Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.2 Ananindeua jun. 2010
http://dx.doi.org/10.5123/S2176-62232010000200002
ARTIGO HISTÓRICO |HISTORICAL ARTICLE | ARTÍCULO HISTÓRICO
The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy
Espécies neotropicais de Leishmania: uma breve revisão histórica sobre sua descoberta, ecologia e taxonomia
Especies neotropicales de Leishmania: una breve revisión histórica sobre su descubrimiento, ecología y taxonomía
Ralph Lainson
Seção de Parasitologia, Instituto Evandro Chagas/SVS/MS, Belém, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
This paper is a review of the major historical events leading to our present classification of the Neotropical Leishmania species, and apart from indicating the basic type of disease these different parasites may cause in humans, it does not discuss the clinical or epidemiological features of the leishmaniases. For each of these species, information is given on the known geographical distribution, recorded phlebotomine sand fly host(s) and the secondary, wild or domestic mammalian hosts. Reasons are given for regarding the parasite referred to as Leishmania (L.) infantum chagasi, the causative agent of American visceral leishmaniasis, as most probably indigenous to the Neotropics rather than imported during the Iberian colonisation.
Keywords: Leishmania; Neotropics; Ecology; Taxonomy.
RESUMO
Este artigo apresenta uma revisão dos mais importantes eventos históricos que levaram à atual classificação das espécies neotropicais de Leishmania e indica as doenças básicas causadas em seres humanos por estes diferentes parasitos, sem discutir os aspectos clínicos e epidemiológicos das leishmanioses. Para cada uma das espécies descritas, são fornecidas informações a respeito de sua conhecida distribuição geográfica, dos flebotomíneos hospedeiros registrados e de seus reservatórios mamíferos secundários, selvagens ou domésticos. Os dados apresentados levam à conclusão de que o parasito Leishmania (L.) infantum chagasi, agente causador da leishmaniose visceral americana, é provavelmente autóctone da região neotropical, e não importado durante a colonização ibérica.
Palavras-chave: Leishmania; Neotrópico; Ecologia; Taxonomia.
RESUMEN
Este artículo presenta una revisión sobre los más importantes eventos históricos que llevaron a la actual clasificación de las especies neotropicales de Leishmania e indica las enfermedades básicas causadas a humanos por estos diferentes parásitos, sin discutir los aspectos clínicos y epidemiológicos de las leishmaniasis. Para cada una de las especies descritas, se suministran informaciones a respecto de su conocida distribución geográfica, de los flebótomos hospederos registrados y de sus reservatorios mamíferos secundarios, salvajes o domésticos. Los datos presentados llevan a la conclusión que el parásito Leishmania (L.) infantum chagasi, agente causador de la leishmaniasis visceral americana, es probablemente autóctono de la región neotropical, y no importado durante la colonización ibérica.
Palabras clave: Leishmania; Neotrópico; Ecología; Taxonomía.
INTRODUCTION
American cutaneous leishmaniasis (ACL) would appear to be an ancient disease afflicting humans in the tropical and sub-tropical areas of the New World, as suggested by early ceramics from Peru and Ecuador (huacos), which often depict human faces with ugly disfigurations very similar to those caused by mucocutaneous leishmaniasis. In addition, historians at the time of the Iberian colonisation often mentioned the frequency of indigenous inhabitants with cutaneous lesions. As long ago as 1571, Pedro Pizarro83 described the destruction of the nose and lips of coca growers working on the lower eastern slopes of the Andes; because mucocutaneous leishmaniasis is now well known to be endemic in this area, it is highly likely that he was giving an early description of this disease.
It slowly became apparent that the skin lesions referred to by the Peruvian Indians as uta and the mucocutaneous disease known as espundia were both widespread throughout most of the Latin American continent, where they were given various names. For the less destructive skin lesions: uta seco, úlcera de Velez, ulcer de los chicleros, buba, úlcera de Baurú, ferida brava, botão do oriente, forest yaws, Bay-sore, pian-bois and bosch-yaws. For the highly destructive mucocutaneous leishmaniasis: espundia, llaga corrosiva, cancro espúndico, nariz de tapir, tiacaraña, gangosa, ferida esponjosa, and cancro fagendênico. The aetiology of these lesions, however, long remained unknown.
American visceral leishmaniasis (AVL) may have an equally ancient history in Latin America, but would clearly offer less visual evidence of its existence. However, the condition known in Brazil as barriga d'água (an abnormally distended abdomen), which is associated with fever and general malaise, was well known, and many such cases in the past were likely to have been undiagnosed AVL.
The following key chronological events in the history of ACL and AVL in the Neotropics, and in particular Brazil, will perhaps help to more readily see how the present classification of their causal agents took shape.
EARLY BEGINNINGS
For many years, Peruvian uta and similar skin lesions in other countries of Latin America were considered to be identical with "oriental sore" in Mediterranean and Asian countries, the aetiology of which was, at that time, also in doubt.
1909-1911
The causative agent of Old World oriental sore was discovered in 1903115 and named Leishmania tropica in 190662. Similar skin lesions in the Neotropics were not associated with a leishmanial parasite until 1909, when Lindenberg60 and Carini and Paranhos6 independently demonstrated "Leishman-Donovan bodies" (amastigotes) in the skin lesions of individuals with "Baurú ulcer" from the State of São Paulo, Brazil. Curiously, Lindenberg (Figure 1) first published his discovery in a newspaper, O Estado de São Paulo, on 30th March 1909, and Carini and Paranhos recorded their findings the next day - in the same newspaper! Finally, in 1911, Splendore demonstrated the presence of the parasite in mucocutaneous lesions of espundia.

At first it was thought that the causative parasite should be referred to as Leishmania tropica (Wright, 1903) Lühe, 1906, but the Brazilian clinician and parasitologist Gaspar Vianna111 (Figure 2) studied amastigotes in the skin lesion of a patient in Além Paraíba, Minas Gerais State, Brazil, and concluded (erroneously, as it was later shown) that their morphology differed from that of L. tropica amastigotes. He therefore named the parasite Leishmania brazilienses, later amended to L. braziliensis by Matta69, in 1916.

1913
For several years, there was a general opinion that all American cutaneous and mucocutaneous leishmaniasis was due to the single parasite, L. braziliensis. Nevertheless, Velez110 decided, if only for patriotic reasons, that the parasite causing Peruvian uta was neither L. tropica nor L. braziliensis and thus named it Leishmania peruviana.
The first report of AVL in the Americas was probably that of Migone74 in 1913, who saw what he considered to be amastigotes in the blood of a patient in Paraguay. The man's symptoms were highly indicative of AVL, and after failing to respond to treatment for malaria, he died. Before his illness, he had worked on the construction of the notorious São Paulo-Corumbá railway in Brazil, where it was thought he probably acquired his infection.
1934-1937
Although sporadic cases of AVL had begun to appear in a number of other South and Central American countries, some time elapsed before definitive proof of the existence of the disease was obtained in Brazil. In 1934, however, 41 cases were diagnosed following the examination of liver tissue removed by viscerotome80 (Figure 3). Three of these were from the State of Pará and represented the first record of Amazonian AVL.

Both L. donovani and L. infantum, the causative agents of Old World visceral leishmaniasis, were known to readily infect laboratory animals. Therefore, in 1937, when Cunha and Chagas12 were, for some reason, unable to infect similar hosts with the parasite from Brazilian cases of AVL, it prompted them to name the parasite Leishmania chagasi.
