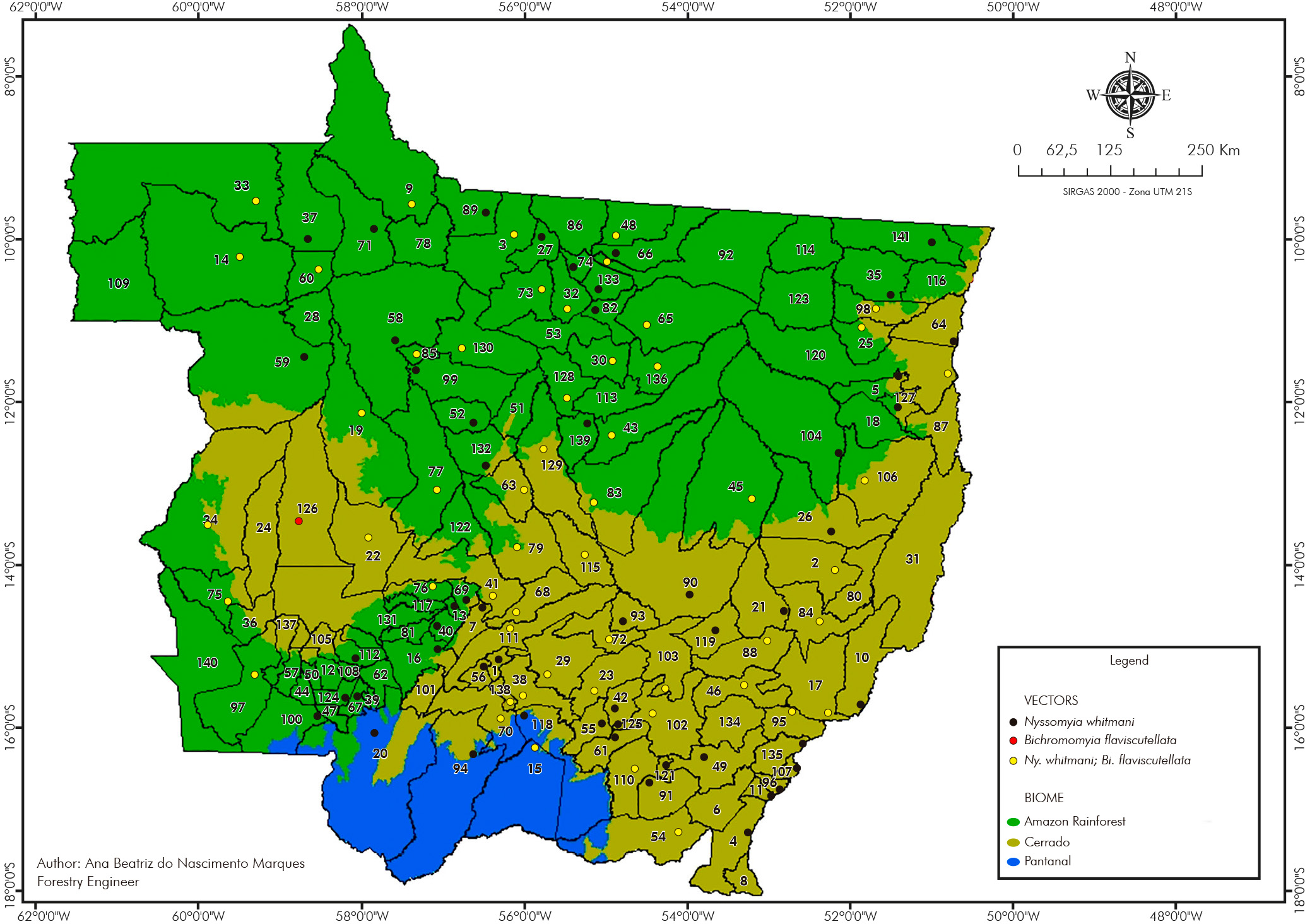
Of the species important in the VL epidemiology, Lu. (Lut.) longipalpis was detected in 48.31% (57/118) of the cities and Lu. (Lut.) cruzi in 34.75% (41/118). These species were found in sympatry in 25.42% (30/118) of the cities analyzed (Figure 3).