1945-1948
A notable event in Brazil was the discovery by Medina71 of an enigmatic parasite causing lesions in the skin of the domestic guinea pig (Cavia porcellus) in 1946; this parasite was later named Leishmania enriettii Muniz & Medina, 1948. This discovery was a clear indication that dermotropic species of Leishmania other than L. braziliensis might be infecting humans in Brazil. Until the 1960s, it was still thought that all cases of human ACL in this country were due to L. braziliensis. This general opinion persisted despite the fact that the causative agent of the disease in the confluent forest of neighbouring French Guyana had been named L. guyanensis Floch, 1954.
In 1946, Convit and Lapenta9 described a strange form of cutaneous leishmaniasis in some patients in Venezuela that was characterised by a large number of nodular lesions scattered over the body and containing enormous numbers of large amastigotes. The patients showed a negative Montenegro skin test and did not respond to the usual antimonial drug treatment. The condition was referred to as diffuse cutaneous leishmaniasis (DCL) and the causative agent in Venezuela was later named Leishmania pifanoi73.
1953-1961
In 1957, researchers in Panama107,106 demonstrated the presence of a Leishmania species in the forest rodent Proechimys semispinosus, and other infections were recorded in forest rodents in the State of São Paulo, Brazil, by Forattini20 in 1960. In neither case, however, was it conclusively shown that the organism was identical to the parasite commonly infecting humans in the same locality.
By now it was becoming clear that different dermotropic leishmanial parasites were probably responsible for cutaneous leishmaniasis in different parts of the Neotropics. That causing "chiclero's ulcer" in the Yucatan, Guatemala and Belize was named Leishmania tropica mexicana by Biagi3, in 1953, and in French Guyana, Floch19 adopted this same trinomial nomenclature by referring to the cause of "pian-bois" as L. tropica guyanensis in 1954. Similarly, in other parts of South America, he regarded cutaneous leishmaniasis as being due to L. tropica braziliensis. However, in 1959, Medina and Romero73, together with several other researchers, rightly disapproved of the specific name "tropica" for these parasites and instead gave the name Leishmania braziliensis pifanoi to the parasite associated with DCL in Venezuela. The Brazilian parasitologist Pessôa81 followed suit in 1961 by listing the known Leishmania species in the Americas as L. braziliensis braziliensis, L. b. guyanensis, L. b. peruviana, L. b. pifanoi, and L. b. mexicana.
1962-1965
In 1962, Garnham23 raised the parasite causing chiclero's ulcer in Belize, Central America, to specific rank as Leishmania mexicana. Additionally, in 1962 and 1964, during studies on the epidemiology of this disease, Lainson and Strangways-Dixon56,57 established that forest rodents were reservoir hosts of the parasite and frequently showed visible lesions, rich in amastigotes, on their tails. A volunteer was successfully infected with the rodent parasite, and a biological and biochemical comparison of the organism with that from cases of ACL showed them to be identical. This represented the first conclusive association of a Neotropical leishmanial parasite known to infect man with a sylvatic reservoir in wild animals.
It was natural to suspect that a similar rodent reservoir of L. braziliensis probably existed in the forests of Brazil. Thus, during a visit to the Instituto Evandro Chagas (IEC) in 1963, the present author discussed the possibility of a collaborative programme on the eco-epidemiology of ACL in the Amazon Region with the late Director, Dr. Orlando Costa, and the late Dr. Otis Causey, at that time head of the lEC's arbovirus programme.
Causey was impressed by the similarity of the tail lesions caused by L. mexicana to similar lesions on the tails of rodents he had noted among animals captured in the Utinga forest in Belém, Brazil. He had, however, thought they were due to bacterial infections of damaged tails, and he promised to examine them more carefully in the future. A few weeks later, he presented the author with a Giemsa-stained smear from a lesion on the tail of a specimen of Oryzomys capito, a common forest rodent in his capture area, and it was rich in leishmanial amastigotes. This unexpectedly rapid discovery prompted a discussion with the Wellcome Trust in London, who agreed to the establishment of the Wellcome Parasitology Unit (WPU) in the Department of Parasitology of the IEC for a provisional period of three years, with the promise of further support should the results of the research warrant it. The IEC/WPU programme lasted until 1992.
1965-1967
Although the parasite of Oryzomys was at first assumed to be L. braziliensis by Nery-Guimaraes and Costa77 in 1964, this conclusion was not supported by the WPU's comparison of the rodent parasite with that from cases of human cutaneous and mucocutaneous leishmaniasis. First, the amastigotes of the rodent parasite were clearly much larger than those of L. braziliensis and, when inoculated into the skin of laboratory hamsters and mice, rapidly produced huge tumour-like lesions packed with amastigotes. In contrast, L. braziliensis produced a small nodule that often required several months to become visible and only contained a small number of relatively tiny amastigotes. In addition, the rodent parasite - by now found to infect a variety of forest rodents (Figure 4) and marsupials - grew luxuriantly in a very simple blood-agar culture medium (NNN), whereas L. braziliensis struggled to survive in the same medium, with successful isolates often dying out after several sub-cultures. In 1969 and 1970, Lainson and Shaw49,45 referred to these differences as the behaviour of "fast and slow strains" of Leishmania.
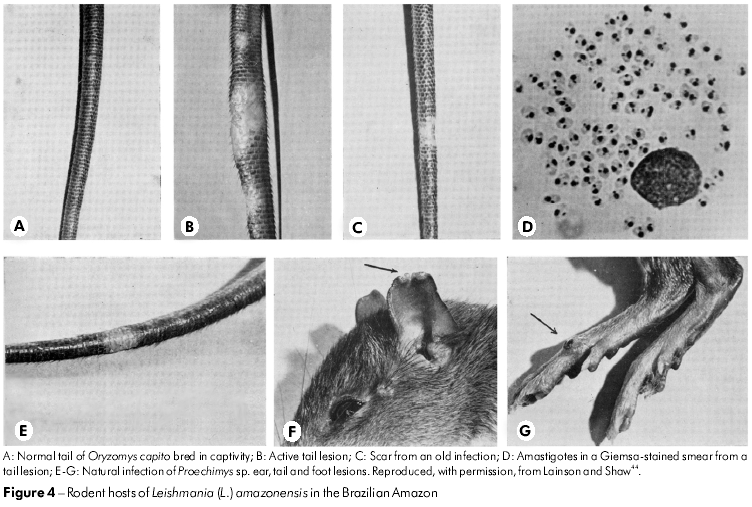
The continued examination of Leishmania isolates from patients coming to the IEC soon showed that a small number of the parasites were the same as those from Oryzomys and other rodents. Importantly, this identification indicated that the parasite was a causal agent of the condition known as DCL or, more correctly, anergic diffuse cutaneous leishmaniasis (ADCL), a very disfiguring infection produced in immunologically incompetent individuals that resists the usual treatment by anti-leishmanial drugs. Because the parasite's biological features closely resembled those of L. mexicana of Central America, it was given the name L. mexicana amazonensis Lainson and Shaw, 1972.
1968
This year saw the first incrimination of the fox Cerdocyon thous as an important reservoir host of the parasite responsible for Amazonian canine and human visceral leishmaniasis, variously referred to as L. chagasi or L. donovani. Three infected foxes52,54 were found near Utinga, on the outskirts of Belém, and ten infected animals, a surprisingly high number, were later found among 25 examined (40%) on the island of Marajó, Pará54. None of these infected animals showed outward signs of disease.
1977
Smears of liver and spleen tissue from a porcupine Coendou prehensilis, captured in a forested area of Pará, revealed the presence of amastigotes measuring up to 6.8 x 4.5 µm43, an unusually large size even when compared with L. m. amazonensis amastigotes. In 1971, Herrer27 had given the name Leishmania hertigi to a parasite of Panamanian porcupines; therefore, the Brazilian Coendou parasite was given the subspecific name Leishmania hertigi deanei in honour of Leonidas Deane. Deane had encountered what was probably the same parasite in porcupines from the State of Piauí, Brazil, but, unsure of its nature, had refrained from naming it. The leishmanial nature of this strange parasite was indicated by its production (albeit only transitory) of amastigotes in the skin of experimentally inoculated hamsters and its formation of typical promastigote stages in blood-agar culture medium. In 1980, Miles et al76 differentiated the parasite from L. (L.) hertigi hertigi and L. mexicana amazonensis by comparative isoenzyme profiles.
1979
A species of Leishmania was isolated from the liver and spleen of a nine-banded armadillo, Dasypus novemcinctus, from the Monte Dourado (Jari) area of northern Pará, Brazil51.
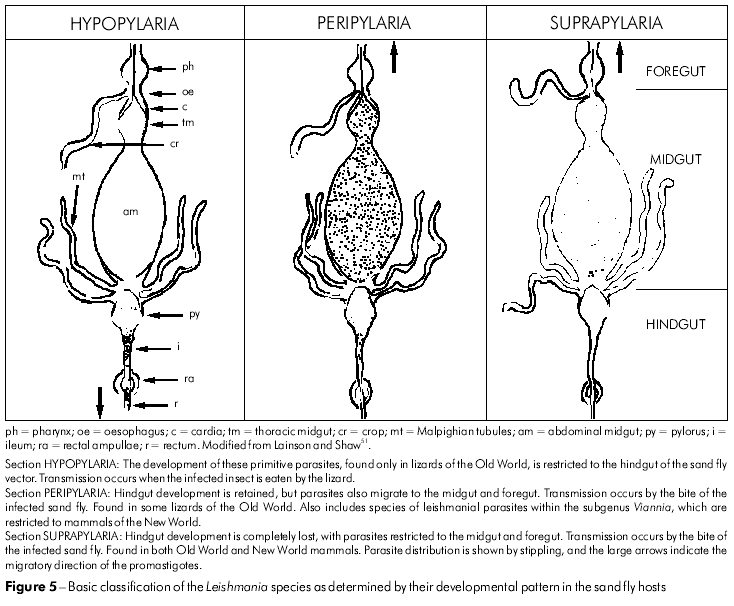
Biological features and the development of biochemical and immunological techniques gradually laid the foundation for preliminary attempts to classify the increasing number of accepted species of the genus Leishmania46,50,42,117,51. Particularly important were observations on the mode of development of these species in their phlebotomine vectors, which enabled the division of the parasites into three distinct groups referred to as Sections (Figure 5).
AN EARLY CLASSIFICATION OF THE LEISHMANIA SPECIES51
SECTION HYPOPYLARIA (from hypo = under, and pyl = gate)
The parasites included in this group were considered the most primitive species, and their development is limited to a posterior position in the pylorus, ileum and rectum of the sand fly gut. The reservoir hosts are apparently restricted to certain lizards of the Old World, in which the parasite may be in the promastigote and/or amastigote form in the viscera or blood. Listed species included Leishmania agamae David, 1929, and L. ceramodactyli Adler & Theodor, 1928. Transmission presumably follows the ingestion of an infected sand fly by the lizard. Some35, including the present author, consider that these parasites are better placed in the genus Sauroleishmania Ranque, 1973.
SECTION PERIPYLARIA (from peri = on all sides, and pyl = gate)
These Leishmania species have maintained an obligate hindgut development in the sand fly, but, in addition, have now developed a migration to the foregut. Included here are the following parasites of Old World lizards: Leishmania adleri Heisch, 1958, and Leishmania tarentolae Wenyon, 1921. The parasite can now be transmitted by the bite of an infected sand fly or when the fly is eaten. This Section, however, was dominated by what Lainson and Shaw referred to as the L. braziliensis complex51 of the New World. At that time, this complex included the following parasites, all of which infect humans: L. braziliensis Vianna, 1911; L. peruviana Velez, 1913; L. guyanensis Floch, 1954; and L. panamensis Lainson & Shaw, 1972.
SECTION SUPRAPYLARIA (from supra = above, and pyl = gate)
These Leishmania species were considered to have lost the primitive hindgut development in the sand fly, with the parasites now restricted to the midgut and foregut. They are found in the skin, viscera or blood of both Old World and Neotropical mammals, and transmission is by the bite of the infected sand fly vector. The Section was divided into four complexes, as follows:
The L. donovani complex
Leishmania donovani (Laveran & Mesnil, 1902) Ross, 1903 (Old World); Leishmania infantum Nicolle, 1908 (Old World); Leishmania chagasi Cunha & Chagas, 1937 (New World).
The L. mexicana complex
L. mexicana mexicana (Biagi, 1953) Lainson & Shaw, 1979; L. mexicana amazonensis Lainson & Shaw, 1972; L. mexicana pifanoi (Medina & Romero, 1959) Medina & Romero, 1962; L. mexicana aristidesi Lainson & Shaw, 1979; L. mexicana enriettii Muniz & Medina, 1948 (All in the New World).
The L. hertigi complex
L. hertigi hertigi Herrer, 1971; L. hertigi deanei Lainson & Shaw, 1977 (New World).
The L. tropica complex
Leishmania tropica (Wright, 1903) Luhe,1906; Leishmania major Yakimov & Schockov, 1914; Leishmania aethiopica Bray, Ashford & Bray, 1973 (All in the Old World).
In 1982, the Russian researcher Saf'janova91 separated the leishmanias of lizards from the true Leishmania species of mammals by the subgeneric use of the names Sauroleishmania Ranque, 1973, and Leishmania Ross, 1903, respectively. Within the subgenus Leishmania, she considered the L. donovani complex to consist of Leishmania (L.) donovani, L. (L.) infantum (Old World) and L. (L.) chagasi (New World). She did not consider the development of L. braziliensis and related Neotropical leishmanias in the sand fly's hindgut (members of the Peripylaria51) to be of taxonomic importance, however, and grouped all the Neotropical parasites together as L. (L.) amazonensis; L. (L.) mexicana; L. (L.) braziliensis and L. (L.) panamensis (dermal leishmaniases); and L. (L.) chagasi (visceral leishmaniasis). Furthermore, Saf'janova was of the opinion that there were insufficient taxonomic criteria to include L. braziliensis peruviana and L. braziliensis guyanensis in her classification. The exclusion of the latter two parasites was most likely due to the unavailability of recent literature that had clearly indicated specific characterisation on biological, biochemical and serological evidence40,70,75.
1987 A REVISED CLASSIFICATION OF THE NEOTROPICAL LEISHMANIA SPECIES
Extensive studies of the ecology and epidemiology of cutaneous leishmaniasis in the Brazilian Amazon Region revealed a steadily increasing number of Leishmania species that were now more adequately characterised by their isoenzyme profiles76, and this prompted a taxonomic revision41.
In 1977, Lainson et al58 had stressed the importance of using the presence or absence of hindgut development in the sand fly to distinguish parasites of the L. braziliensis complex (hindgut development present) from those of the L. mexicana complex (hindgut development absent)58. Accordingly, in the revised classification, all species with hindgut development were placed in the new subgenus Viannia, which was named in honour of Gaspar Vianna, who had described L. (V.) braziliensis, now the type species of the subgenus. It followed that all species lacking hindgut development were housed in the subgenus Leishmania Ross, 1903 used by Saf'janova91 in 1982. In addition, while commenting on "the cumbersome combination of geographic names of parasites, which at times entered into absurd conflict with each other" (e.g., L. braziliensis guyanensis and L. mexicana venezuelensis), it was also proposed to raise the subspecific names to specific level. With these modifications, the following classification was given for Leishmania species of the Neotropics.