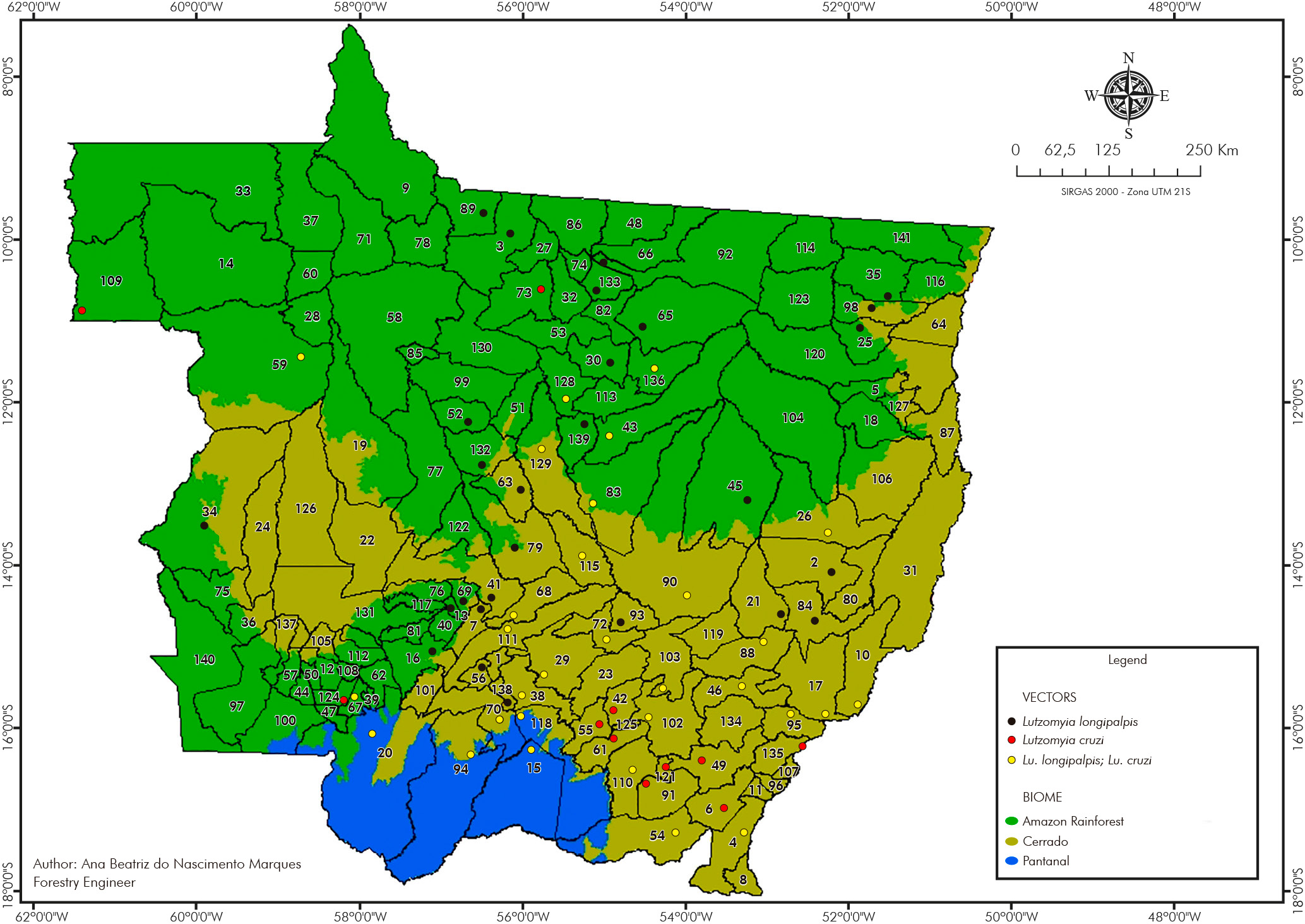
List of cities: 1-Acorizal, 2-Água Boa, 3-Alta Floresta, 4-Alto Araguaia, 5-Alto Boa Vista, 6-Alto Garças, 7-Alto Paraguai, 8-Alto Taquari, 9-Apiacás, 10-Araguaiana, 11-Araguainha, 12-Araputanga, 13-Arenápolis, 14-Aripuanã, 15-Barão de Melgaço, 16-Barra do Bugres, 17-Barra do Garças, 18-Bom Jesus do Araguaia, 19-Brasnorte, 20-Cáceres, 21-Campinápolis, 22-Campo Novo do Parecis, 23-Campo Verde, 24-Campos de Júlio, 25-Canabrava do Norte, 26-Canarana, 27-Carlinda, 28-Castanheira, 29-Chapada dos Guimarães, 30-Cláudia, 31-Cocalinho, 32-Colíder, 33-Colniza, 34-Comodoro, 35-Confresa, 36-Conquista D'Oeste, 37-Cotriguaçu, 38-Cuiabá, 39-Curvelândia, 40-Denise, 41-Diamantino, 42-Dom Aquino, 43-Feliz Natal, 44-Figueirópolis D'Oeste, 45-Gaúcha do Norte, 46-General Carneiro, 47-Glória D'Oeste, 48-Guarantã do Norte, 49-Guiratinga, 50-Indiavaí, 51-Ipiranga do Norte, 52-Itanhangá, 53-Itaúba, 54-Itiquira, 55-Jaciara, 56-Jangada, 57-Jauru, 58-Juara, 59-Juína, 60-Juruena, 61-Juscimeira, 62-Lambari D'Oeste, 63-Lucas do Rio Verde, 64-Luciara, 65-Marcelândia, 66-Matupá, 67-Mirassol D'Oeste, 68-Nobres, 69-Nortelândia, 70-Nossa Senhora do Livramento, 71-Nova Bandeirantes, 72-Nova Brasilândia, 73-Nova Canaã do Norte, 74-Nova Guarita, 75-Nova Lacerda, 76-Nova Marilândia, 77-Nova Maringá, 78-Nova Monte Verde, 79-Nova Mutum, 80-Nova Nazaré, 81-Nova Olímpia, 82-Nova Santa Helena, 83-Nova Ubiratã, 84-Nova Xavantina, 85-Novo Horizonte do Norte, 86-Novo Mundo, 87-Novo Santo Antônio, 88-Novo São Joaquim, 89-Paranaíta, 90-Paranatinga, 91-Pedra Preta, 92-Peixoto de Azevedo, 93-Planalto da Serra, 94-Poconé, 95-Pontal do Araguaia, 96-Ponte Branca, 97-Pontes e Lacerda, 98-Porto Alegre do Norte, 99-Porto dos Gaúchos, 100-Porto Esperidião, 101-Porto Estrela, 102-Poxoréu, 103-Primavera do Leste, 104-Querência, 105-Reserva do Cabaçal, 106-Ribeirão Cascalheira, 107-Ribeirãozinho, 108-Rio Branco, 109-Rondolândia, 110-Rondonópolis, 111-Rosário Oeste, 112-Salto do Céu, 113-Santa Carmem, 114-Santa Cruz do Xingu, 115-Santa Rita do Trivelato, 116-Santa Terezinha, 117-Santo Afonso, 118-Santo Antônio de Leverger, 119-Santo Antônio do Leste, 120-São Félix do Araguaia, 121-São José do Povo, 122-São José do Rio Claro, 123-São José do Xingu, 124-São José dos Quatro Marcos, 125-São Pedro da Cipa, 126-Sapezal, 127-Serra Nova Dourada, 128-Sinop, 129-Sorriso, 130-Tabaporã, 131-Tangará da Serra, 132-Tapurah, 133-Terra Nova do Norte, 134-Tesouro, 135-Torixoréu, 136-União do Sul, 137-Vale de São Domingos, 138-Várzea Grande, 139-Vera, 140-Vila Bela da Santíssima Trindade, 141-Vila Rica.