SUBGENUS LEISHMANIA ROSS, 1903
Definition: With the characters of the genus Leishmania. Life cycle in the insect host limited to the midgut and foregut. Type species: Leishmania (Leishmania) donovani (Laveran & Mesnil, 1903) Ross, 1903. It contained the following Neotropical parasites: Leishmania (L.) chagasi Cunha & Chagas, 1937; L. (L.) enriettii Muniz & Medina, 1948; L. (L.) mexicana Biagi, 1953 emend. Garnham, 1962; L. (L.) amazonensis Lainson & Shaw, 1972; L. (L.) aristidesi Lainson & Shaw, 1979; L. (L.) venezuelensis Bonfante-Garrido, 1980; L. (L.) garnhami Scorza et al, 1979; L. (L.) pifanoi (Medina & Romero, 1959) Medina & Romero, 1962; L. (L.) hertigi Herrer, 1971; L. (L.) deanei Lainson & Shaw, 1977.
SUBGENUS VIANNIA LAINSON & SHAW, 1987
Definition: With the characters of the genus Leishmania. Life cycle in the insect host including a prolific phase of development as rounded or stumpy paramastigotes and promastigotes attached to the wall of the hindgut (pylorus and/or ileum) by flagellar hemidesmosomes, but with later migration of the parasites to the midgut and foregut. Type species: Leishmania (Viannia) braziliensis.
Species of this subgenus are known only in the New World and were listed as follows: L. (V.) braziliensis Vianna, 1911, emend Matta, 1916; L. (V.) peruviana Velez, 1913; L. (V.) guyanensis Floch, 1954; L. (V.) panamensis Lainson & Shaw, 1972.
CONTINUING RESEARCH ON NEOTROPICAL LEISHMANIA SPECIES
1987
The use of monoclonal antibodies became an established method for the identification of Leishmania (Viannia) braziliensis in infected sand flies97.
1988-1989
A new species of the subgenus Viannia was isolated from a sloth, a procyonid and two species of monkeys in lowland forest at the foot of the Carajás hills, Pará State, Brazil38. This parasite was named Leishmania (Viannia) shawi Lainson et al, 1989.
The parasite that had been isolated from the nine-banded armadillo (Dasypus novemcinctus) in 1979 was characterised and finally named as Leishmania (Viannia) naiffi Lainson & Shaw, 1989.
1991
Kreutzer et al36 described a new species of Leishmania infecting humans in Colombia and Panama and named it L. (Viannia) colombiensis.
1992
Grimaldi et al26 isolated another previously undescribed parasite from the sloth Choloepus hoffmanni and the squirrel Sciurus granatensis in Ecuador and gave it the name L. (V.) equatorensis.
2002
A parasite isolated from cases of ACL in soldiers engaged in manoeuvres in degraded forest in Belém, Pará State, Brazil, was found to differ from all previous Leishmania species in the Amazon region99 and was given the name Leishmania (Viannia) lindenbergi Silveira et al, 2002.
2003
In 1977, a Leishmania of the subgenus Viannia was isolated from a single specimen of the sand fly Lutzomyia tuberculata taken from the trunk of a large tree in the Utinga forest. It remained for a long period in the IEC cryobank until it was finally characterised and named4 Leishmania (Viannia) utingensis Braga et al, 20034.
1998/2005
The establishment of the subgenus Viannia and characterisation of additional leishmanial parasites isolated from sand flies, wild mammals and patients with ACL necessitated two further publications that updated and modified the classification48,47.
A major change to the previous listing of parasites in the subgenus Leishmania was the proposal of the authors Lainson and Shaw47, in 2005, to divide Leishmania (L.) infantum into two subspecies: L. (L.) infantum infantum (Old World) and L. (L.) infantum chagasi (New World). In addition, the new classification included Leishmania (L.) forattinii Yoshida et al, 1993, a parasite found in Brazil in an opossum, Didelphis marsupialis aurita, and a rodent, Proechimys iheringi denigratus.
All the presently recognised Neotropical species of Leishmania, their recorded geographical distribution, proven or suspected sand fly hosts, recorded mammalian reservoir hosts, and clinical data concerning those known to infect humans are given below.
PRESENT CLASSIFICATION OF THE NEOTROPICAL LEISHMANIA SPECIES
Adapted from Cox11 and Lainson and Shaw47.
Kingdom: Protozoa Goldfuss, 1818
Phylum: Euglenozoa Cavalier-Smith, 1998
Class: Kinetoplastea: Honigberg, 1963
Order: Trypanosomatida Kent, 1880
Family: Trypanosomatidae Doflein, 1901
Genus: Leishmania Ross, 1903
Subgenus: Leishmania Ross, 1903
Subgenus: Viannia Lainson & Shaw, 1987
SUBGENUS LEISHMANIA
LEISHMANIA (LEISHMANIA) INFANTUM CHAGASI (CUNHA & CHAGAS, 1937) SHAW, 2002
Known geographical distribution
Most of the Latin American continent, including Argentina, Bolivia, Brazil, Colombia, Ecuador, El Salvador, Guadeloupe, Guatemala, Honduras, Martinique, Mexico, Nicaragua, Paraguay, Surinam and Venezuela.
Known sand fly hosts
Lutzomyia (Lutzomyia) longipalpis is the principal vector throughout the range of AVL14,37, but Lu. evansi has also been incriminated in Colombia and Venezuela109,17. Lu. (Lu.) cruzi became highly suspected as an alternative vector in the State of Mato Grosso do Sul, Brazil, when L. (L.) infantum chagasi was isolated from 14 specimens93. The females of Lu. cruzi, however, are indistinguishable from those of Lu. longipalpis, and even the males of the two species can only be separated based on small differences. The authors concluded that because Lu. longipalpis males were apparently absent at the time of their study, the infected females were Lu. cruzi. Although the presence of Lu. longipalpis in the same area has since been established94, there now seems to be little doubt that Lu. (Lu.) cruzi may be an alternative vector of Leishmania (L.) infantum chagasi in the State of Mato Gosso do Sul.
Recorded mammalian hosts
The sylvatic canids Cerdocyon thous ("crab-eating fox")52,54 and Speothos venaticus ("bush-dog")18; the felids Panthera onca (jaguar) and Felis concolor (puma)13; the opossums Didelphis marsupialis10,108 and D. albiventris98; the domestic dog; and humans.
Human infection
L. (L.) chagasi predominantly produces visceral leishmaniasis, which is often fatal unless adequately treated, but infection can be asymptomatic in some individuals. In Costa Rica, infection is largely in the form of non-ulcerative cutaneous lesions116, and in Honduras and Nicaragua, infection is both visceral and cutaneous84,2.
Opinions have been divided as to whether the parasite named L. (L.) chagasi is indigenous to the American tropics or if the disease in the New World is due to L. (L.) infantum, which was introduced by Iberian immigrants, or their dogs, as recently as about 500 years ago. Arguments favouring the indigenous hypothesis have been given as follows37,54,48,47:
1. In terms of geological time, 500 years is a very short period for the parasite to have achieved such a wide distribution throughout the Latin American continent, from Mexico to Argentina.