Figure 3 - Lu. (Lut.) longipalpis and Lu. (Lut.) cruzi spatial distribution in Mato Grosso State, Brazil, from 2006 to 2021
Lu. (Lut.) longipalpis and Lu. (Lut.) cruzi were found in all three biomes. In the Amazon Rainforest biome, Lu. (Lut.) longipalpis occurred in 36.84% (21/57) and Lu. (Lut.) cruzi in 15.79% (9/57) of the cities predominantly in this biome. For the Cerrado, Lu. (Lut.) longipalpis was detected in 32/56 (57.14%) and Lu. (Lut.) cruzi in 28/56 (50.00%) of the cities in this biome. Both species were recorded in all cities (4/4) of the Pantanal biome.
Among the 118 cities analyzed in this study, only 10 (8.47%) did not have the main species of epidemiological importance for ATL and VL (Figure 1).
DISCUSSION
The findings of this study reveal the expansion of entomological surveillance for phlebotomines in Mato Grosso, from the 68 cities surveyed by Missawa and Lima24 (1996-2004) to 118 (2006-2021). However, there is still a need to expand entomological surveillance actions to the other cities that have not been surveyed/silenced (Alto Taquari, Araputanga, Bom Jesus do Araguaia, Castanheira, Cocalinho, Conquista D'Oeste, Curvelândia, Figueirópolis D'Oeste, Glória D'Oeste, Indiavaí, Itaúba, Jauru, Lambari D'Oeste, Nova Monte Verde, Nova Nazaré, Nova Olímpia, Rio Branco, Santa Cruz do Xingu, Santo Afonso, Santo Antônio do Leste, Santa Terezinha, Tesouro, and Vale de São Domingos), in order to encompass the entire state.
In addition to the increase in the number of cities surveyed, there was an increase in the number of phlebotomine species recorded, from 106 species described by Missawa and Maciel22 to 132. This growth may be due to the expansion of entomological monitoring carried out by health services and research groups, revealing the importance of these activities for understanding the phlebotomine fauna. This information is necessary for planning prevention and control measures to reduce the risk of leishmaniasis transmission in Mato Grosso since the presence of vectors and their spatial density are associated with the lower or higher risk of leishmaniasis occurrence in each geographical area. Thus, this study contributes to identifying locations at risk of disease transmission in the state.
Missawa and Maciel22, using Young and Duncan31 for taxonomic classification, identified Lutzomyia goiana (Martins, Falcão & Silva, 1962) and Migonemyia baityi Damasceno, Causey & Arouck, 1945 in Mato Grosso. Using the taxonomic classification proposed by Galati32, these species were reclassified as Micropygomyia (Sauromyia) vonatzingeni (Galati, 2007) and Migonemyia (Blancasmyia) bursiformis (Floch & Abonnenc, 1944), respectively, qualitatively altering the list of phlebotomine species found in Mato Grosso (Table 1).
Queiroz et al.36 and Moraes38 identified Ps. wellcomei and Micropygomyia (Sauromyia) ferreirana (Barreto, Martins & Pellegrino, 1956) in Barra do Garças city, respectively, marking the first records of these species in Mato Grosso. In Cáceres, Alves et al.37 detected: Brumptomyia avellari (Costa Lima, 1932); Brumptomyia mangabeirai (Barreto & Coutinho, 1941); Evandromyia (Aldamyia) aldafalcaoae (Santos, Andrade Filho & Honer, 2001); Psathyromyia (Forattiniella) campograndensis (Oliveira, Andrade Filho, Falcão & Brazil, 2001); Expapillata cerradincola (Galati, Nunes, Oshiro & Dorval, 1995); and Micropygomyia (Silvamyia) echinatopharynx Andrade Filho, Galati, Andrade & Falcão, 2004. Thies et al.39 reported Viannamyia caprina (Osorno-Mesa, Morales & Osorno, 1972) and Psychodopygus dorlinsis (Le Pont & Desjeux, 1982) in Sinop, marking the first record of these species in the state.
In addition to the main species involved in leishmaniasis epidemiology1,12, others also implicated in the dissemination cycle were recorded in Mato Grosso, such as Bichromomyia olmeca bicolor (Fairchild & Theodor, 1971), Bichromomyia olmeca nociva (Young & Arias, 1982), Ny. umbratilis, Nyssomyia yuilli yuilli (Young & Porter, 1972), Trichophoromyia ubiquitalis (Mangabeira, 1942), Mg. (Mig.) migonei, Nyssomyia antunesi (Coutinho, 1939), among others.
The diversity of phlebotomine species recorded in Mato Grosso (132 species) quantitatively surpasses its neighboring states such as Pará (130)40, Amazonas (131)41, Tocantins (43)42, Goiás (47)43, and Mato Grosso do Sul (54)14; except for Rondônia, which surpassed this record, with 143 species44. In Bolivia, a country bordering Mato Grosso, 121 species were recorded45.