2. The host-specificity of the Leishmania species in nature is most pronounced in the sand fly, which is generally regarded as the primary host of Leishmania species. Therefore, it seems unlikely that introduced L. (L.) infantum could have made a sudden jump from the genus Phlebotomus in the Old World to the genus Lutzomyia in the Americas. Lutzomyia (Lutzomyia) longipalpis is the principal vector of L. (L.) infantum chagasi throughout the geographical range14,37, and it is not known to naturally transmit any other species of Leishmania.
3. Based on molecular data, it is frequently stated that the parasites referred to as L. (L.) infantum and L. (L.) chagasi are identical. There have been, however, a few publications (conveniently disregarded) claiming the demonstration of some differences between the two organisms. These, it has been claimed, have been demonstrated by restriction endonuclease digestion and hybridisation of kinetoplast DNA33,32 and in radiorespirometry profiles of the two parasites16,15; finally, antigenic differences have been claimed for their respective promastigotes92. Unless these findings are disproved, it would therefore seem necessary to consider them in any discussion on the taxonomy of the two organisms.
4. In the transmission cycle of L. (L.) infantum chagasi among wild animals by the sylvatic population of Lutzomyia longipalpis, there is a high prevalence of infection in the native fox Cerdocyon thous in Brazil54 and in the opossum Didelphis marsupialis in Colombia10,108. Infections have also been sporadically reported in other wild animals, including the opossum Didelphis albiventris98 the "bush dog" Speothos venaticus18,the jaguar Panthera onca and the puma Felis concolor13. All of the infections recorded in these wild animals have consistently been of a benign inapparent nature, which is more suggestive of a very ancient host-parasite relationship rather than infection with a strange and recently introduced parasite.
The great diversity of Leishmania species in the New World has prompted the suggestion that leishmanial parasites originated in the American tropics78,79, and that the genus Leishmania gained entrance to the Old World via the Bering land bridge. Other authors63, while agreeing with this hypothesis, have postulated that, following the introduction of the ancestral leishmanial parasite into the Old World and the evolution of Leishmania donovani and Leishmania infantum (an estimated 14-24 million years ago), the latter parasite gained entrance to the New World by way of the Iberian colonists.
To the present author, it seems equally reasonable to suggest that while this evolution of the ancestral parasite was taking place in the Old World, giving rise not only to the viscerotropic parasites L. (L.) donovani and L. (L.) infantum, but also to the dermotropic members of the L. (L.) tropica complex, another such evolutionary process of the ancestral parasite continued in the New World, producing the viscerotropic parasite named as L. chagasi and dermotropic parasites of the subgenus Leishmania (e.g., those of the L. mexicana and L. hertigi complexes). At the same time, another ancient line diverged to form the subgenus Viannia, the members of which retained the primitive characteristic of hindgut development in the sand fly gut. This group of leishmanial parasites is unknown in the Old World, possibly because their ancestral form never gained entrance via the Bering land bridge due to a restricted locomotor capacity of the mammalian reservoir hosts.
The name L. (L.) infantum Nicolle, 1908 clearly has chronological priority over the name L. (L.) chagasi Cunha & Chagas, 1937, and we are obliged to accept the specific name of infantum for the parasite in both hemispheres. The prolonged geographical separation might explain the above-mentioned recorded differences between the two populations, leading to the view that it is best to now regard them as the subspecies L. (L.) infantum chagasi and L. (L.) infantum infantum95,39,47,11.
Some confusion has occurred regarding the authorship of this proposal. It was first made by Lainson and Shaw47 when their chapter "New World Leishmaniasis" was submitted for publication in the 10th edition of "Topley & Wilson's Microbiology and Microbial Infections". There was an unusually long delay, however, before this edition finally appeared in print in 2005, and during this time, both Shaw95, in 2002, and Lainson and Rangel39, in 2003 used the new subspecific names in other publications. Chronologically, therefore, the correct subspecific names of L. infantum should be written as L. (L.) infantum infantum (Nicolle, 1908) Shaw, 2002 and L. (L.) infantum chagasi (Cunha & Chagas, 1937) Shaw, 2002.
LEISHMANIA (L.) ENRIETTII MUNIZ & MEDINA, 1948
Known geographical distribution
Known only in the States of Paraná71 and São Paulo65, Brazil.
Known sand fly hosts
Lu. monticola and Lu. correalimai are suspected, the former having been experimentally infected when fed on the lesions of guinea pigs64.
Recorded mammalian hosts
Natural infections only recorded in the domestic guinea pig (Cavia porcellus).
Human infection
Not yet reported, and attempts to infect volunteers failed.
LEISHMANIA (L.) MEXICANA (BIAGI, 1953) GARNHAM, 1962
Known geographical distribution
Belize, Guatemala, Honduras, Costa Rica, southern USA. Reports in geographically widely separated South American countries must be viewed with caution.
Known sand fly hosts
Lutzomyia olmeca olmeca is a proven vector. Lu. diabolica is suspected in northern Mexico and southern Texas, and Lu. anthophora is suspected in Arizona.
Recorded mammalian hosts
The forest rodents Ototylomys phyllotis, Nyctomys sumichrasti, Heteromys desmarestianus and Sigmodon hispidus, and Neotoma albigula in the southern USA (Arizona); humans.
Human infection
Cutaneous leishmaniasis, with a pronounced tendency to cause lesions of the external ear ("chiclero's ulcer" or "chiclero's ear"). Occasional cases of ADCL.
LEISHMANIA (L.) PIFANOI (MEDINA & ROMERO, 1959) MEDINA & ROMERO, 1962
Known geographic distribution
Apparently limited to Venezuela, particularly in the States of Yaracuy, Lara and Miranda.
Known sand fly hosts
Uncertain, but possibly Lutzomyia flaviscutellata.
Recorded mammalian hosts
Humans. Although the wild animal reservoir hosts of L. (L.) pifanoi remain unknown, Lima et al59 suggested that the rodents Sigmodon hispidus and Rattus rattus could be reservoirs of various Leishmania spp., including, presumably, L. (L.) pifanoi.
Human infection
So far, all cases recorded have been ADCL.
LEISHMANIA (LEISHMANIA) AMAZONENSIS LAINSON & SHAW, 1972
Known geographical distribution
Bolivia, Brazil, Colombia, French Guyana and Paraguay. Probably occurs in other Neotropical countries where the sand fly vector exists.
Known sand fly hosts
Lutzomyia (Nyssomyia) flaviscutellata is the major vector44,96,113,112, with occasional infections reported in the closely related Lu. (N.) olmeca olmeca and Lu. (N.) reducta. A parasite identified as L. (L.) amazonensis was isolated from 16 of 1,715 specimens of Lu. nuneztovari68 in Bolivia.
Recorded mammalian hosts
The terrestrial forest rodents Proechimys spp., Oryzomys spp., Nectomys, Neacomys, and Dasyprocta; the marsupials Marmosa, Metachirus, Didelphis and Philander; the fox Cerdocyon thous; humans.
Human infection
Localised single-sore cutaneous leishmaniasis and, in patients with a defective cell-mediated immune system, ADCL. Rare cases of visceral leishmaniasis have been attributed to this parasite in the State of Bahia, Brazil1, but not elsewhere. The clinical and immunopathological spectrum of American cutaneous leishmaniasis, with particular reference to the disseminated form of the disease due to L. (L.) amazonensis and L. (V.) braziliensis and illustrating the extreme pathogenicity at the poles of ADCL and mucocutaneous leishmaniasis, has been described elsewhere100.
LEISHMANIA (LEISHMANIA) ARISTIDESI (LAINSON & SHAW, 1979) EMEND LAINSON & SHAW, 1987
Known geographical distribution
Sasardi forest in the San Blas Territory of Eastern Panama.
Known sand fly hosts
A putative vector is Lutzomyia (Nyssomyia) olmeca bicolor based on its predominance in rodent and marsupial-baited Disney traps in areas where infected animals were also obtained7.
Recorded mammalian hosts
The opossum Marmosa robinsoni and the rodents Proechimys semispinosus and Dasyprocta punctata29,30.
Human infection
Not known, although Lu. olmeca bicolor has occasionally been found to feed on humans.
LEISHMANIA (LEISHMANIA) GARNHAMI SCORZA ET AL, 1979
Known geographical distribution The Venezuelan Andes.
Known sand fly hosts
The most suspected vector is Lu. youngi. A parasite found in an infected specimen produced amastigotes in the skin of an inoculated hamster that were thought to be L.(L.) garnhami, but the parasite was not definitively identified66.
Recorded mammalian hosts
The opossum Didelphis marsupialis and humans.
Human infection
ACL, of the simple localised lesion type.
LEISHMANIA (LEISHMANIA) VENEZUELENSIS BONFANTE-GARRIDO, 1980
Known geographical distribution
Venezuela, in the States of Lara and Yaracuy.
Known sand fly hosts
A definite vector has not been identified, but Lu. olmeca bicolor and Lu. rangeliana are suspected to be involved.
Recorded mammalian hosts
The wild mammalian hosts remain uncertain, but the parasite has been recorded in the domestic cat and humans. It has been suggested that the rodents Sigmodon hispidus and Rattus rattus are potential reservoir hosts of various Leishmania spp.59, including, presumably, L. (L.) venezuelensis.
Human infection
Single or multiple skin lesions. Sometimes disseminated nodules that can be confused with ADCL, but the infection responds well to antimonial treatment.
LEISHMANIA (LEISHMANIA) FORATTINII YOSHIDA ET AL, 1993
Known geographical distribution
Brazil, in the States of São Paulo, Bahia and Espírito Santo.
Known sand fly hosts
Not yet identified, but Lu. (Psychodopygus) ayrozai and Lu. yuilli have been experimentally infected.
Recorded mammalian hosts
The rodent Proechimys iheringi and the marsupial Didelphis marsupialis in the State of São Paulo.
Human infection
Not yet recorded, but as the suspected vectors are known to feed on humans, infections might be found in the future.
LEISHMANIA (LEISHMANIA) HERTIGI HERRER, 1971
Known geographical distribution
Panama and Costa Rica.
Known sand fly hosts
The sand fly vector has yet to be discovered. The high rate of infection in the mammalian host suggests that it lives in close proximity to the vector(s), possibly in hollow trees.
Recorded mammalian hosts
The tropical porcupine Coendou rothschildi. Extensive examinations of other wild animals have revealed no other mammalian reservoir host.
Human infection
Unrecorded, possibly due to the inability of the parasite to survive in human tissues or because the vector never bites humans.
LEISHMANIA (LEISHMANIA) DEANEI LAINSON & SHAW, 1977
Known geographical distribution
To date, only recorded in the Brazilian Amazon Region.
Known sand fly hosts
The invertebrate host remains unknown. Tree-inhabiting sand flies of the species Lutzomyia (Viannamyia) furcata were taken from a hollow tree inhabited by an infected porcupine in Utinga forest, Belém, Pará, Brazil, and were shown to contain promastigotes in their undigested bloodmeals. However, there was no evidence that the parasites had migrated to the foregut, and they disappeared with the complete digestion of the blood41.
Recorded mammalian hosts
The tree porcupine Coendou p. prehensilis. Like L. (L.) hertigi, L. (L.) deanei has a very high infection rate in this porcupine, and an exhaustive examination of other wild animals suggests that it is the sole mammalian reservoir host of the parasite.
Human infection
Unrecorded. Again, as is the case with L. (L.) hertigi, this may be because the organism cannot survive in human tissues or simply because the vector never bites humans.
In the 2005 classification47 these two enigmatic parasites were placed under the heading of "Leishmania-like parasites of uncertain taxonomic position", largely because molecular studies79 had suggested them to be more closely related to Endotrypanum (an endoerythrocytic flagellate of sloths) than to Leishmania. However, their present names were retained47 until further information could be obtained, and, for this reason, I am grouping them here with members of the subgenus Leishmania. Based on the absence of attached hindgut forms of L. (L.) deanei in Lutzomyia furcata, albeit in transitory infections, this parasite certainly does not appear to be a member of the subgenus Viannia. Knowledge of the complete life cycles of these two organisms in their natural invertebrate hosts will hopefully indicate their exact taxonomic status.
Although both members of the L. hertigi complex appear to be peculiar to porcupines, they are easily distinguished by isoenzyme profiles and a marked difference in the morphology of their amastigotes: those of L. (L.) hertigi are strangely elongated and measure only from 3.5 x 1.2 to 4.8 x 2.5 μm, while those of L. (L.) deanei are rounded in form and, at 5.1 x 3.1 to 6.8 x 3.7 μm, are the largest of all recorded species of Leishmania.
THE SUBGENUS VIANNIA
LEISHMANIA (VIANNIA) BRAZILIENSIS (VIANNA, 1911) EMEND MATTA, 1916
Known geographical distribution
Although parasites variously referred to as L. braziliensis or L. braziliensis sensu lato have been reported in almost all Latin American countries from Argentina to Mexico, doubt remains as to the true nature of many records due to inadequate methods of identification in the past. Some may be simple zymodemes of L. (V.) braziliensis, but others may prove to be different, unidentified species of the subgenus Viannia.
Known sand fly hosts
The existing uncertainties regarding the exact geographical distribution of L. (V.) braziliensis make it difficult to identify all of its vectors. However, at least in Brazil, where the parasite has been recorded in every State, it is clear that there are numerous sand fly species involved in its transmission. These include Lutzomyia (Nyssomyia) intermedia, Lu. (N.) whitmani sensu stricto, Lu. (Psychodopygus) wellcomei, Lu. migonei85 and Lu. (N.) neivae (Pinto, 1926) (for reference, see "Concluding Remarks").
In a recent study in the Salobo area of the Serra dos Carajás, Pará, Brazil, promastigotes from four specimens of Lu. (Psychodopygus) davisi were identified as L. (V.) braziliensis, while others from specimens of Lu. (Psychodopygus) hirsuta (3 infected), Lu. (Nyssomyia) umbratilis (3), Lu. (N.) richardwardi (2), Lu. (Trichophoromyia) brachipyga (2), Lu. (T.) ubiquitalis (2), Lu. trinidadensis (1) and Lu. migonei (1) remain to be identified104. Lu. (P.) davisi has previously been indicated as an important vector of zoonotic cutaneous leishmaniasis in the State of Rondônia25.
In Pará State (near Paragominas), a parasite identified as L. (V.) braziliensis was isolated from a sand fly with the dual female morphology of Lu. (P.) complexa and Lu. (P.) wellcomei103. The females of these two species are morphologically indistinguishable, but it was concluded that the infected specimen was Lu. (P.) complexa due to the apparent absence of any males of Lu. (P.) wellcomei.
Recorded mammalian hosts
The low-level flight and high attraction to rodent-baited traps of the sand fly Lu. (Psychodopygus) wellcomei, an important vector of L. (V.) braziliensis in the Serra dos Carajás, Pará55, led to early suspicions that terrestrial rodents and some marsupials might be the reservoir hosts of this parasite114,55.