This diversity demonstrates the adaptation of these vectors to different environments (ecotopes, biomes), with varying levels of anthropogenic interference, whether through forms of land use and occupation, deforestation, fires, or resource extraction, factors that can cause changes in phlebotomine ecology26,46. The size of Mato Grosso's geographical area and the variety of biomes (Cerrado, Amazon Rainforest, and Pantanal), as well as transition zones, can favor the diversification of the phlebotomine fauna, as has already been observed for triatomine species47. Knowing the phlebotomine fauna is important, as it is estimated that 10% of them are incriminated or suspected of transmitting the agents that cause leishmaniasis48.
Brazil is a vast country with five biomes, which may explain the diversity of phlebotomines43. Mato Grosso has three biomes throughout its territorial area, which may have contributed to a greater richness of recorded species compared to other Brazilian states20,21.
Regarding the importance of recording vectors in ATL epidemiology, this study highlighted the wide distribution of Ny. whitmani in Mato Grosso, found in 88.98% of the surveyed cities, results that corroborate the Zeilhofer et al.34 and Missawa et al.33 findings.
The wide distribution of Ny. whitmani is reflected in the widespread occurrence of human cases in all Mato Grosso cities, as well as the maintenance and growth of ATL cases in the state26. Ny. whitmani is involved in ATL transmission in both old colonization areas and recent urbanization areas49. According to Souza et al.50, this species is adapted to urban areas and can transmit ATL both in the peridomicile and intradomicile, often found in chicken coops, suggesting a process of adaptation to the anthropic environment36,51; it is opportunistic and has eclectic feeding habits, adjusting its habits to the availability of hosts in anthropic environments52.
Ny. whitmani can be found in five regions of Brazil, associated with a variety of vegetation types, such as forests, cerrado, and caatinga, widely distributed in Roraima, Acre, Tocantins, and Mato Grosso do Sul53. This wide distribution and association with Leishmania braziliensis in different regions show the importance of this species in ATL epidemiology, reinforcing its status as the main vector in Mato Grosso, corroborating its findings in other areas54,55,56.
These vector findings demonstrate the relevance of entomological research and reinforce the need to develop public health policies aimed at preventing and promoting health, as well as timely diagnosis and treatment of ATL human cases.
Although recorded in all studied biomes, Bi. flaviscutellata was more prevalent in the Cerrado cities of Mato Grosso, reinforcing the need for attention to its dispersion and importance in the state's ATL epidemiology. This species is zoophilic and feeds on small rodents of the genera Oryzomys and Proechimys and is considered a vector of Leishmania amazonensis57. It inhabits forest areas, found in openings of fallen trees that offer conditions for both the development of immature forms and the permanence of vertebrate hosts58. Occasionally, it invades the peridomestic habitats59 and shelters of domestic animals60,61, exploiting degraded areas usually occupied by human populations, facilitating human-vector contact42,59.
When assessing the dispersion of important species for VL in Mato Grosso, its primary vector, Lu. (Lut.) longipalpis, occurred in all the Pantanal biome cities and in 57.14% of those in Cerrado. Missawa and Lima24 reported the association of this species with the Amazon Rainforest and Cerrado biomes. The maintenance of Lu. (Lut.) longipalpis is possibly associated with its dietary eclecticism7,62,63,64,65,66.
Lu. (Lut.) cruzi was also found in 100% of the cities with the Pantanal biome and in 50% of the Cerrado cities, corroborating the Ribeiro and Missawa67 and Missawa and Lima24 findings, who reported a higher frequency of this species in Cerrado and Pantanal cities, suggesting the Cerrado as the preferential environment for this species.
Lu. (Lut.) cruzi is important to VL epidemiology in Mato Grosso35 and Mato Grosso do Sul68. Cases of VL and ATL have been reported in the absence of the main vector species, Lu. (Lut.) longipalpis, and there are already records of natural infection of Lu. (Lut.) cruzi by Leishmania chagasi in both states35,68, demonstrating that this species may be part of the leishmaniasis transmission chain.
The occurrence of Lu. (Lut.) longipalpis and Lu. (Lut.) cruzi, with a wide distribution in the different biomes of Mato Grosso, confirms the generalist nature of these species, adapted to diversified habitats69.
Another species constantly present in ATL endemic areas is Ny. umbratilis70, considered one of the main vectors of Leishmania (Viannia) guyanensis in most of Latin American countries, such as Brazil, Bolivia, Colombia, Peru, Venezuela, Suriname, and French Guiana, and in the Amazon Region71. Mg. (Mig.) migonei is a permissive vector, capable of being infected with various species of Leishmania72, also found in the state.
A species of Leishmania can be transmitted by different species of sand flies (Diptera: Psychodidae: Phlebotominae) in different geographical regions and biomes43. The presence of medically important phlebotomine species in the state's different biomes reveals vulnerable and/or receptive areas for transmitting leishmaniasis in all the Mato Grosso cities surveyed.
CONCLUSION
This study revealed a high diversity of phlebotomines in Mato Grosso, ranking it the second Brazilian state with the highest number of distinct species.
It was possible to verify the expansion of entomological surveillance in the state over the years and an increase in the number of identified species that may be involved and/or incriminated in the transmission of leishmaniasis in Mato Grosso.
Thus, entomological and epidemiological surveillance studies, especially those focused on the knowledge of phlebotomine fauna and its behavior, will provide subsidies to support health services in effective prevention and health promotion activities aimed at controlling leishmaniasis.
The importance of integrated work between the Municipal Health Departments and the State Health Department is also emphasized. This allows the optimization of resources and the prompt effectiveness of vector control actions, thereby reducing the risk of disease transmission and ensuring the improvement of the population's quality of life.