Before the development of biochemical, serological and molecular techniques for the characterisation and identification of Leishmania isolates, it was only possible to use biological ("extrinsic") characters of the parasites, such as the size of amastigotes and their behaviour in a standardised culture medium and in inoculated laboratory animals. Using such characters together with the pattern of development of parasites in experimentally infected sand flies (to indicate members of the subgenus Viannia), early researchers could at least say that isolates made from wild animals in an area highly endemic for human ACL due to L. (V) braziliensis were probably this parasite. These records include the following wild animals.
Oryzomys concolor, O. capito, O. nigripes, Akodon arviculoides, Proechimys spp., Rattus rattus, Rhipidomys leucodactylus (Rodentia) and Didelphis marsupialis (Marsupialia)21,22,45,51,53,88, all in Brazil; in Venezuela, Rattus rattus and Sigmodon hispidus (Rodentia)59. Finally, parasites from the Brazilian rodents Bolomys lasiurus and Rattus rattus were conclusively shown to be L. (V.) braziliensis by multilocus enzyme electrophoresis5.
Domestic animals, including equines, dogs and cats, have been found with skin lesions due to L. (V) braziliensis in areas that suggest a peridomestic transmission cycle. These reports have come primarily from Argentina, Bolivia, Brazil, Colombia and Venezuela. Humans are commonly infected.
Human infection
Cutaneous leishmaniasis, usually with one or a few lesions. Infection commonly leads to mucocutaneous disease. The clinical and immunopathological spectrum of American cutaneous leishmaniasis, with particular reference to the disseminated and mucocutaneous diseases, has been described elsewhere100.
LEISHMANIA (VIANNIA) PERUVIANA VELEZ, 1913
Known geographical distribution
Peru, on the western side of the Andes, in areas with scant vegetation and a restricted population of wild animals. Could extend into the Argentinean highlands and other Andean countries.
Known sand fly hosts
Lutzomyia (Helcocyrtomyia) peruensis and Lutzomyia verrucarum are highly suspected, and a parasite with biological features similar to those obtained from humans and dogs has been isolated from the former28.
Recorded mammalian hosts
Dogs and humans. A recent study reported the isolation of this parasite from the rodent Phyllotis andinum and the opossum Didelphis marsupialis61.
Human infection
Simple cutaneous leishmaniasis with one or few lesions. The parasite is not known to produce the mucocutaneous disease.
LEISHMANIA (VIANNIA) GUYANENSIS FLOCH, 1954
Known geographical distribution
This sylvatic species commonly infects humans in Brazil, particularly north of the Amazon River, and in the neighbouring countries of French Guyana and Surinam. Also reported in Colombia, Ecuador, Venezuela and the lowland forest of Peru.
Known sand fly hosts
The principal vector is Lutzomyia (Nyssomyia) umbratilis, with relatively infrequent infections recorded in Lu. (N.) anduzei (Reviews47,85). Some early reports of infection in Lu. (N.) whitmani s.l. may have actually been Leishmania (Viannia) shawi.
Recorded mammalian hosts
Major sylvatic hosts are the sloth Choloepus didactylus and the lesser anteater Tamandua tetradactyla53,24, with occasional infections in rodents and opossums. Infection in wild animals is benign and inapparent.
Human infection
Cutaneous leishmaniasis with one or multiple lesions. The latter may arise from multiple bites of infected sand flies or metastatic lymphatic spread. Rare cases of mucocutaneous involvement have been reported.
LEISHMANIA (VIANNIA) PANAMENSIS LAINSON & SHAW, 1972
Known geographical distribution
Canal Zone, Panama; also recorded in Colombia, Costa Rica, Ecuador, Honduras, Nicaragua and Venezuela.
Known sand fly hosts
The major vector is considered to be Lutzomyia (N.) trapidoi. A number of other species are thought to act as secondary vectors, including Lu. (N.) ylephiletor, Lu. (Lu.) gomez and Lu. (Psychodopygus) panamensis34,8.
Recorded mammalian hosts
The sloth Choloepus hoffmanni is the major host, with occasional infections reported in the sloths Bradypus infuscatus and B. griseus. This parasite has also been reported in a number of other arboreal animals, including Bassaricyon gabbi, Nasua nasua and Potos flavus (Procyonidae), the monkeys Aotus trivirgatus and Saguinus geoffroyi and the terrestrial rodent Heteromys sp.29,30. Hunting dogs, like humans, often become "victim hosts" with visible skin lesions.
Human infection
Cutaneous leishmaniasis, with one to several lesions; rare cases of the mucocutaneous disease have been reported.
LEISHMANIA (VIANNIA) LAINSONI SILVEIRA ET AL, 1987
Known geographical distribution
Forested areas of Brazil, Peru and Bolivia.
Known sand fly hosts
To date, the only known vector is Lutzomyia (Trichophoromyia) ubiquitalis102. This insect is the first representative of the subgenus Trichophoromyia to be incriminated as a vector of a Leishmania species. Lu. (T.) velascoi is highly suspected as a vector in Bolivia67.
Recorded mammalian hosts
So far, only the large rodent Agoutipaca101 and humans have been identified as hosts.
Human infection
Infection by this parasite usually presents as a single lesion, and no case of the mucocutaneous disease has yet been recorded.
LEISHMANIA (VIANNIA) NAIFFI LAINSON & SHAW, 1989
Known geographical distribution
This species has been isolated in the States of Pará and Amazonas, Brazil, and in French Guyana. However, it will almost certainly be reported in other parts of Latin America where the mammalian reservoir host and sand fly vectors coexist.
Known sand fly hosts
The principal vector of infection among the armadillo reservoir hosts appears to be Lutzomyia (Psychodopygus) ayrozai. This sand fly is not greatly anthropophilic, however, which probably accounts for the low rate of human infection. Rare infections have been recorded in Lu. (P.) paraensis and Lu. (P.) squamiventris, which are highly anthropophilic and are therefore likely vectors of the parasite to humans.
Recorded mammalian hosts
The only wild animal host known at present is the nine-banded armadillo Dasypus novemcinctus, in which infection is very common in apparently normal skin and viscera.
Human infection
Cutaneous leishmaniasis, usually in the form of a single lesion. Unlike most Neotropical Leishmania species, L. (V.) naiffi rarely produces a visible lesion in the skin of the laboratory hamster. If this parasite also produces occult infection in the skin of humans, it is possible that transmission to man is much more frequent than is generally thought.
LEISHMANIA (VIANNIA) SHAWI LAINSON ET AL, 1989
Known geographic distribution
Various areas of the Brazilian Amazon Region.
Known sand fly hosts
Lutzomyia (N.) whitmani sensu lato. Morphometric differences have been recorded between Lu. (N.) whitmani sensu stricto from the type locality in Bahia State, northeast Brazil, and the vector of L. (V.) shawi in the State of Pará, Brazilian Amazon86. These differences, together with separation of the two populations by DNA probes87, suggest that the vector of L. (V.) shawi might be a "cryptic species" of a Lu. (N.) whitmani complex. This suggestion has been disputed, however, following a phylogenetic analysis of the mitochondrial (cytochrome b) haplotypes of Lu. (N.) whitmani, which led to the conclusion that clades of haplotypes and a continuum of interbreeding populations of this sand fly exist in the forests of Brazil31. Nevertheless, the behaviours of the type species of the sand fly in Bahia and of Lu. (N.) whitmani s.l. in the State of Pará are very different. In the former locality, the insect is highly anthropophilic, is commonly found in houses and is a vector of L. (V.) braziliensis. In the primary forest of Pará, however, the fly very rarely bites humans, has not been found to enter houses even when they are close to the forest, and is a vector of L. (V.) shawi.