REFERENCES
1 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Articulação Estratégica de Vigilância em Saúde. Guia de vigilância em saúde. 5. ed. rev. atual. Brasília: Ministério da Saúde; 2022. [Link] [ Links ]
2 Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013 Jan;58:227-50. Doi: 10.1146/annurev-ento-120811-153557 [Link] [ Links ]
3 Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Mem Inst Oswaldo Cruz. 2005 Dec;100(8):811-27. Doi: 10.1590/s0074-02762005000800001 [Link] [ Links ]
4 Brazil RP. The dispersion of Lutzomyia longipalpis in urban areas. Rev Soc Bras Med Trop. 2013 Mai-Jun;46(3):263-4. Doi: 10.1590/0037-8682-0101-2013 [Link] [ Links ]
5 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Sistema de Informação de Agravos de Notificação. Casos confirmados notificados no Sistema de Informação de Agravos de Notificação [Internet]. Brasília: Ministério da Saúde ; 2006-2021 [citado 2021 mar 14]. Disponível em: Disponível em: http://dtr2004.saude. gov.br/sinanweb/tabnet/dh?sinannet/lta/bases/ltabrnet.def . [ Links ]
6 Mestre GLC, Fontes CJF. A expansão da epidemia da leishmaniose visceral no Estado de Mato Grosso, 1998-2005. Rev Soc Bras Med Trop. 2007 jan-fev;40(1):42-8. Doi: 10.1590/S0037-86822007000100008 [Link] [ Links ]
7 Rangel EF, Lainson R. Transmissores de leishmaniose tegumentar americana. In: Rangel EF, Lainson R, organizadores. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. p. 291-309. [ Links ]
8 Rangel EF, Lainson R, Carvalho BM, Costa SM, Shaw JJ. Sand fly vectors of American cutaneous leishmaniasis in Brazil. In: Rangel EF, Shaw JJ, editors. Brazilian sand flies: biology, taxonomy, medical importance and control. Cham: Springer; 2018. p. 341-80. [ Links ]
9 Shimabukuro PHF, Tolezano JE, Galati EAB. Chave de identificação ilustrada dos Phlebotominae (Diptera, Psychodidae) do estado de São Paulo, Brasil. Pap Avulsos Zool. 2011;51(27):399‑441. Doi: 10.1590/S0031-10492011002700001 [Link] [ Links ]
10 Costa SM, Cordeiro JLP, Rangel EF. Environmental suitability for Lutzomyia (Nyssomyia) whitmani (Diptera: Psychodidae: Phlebotominae) and the occurrence of American cutaneous leishmaniasis in Brazil. Parasit Vectors. 2018 Mar;11:155. Doi: 10.1186/s13071-018-2742-7 [Link] [ Links ]
11 Sherlock IA. A importância dos flebotomíneos. In: Rangel EF, Lainson R, organizadores. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz ; 2003. p. 15-21. [ Links ]
12 Rangell EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009 Nov;104(7):937-54. Doi: 10.1590/S0074-02762009000700001 [Link] [ Links ]
13 Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy. Rev Pan-Amaz Saude. 2010 Jun;1(2):13-32. Doi: 10.5123/S2176-62232010000200002 [Link] [ Links ]
14 Almeida PS, Nascimento JC, Ferreira AD, Minzão LD, Portes F, Miranda AM, et al. Espécies de flebotomíneos (Diptera, Psychodidae) coletadas em ambiente urbano em municípios com transmissão de leishmaniose visceral do Estado de Mato Grosso do Sul, Brasil. Rev Bras Entomol. 2010 jun;54(2):304-10. Doi: 10.1590/S0085-56262010000200014 [Link] [ Links ]
15 Dorval MEMC, Oshiro ET, Cupollilo E, Castro ACC, Alves TP. Ocorrência de leishmaniose tegumentar americana no Estado do Mato Grosso do Sul associada à infecção por Leishmania (Leishmania) amazonensis. Rev Soc Bras Med Trop. 2006 jan-fev;39(1):43-6. Doi: 10.1590/S0037-86822006000100008 [Link] [ Links ]
16 Harhay MO, Olliaro PL, Costa DL, Costa CHN. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 2011 Sep;27(9):403-9. Doi: 10.1016/j.pt.2011.04.001 [Link] [ Links ]
17 Brito VN, Almeida ABPF, Nakazato L, Duarte R, Souza CO, Sousa VRF. Phlebotomine fauna, natural infection rate and feeding habits of Lutzomyia cruzi in Jaciara, state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2014 Nov;109(7):899-904. Doi: 10.1590/0074-0276140112 [Link] [ Links ]
18 Feitosa MM, Ikeda FA, Luvizotto MCR, Perri SH. Aspectos clínicos de cães com leishmaniose visceral no município de Araçatuba, São Paulo (Brasil). Clin Vet. 2000;5(28):36-44. [ Links ]
19 Romero GAS, Boelaert M. Control of visceral leishmaniasis in Latin America - a systematic review. PLoS Negl Trop Dis. 2010 Jan;4(1):e584. Doi: 10.1371/journal.pntd.0000584 [Link] [ Links ]
20 Instituto Brasileiro de Geografia e Estatística (BR). Mapas interativos [Internet]. 2022? [citado 2023 ago 30]. Disponível em: Disponível em: https://mapasinterativos.ibge.gov.br/sigibge/ . [ Links ]
21 Schwenk LM. Domínios biogeográficos. In: Moreno G, Higa TCCS, organizadores. Geografia de Mato Grosso: território, sociedade e ambiente. 2. ed. rev. atual. Cuiabá: Entrelinhas; 2017. p. 254-75. [ Links ]
22 Missawa NA, Maciel GBML. List of species in the genus Lutzomyia, França, 1924 (Psychodidae, Phlebotominae) from the State of Mato Grosso. Rev Soc Bras Med Trop. 2007 Jan-Feb;40(1):11-4. Doi: 10.1590/S0037-86822007000100002 [Link] [ Links ]
23 Ribeiro ALM, Missawa NA, Zeilhofer P. Distribution of phlebotomine sandflies (Diptera: Psychodidae) of medical importance in Mato Grosso State, Brazil. Rev Inst Med Trop S Paulo. 2007 Sep-Oct;49(5):317-21. Doi: 10.1590/S0036-46652007000500008 [Link] [ Links ]
24 Missawa NA, Lima GBM. Distribuição espacial de Lutzomyia longipalpis (Lutz & Neiva, 1912) e Lutzomyia cruzi (Mangabeira, 1938) no estado de Mato Grosso. Rev Soc Bras Med Trop. 2006 jul-ago;39(4):337-40. Doi: 10.1590/S0037-86822006000400004 [Link] [ Links ]
25 Dias ES, França-Silva JC, Silva JC, Monteiro EM, Paula KM, Gonçalves CM, et al. Flebotomíneos (Diptera: Psychodidae) de um foco de leishmaniose tegumentar no estado de Minas Gerais. Rev Soc Bras Med Trop. 2007 jan-fev;40(1):49-52. Doi: 10.1590/S0037-86822007000100009 [Link] [ Links ]
26 Costa JML. Epidemiologia das leishmanioses no Brasil. Gaz Med Bahia. 2005 jan-jun;75(1):3-17. [Link] [ Links ]
27 Leonardo FS, Rebêlo JMM. A periurbanização de Lutzomyia whitmani em área de foco de leishmaniose cutânea, no Estado do Maranhão, Brasil. Rev Soc Bras Med Trop. 2004 mai-jun;37(3):282-4. Doi: 10.1590/S0037-86822004000300016 [Link] [ Links ]
28 Instituto Brasileiro de Geografia e Estatística. Cidades e Estados: Mato Grosso [Internet]. Rio de Janeiro: IBGE; 2022 [citado 2023 ago 31]. Disponível em: Disponível em: https://www.ibge.gov.br/cidades-e-estados/mt.html . [ Links ]
29 Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq News. 1962;22:126-9. [Link] [ Links ]
30 Shannon RC. Methods for collecting and feeding mosquitoes in jungle yellow fever studies. Am J Trop Med Hyg. 1939;19(2):131-40. Doi: 10.4269/ajtmh.1939.s1-19.131 [Link] [ Links ]
31 Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Gainesville (FL): American Entomological Institute; 1994. Memoirs of the American Entomological Institute, n. 54). [ Links ]
32 Galati EAB. Morfologia e terminologia de Phlebotominae (Diptera: Psychodidae). Classificação e identificação de táxons das Américas [Internet]. Vol I. Apostila da Disciplina Bioecologia e Identificação de Phlebotominae do Programa de Pós-Graduação em Saúde Pública. São Paulo: Faculdade de Saúde Pública, Universidade de São Paulo; 2021 [citado 2023 ago 31]. 133 p. Disponível em: Disponível em: http://www.fsp.usp.br/egalati . [ Links ]
33 Missawa NA, Maciel GBML, Rodrigues H. Distribuição geográfica de Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) no Estado de Mato Grosso. Rev Soc Bras Med Trop. 2008 jul-ago;41(4):369-73. Doi: 10.1590/s0037-86822008000400009 [Link] [ Links ]
34 Zeilhofer P, Kummer OP, Santos ES, Ribeiro ALM, Missawa NA. Spatial modelling of Lutzomyia (Nyssomyia) whitmani s.l. (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae) habitat suitability in the state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2008 Nov;103(7):653-60. Doi: 10.1590/s0074-02762008000700005 [Link] [ Links ]
35 Missawa NA, Veloso MAE, Maciel GBML, Michalsky EM, Dias ES. Evidência de transmissão de leishmaniose visceral por Lutzomyia cruzi no município de Jaciara, Estado de Mato Grosso, Brasil. Rev Soc Bras Med Trop. 2011 jan-fev;44(1):76-8. Doi: 10.1590/s0037-86822011000100017 [Link] [ Links ]
36 Queiroz MFM, Varjão JR, Moraes SC, Salcedo GE. Analysis of sandflies (Diptera: Psychodidae) in Barra do Garças, State of Mato Grosso, Brazil, and the influence of environmental variables on the vector density of Lutzomyia longipalpis (Lutz & Neiva, 1912). Rev Soc Bras Med Trop. 2012 May-Jun;45(3):313-7. Doi: 10.1590/s0037-86822012000300007 [Link] [ Links ]
37 Alves GB, Oshiro ET, Leite MC, Melão AV, Ribeiro LM, Mateus NLF, et al. Phlebotomine sandflies fauna (Diptera: Psychodidae) at rural settlements in the municipality of Cáceres, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2012 Jul-Aug;45(4):437-43. Doi: 10.1590/s0037-86822012005000010 [Link] [ Links ]
38 Moraes SC. Ecoepidemiologia da leishmaniose tegumentar americana no município de Barra do Garças, Mato Grosso, Brasil [Tese]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas; 2015. 124 p. [Link] [ Links ]
39 Thies SF, Bronzoni RVM, Espinosa MM, Souza CO, Ribeiro ALM, Santos ES, et al. Frequency and diversity of phlebotomine sand flies (Diptera: Psychodidae) in Sinop, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2016 Sep-Oct;49(5):544-52. Doi: 10.1590/0037-8682-0251-2016 [Link] [ Links ]
40 Galati EAB. Phlebotominae (Diptera, Psychodidae): classification, morphology and terminology of adults and identification of American taxa. In: Rangel E, Shaw J, editors. Brazilian sand flies. Gewerbestrasse: Springer; 2018. p. 9-212. Doi: 10.1007/978-3-319-75544-1_2 [Link] [ Links ]
41 Alves VR, Freitas RA, Santos FL, Oliveira AFJ, Barrett TV, Shimabukuro PHF. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012 Jun;56(2):220-7. Doi: 10.1590/S0085-56262012005000020 [Link] [ Links ]
42 Vilela ML, Pita-Pereira D, Azevedo CG, Godoy RE, Britto C, Rangel EF. The phlebotomine fauna (Diptera: Psychodidae) of Guaraí, state of Tocantins, with an emphasis on the putative vectors of American cutaneous leishmaniasis in rural settlement and periurban areas. Mem Inst Oswaldo Cruz. 2013 Aug;108(5):578-85. Doi: 10.1590/s0074-02762013000500007 [Link] [ Links ]
43 Almeida PS, Andrade AJ, Sciamarelli A, Raizer J, Menegatti JA, Hermes SCNM, et al. Geographic distribution of phlebotomine sandfly species (Diptera: Psychodidae) in Central-West Brazil. Mem Inst Oswaldo Cruz. 2015 Jun;110(4):551-9. Doi: 10.1590/0074-02760140462 [Link] [ Links ]
44 Costa GS, Pereira Júnior AM, Pessoa FAC, Shimabukuro PHF, Medeiros JF. New records of phlebotomine sand flies (Diptera: Psychodidae) from the Western Brazilian Amazon and the description of the female of Pintomyia fiocruzi. J Med Entomol. 2020 Jul;57(4):1328-33. Doi: 10.1093/jme/tjaa030 [Link] [ Links ]
45 Brazil RP, Passos WL, Brazil BG, Temeljkovitch M, Andrade Filho JD. Diptera, Psychodidae, Phlebotominae Rondani, 1840: range extension and new records from lowland Bolivia. Check List. 2010 Oct;6(4):587-8. Doi: 10.15560/6.4.587 [Link] [ Links ]
46 Tonelli GB, Binder C, Nogueira VLC, Prado MH, Theobaldo GG, Campos AM, et al. The sand fly (Diptera: Psychodidae) fauna of the urban area of Lassance, Northeast Minas Gerais, Brazil. PLoS One. 2021 Oct;16(10):e0257043. Doi: 10.1371/journal.pone.0257043 [Link] [ Links ]
47 Pereira JM, Almeida PS, Sousa AV, Paula AM, Machado RB, Gurgel-Gonçalves R. Climatic factors influencing triatomine occurrence in Central-West Brazil. Mem Inst Oswaldo Cruz. 2013 May;108(3):335-41. Doi: 10.1590/S0074-02762013000300012 [Link] [ Links ]
48 Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013 Jun;27(2):123-47. Doi: 10.1111/j.1365-2915.2012.01034.x [Link] [ Links ]
49 Costa SM, Cordeiro JLP, Rangel EF. Environmental suitability for Lutzomyia (Nyssomyia) whitmani (Diptera: Psychodidae: Phlebotominae) and the occurrence of American cutaneous leishmaniasis in Brazil. Parasit Vectors. 2018 Mar;11(1):155. Doi: 10.1186/s13071-018-2742-7 [Link] [ Links ]
50 Souza CM, Pessanha JE, Barata RA, Monteiro EM, Costa DC, Dias ES. Study on phlebotomine sand fly (Diptera: Psychodidae) fauna in Belo Horizonte, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2004 Dec;99(8):795-803. Doi: 10.1590/s0074-02762004000800003 [Link] [ Links ]
51 Oliveira AG, Andrade Filho JD, Falcão AL, Brazil RP. Estudo dos flebotomíneos (Diptera, Psychodidae, Phlebotominae) na zona urbana da cidade de Campo Grande, Mato Grosso do Sul, Brasil, 1999-2000. Cad Saude Publica. 2003 jul-ago;19(4):933-44. Doi: 10.1590/s0102-311x2003000400016 [Link] [ Links ]
52 Muniz LHG, Rossi RM, Neitzke HC, Monteiro WM, Teodoro U. Estudo dos hábitos alimentares de flebotomíneos em área rural no sul do Brasil. Rev Saude Publica. 2006 dez;40(6):1087-93. Doi: 10.1590/s0034-89102006000700018 [Link] [ Links ]
53 Costa SM, Cechinel M, Bandeira V, Zannuncio JC, Lainson R, Rangel EF. Lutzomyia (Nyssomyia) whitmani s.l. (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae): geographical distribution and the epidemiology of American cutaneous leishmaniasis in Brazil Mini-review. Mem Inst Oswaldo Cruz. 2007 Mar;102(2):149-53. Doi: 10.1590/s0074-02762007005000016 [Link] [ Links ]
54 Azevedo ACR, Rangel EF, Costa EM, David J, Vasconcelos AW, Lopes UG. Natural infection of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) by Leishmania of the braziliensis complex in Baturité, Ceará state, northeast Brazil. Mem Inst Oswaldo Cruz. 1990 Apr-Jun;85(2):251. Doi: 10.1590/s0074-02761990000200021 [Link] [ Links ]
55 Azevedo ACR, Vilela ML, Souza NA, Andrade-Coelho CA, Barbosa AF, Firmo ALS, et al. The sand fly fauna (Diptera: Psychodidae: Phlebotominae) of a focus of cutaneous leishmaniasis in Ilhéus, state of Bahia, Brazil. Mem Inst Oswaldo Cruz. 1996 Jan-Feb;91(1):75-9. Doi: 10.1590/s0074-02761996000100012 [Link] [ Links ]
56 Saraiva L, Lopes JS, Oliveira GBM, Batista FA, Falcão AL, Andrade Filho JD. Estudo dos flebotomíneos (Diptera: Psychodidae) em área de leishmaniose tegumentar americana nos municípios de Alto Caparaó e Caparaó, Estado de Minas Gerais. Rev Soc Bras Med Trop. 2006 jan-fev;39(1):56-63. Doi: 10.1590/s0037-86822006000100011 [Link] [ Links ]
57 Shaw JJ, Lainson R, Ward RD. Leishmaniasis in Brazil: VII. Further observations on the feeding habitats of Lutzomyia flaviscutellata (Mangabeira) with particular reference to its biting habits at different heights. Trans R Soc Trop Med Hyg. 1972;66(5):718-23. Doi: 10.1016/0035-9203(72)90085-5 [Link] [ Links ]
58 Ready PD, Lainson R, Shaw JJ. Leishmaniasis in Brazil: XX. Prevalence of “enzootic rodent leishmaniasis” (Leishmania mexicana amazonensis), and apparent absence of “pian bois” (Le. braziliensis guyanensis), in plantations of introduced tree species and in other non-climax forests in eastern Amazônia. Trans R Soc Trop Med Hyg. 1983;77(6):775-85. Doi: 10.1016/0035-9203(83)90288-2 [Link] [ Links ]
59 Lainson R, Shaw JJ, Silveira FT, Souza AAA, Braga RR, Ishikawa EAY. The dermal leishmaniases of Brazil, with special reference to the eco-epidemiology of the disease in Amazonia. Mem Inst Oswaldo Cruz. 1994 Jul-Sep;89(3):435-43. Doi: 10.1590/s0074-02761994000300027 [Link] [ Links ]
60 Vilela ML, Azevedo ACR, Costa SM, Costa WA, Motta-Silva D, Grajauskas AM, et al. Sand fly survey in the influence area of Peixe Angical Hydroelectric Plant, state of Tocantins, Brazil. 6th International Symposium on Phlebotomine Sandflies; 2008 Oct; Lima, Peru. [ Links ]
61 Shaw J. How climatic and environmental variations affect the eco-epidemiology of the leishmaniases and their control. III Workshop de Genética e Biologia Molecular de Insetos Vetores de Doenças Tropicais; 2008 Sep 2; Recife, Pernambuco. p. 13. [ Links ]
62 Brazil RP, Brazil BG. Biologia de flebotomíneos neotropicais. In: Rangel EF, Lainson R, organizadores. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz ; 2003. p. 257-74. [ Links ]
63 Rangel EF, Vilela ML. Lutzomyia longipalpis (Diptera, Psychodidae, Phlebotominae) and urbanization of visceral leishmaniasis in Brazil. Cad Saude Publica. 2008 Dec;24(12):2948-52. Doi: 10.1590/s0102-311x2008001200025 [Link] [ Links ]
64 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância da leishmaniose tegumentar americana. 2. ed. atual. Brasília: Ministério da Saúde ; 2013. [Link] [ Links ]
65 Salomón OD, Feliciangeli MD, Quintana MG, Afonso MMS, Rangel EF. Lutzomyia longipalpis urbanisation and control. Mem Inst Oswaldo Cruz. 2015 Nov;110(7):831-46. Doi: 10.1590/0074-02760150207 [Link] [ Links ]
66 Lana RS, Michalsky EM, Lopes LO, Lara-Silva FO, Nascimento JL, Pinheiro LC, et al. Ecoepidemiological aspects of visceral leishmaniasis in an endemic area in the Steel Valley in Brazil: an ecological approach with spatial analysis. PLoS One. 2018 Oct;13(10):e0206452. Doi: 10.1371/journal.pone.0206452 [Link] [ Links ]
67 Ribeiro ALM, Missawa NA. Spatial distribution of phlebotomine species in the state of Mato Grosso, Brazil, in the period of 1996 to 2001. Entomol Vect. 2002;9:33-4. [ Links ]
68 Santos SO, Arias J, Ribeiro AA, Hoffmann MP, Freitas RA, Malacco MAF. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis. Med Vet Entomol. 1998 Jul;12(3):315-7. Doi: 10.1046/j.1365-2915.1998.00104.x [Link] [ Links ]
69 Aguiar GM, Medeiros WM. Distribuição regional e hábitats das espécies de flebotomíneos do Brasil. In: Rangel EF, Lainson R, organizadores. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz ; 2003. p. 207-55. [ Links ]
70 Pinheiro FG, Luz SLB, Franco AMR. Infecção natural por tripanosomatídeos (Kinetoplastida: Trypanosomatidae) em Lutzomyia umbratilis (Diptera: Psychodidae) em áreas de leishmaniose tegumentar americana no Amazonas, Brasil. Acta Amaz. 2008;38(1):165-72. Doi: 10.1590/S0044-59672008000100019 [Link] [ Links ]
71 Brazil RP, Rodrigues AAF, Andrade Filho JD. Sand fly vectors of Leishmania in the Americas - a mini review. Entomol Ornithol Herpetol. 2015;4(2):144. Doi: 10.4172/2161-0983.1000144 [Link] [ Links ]
72 Guimarães VCFV, Pruzinova K, Sadlova J, Volfova V, Myskova J, Brandão Filho SP, et al. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit Vectors. 2016 Mar;9:159. Doi: 10.1186/s13071-016-1444-2 [Link] [ Links ]
How to cite this article / Como citar este artigo: Thies SF, Menegatti JA, Moraes SC, Martins MF. List of sand fly (Diptera: Psychodidae) and spatial distribution of leishmaniasis main vector species in Mato Grosso State, Brazil. Rev Pan Amaz Saude. 2023;14:e202301427. Doi: http://dx.doi.org/10.5123/S2176-6223202301427
Received: December 13, 2022; Accepted: July 04, 2023











 texto em
texto em