Recorded mammalian hosts
The monkeys Cebus apella and Chiropotes satanas (Cebidae), the sloths Choloepus didactylus and Bradypus tridactylus (Xenarthra), the coatimundi Nasua nasua (Procyonidae), and humans.
Human infection
The parasite is responsible for cutaneous leishmaniasis, usually of the single lesion type, but cases of multiple lesions, clearly due to metastases, are occasionally seen. Mucocutaneous disease due to L. (V.) shawi has not yet been reported.
LEISHMANIA (VIANNIA) COLOMBIENSIS KREUTZER ET AL, 1991
Known geographical distribution
First recorded in Colombia and Panama, this parasite was also subsequently found in Venezuela. Its distribution likely extends into the forests of Brazil and of the Peruvian lowlands, as well as into other Latin American countries where the sylvatic mammalian and sand fly hosts coexist.
Known sand fly hosts
Lutzomyia (Helcocyrtomyia) hartmanni in Colombia; Lu. (Lu.) gomezi and Lu. (Psychodopygus) panamensis in Panama.
Recorded mammalian hosts
The sloth Choloepus hoffmanni and humans (Panama).
Human infection
Single or multiple cutaneous lesions. No case of the mucocutaneous disease due to this parasite has been reported.
LEISHMANIA (VIANNIA) EQUATORENSIS GRIMALDI ET AL, 1992
Known geographical distribution
To date, this parasite appears to be limited to the Pacific coast of Ecuador.
Known sand fly hosts
Lutzomyia (N.) hartmanni.
Recorded mammalian hosts
The sloth Choloepus hoffmanni and the squirrel Sciurus granatensis.
Human infection
Not yet recorded.
LEISHMANIA (VIANNIA) LINDENBERGI SILVEIRA ET AL, 2002
Known geographic distribution
This parasite has only been found in degraded forest in Belém, Pará, Brazil.
Known sand fly hosts
The vector is currently unknown, but Lutzomyia (N.) antunesi is highly suspected. This insect was shown to be the predominant anthropophilic sand fly in an area where a number of soldiers acquired L. (V.) lindenbergi infections while carrying out manoeuvres in the forest. In addition, the low-level flight of Lu. (N.) antunesi would explain why the skin lesions of these men were mostly on their faces and arms. Because the men spent most of their time standing in trenches, these parts of the body would be the most exposed to the bites of a low-flying sand fly. An unidentified Leishmania species was found in specimens of Lu. (N.) antunesi on the island of Marajó, Pará90, but its development in the sand fly was suprapylarian. In contrast, in experimentally infected sand flies, the development of L. lindenbergi is peripylarian, which is typical of parasites in the subgenus Viannia.
Recorded mammalian hosts
To date, humans are the only known hosts. It is suspected that the wild animal reservoirs are probably terrestrial.
Human infection
Localised cutaneous lesions: to date, no case of the mucocutaneous disease has been reported.
LEISHMANIA (VIANNIA) UTINGENSIS BRAGA ET AL, 2003
Known geographic distribution
Belém, Pará, Brazil.
Known sand fly hosts
Only recorded from a single specimen of the sand fly Lutzomyia (Viannamyia) tuberculata that was taken from the trunk of a large tree in the Utinga forest, Belém, Pará, Brazil.
Recorded mammalian hosts
Currently unknown.
Human infection
The parasite has not been recorded in humans.
"HYBRIDS" OF LEISHMANIA SPECIES WITHIN THE SUBGENUS VIANNIA
These include L. (V.) braziliensis / L. (V.) panamensis; L. (V.) braziliensis / L. (V.) guyanensis; and L. (V.) braziliensis / L. (V.) peruviana, all of which have only been isolated from cases of human ACL. Only the latter "hybrid" has been associated with the mucocutaneous disease. It has been suggested that these "hybrids" are the result of genetic exchange. For more details and references, consult Lainson and Shaw47.
CONCLUDING REMARKS
Since preparing this paper, I have been informed that the sand fly Lutzomyia (Nyssomyia) neivae (Pinto, 1926) has now been found to be naturally infected by Leishmania (V.) braziliensis in southern Brazil82. I am indebted to the reviewer of my paper for this information.
The difficulties in obtaining irrefutable proof of the participation of Lutzomyia cruzi in the transmission of Leishmania (L.) infantum chagasi, due to the fact that the females of this sand fly cannot be morphologically distinguished from those of Lu. (Lu.) longipalpis, is paralleled by a similar problem that arose during the search for the vector(s) of Leishmania (V.) braziliensis in the Serra dos Carajás, Pará, Brazil. The two predominant anthropophilic sand fly species in the area were found to be Lutzomyia (Psychodopygus) wellcomei and Lu (P.) complexa, the females of which are also morphologically indistinguishable. Numerous infected females were found to be infected by L. (V.) braziliensis, and the problem was in deciding to which species they belonged. This dilemma was eventually solved by breeding out the adult flies from the eggs of infected females; this strategy provided the all-important males and conclusively showed the infected flies to be Lu. (P.) welcomei89. This method could perhaps also be used to identify infected Lu. cruzi / Lu. longipalpis in the State of Mato Grosso do Sul.
Considering the remarkable number of Leishmania species that have now been recorded in the Neotropics, and particularly in the Amazon region, this area might well be the birthplace of this genus. This hypothesis is supported by the observation that many of these parasites (species of the subgenus Viannia) have retained a hindgut development in the sand fly host, which is reminiscent of the life cycle of the monoxenous flagellates of insects from which Leishmania is thought to have evolved.
The existence of species of Leishmania that are known only in the sand fly host (e.g., L. (V.) utingensis in Lutzomyia tuberculata) suggests that others remain undetected among the numerous sand fly species that are non-anthropophilic. The continued search for these parasites and their wild mammalian reservoir hosts will be essential in generating an even more complete picture of the ecology of this fascinating group of parasites.
ACKNOWLEDGEMENTS
The author is indebted to the Wellcome Trust, London, for their continued support of the Wellcome Parasitology Unit over nearly 30 years, and to the succession of Directors of the Instituto Evandro Chagas, Secretaria de Vigilância em Saúde, Ministério da Saúde, Brazil, where this work was carried out. There is not enough space to list all the participants during this long period, but thanks must be given to the following: to Prof. Jeffrey J. Shaw, old friend and colleague who worked with the author from the date of the establishment of the WPU in 1965 until it was disbanded in 1992. To those who pioneered the biochemical, serological and molecular techniques for the identification of Leishmania species and enabled their installation in the IEC/WPU Leishmaniasis Programme, including Drs. M. L. Chance, P J. Gardener, Prof. M. A. Miles, Prof. D. C. Barker, Dr. Diane McMahon-Pratt and Dr. J. David; and to Drs. Marinete M. Póvoa, Roseli R. Braga and Edna A. Y. Ishikawa, who so effectively used them. To our highly productive line of entomologists, Dr. Habib Fraiha, Prof. Richard Ward, Drs. Paul Ready, Adelson A. A. Souza, and Lee Ryan, and to clinician and parasitologist Fernando T. Silveira who, as the present head of the lEC's Leishmaniasis Programme, is using his long experience in the field and laboratory, with past members of the WPU, to ensure that it continues to flourish. Finally, to all past and present technical staff, in particular Maria das Graças S. da Silva, Sued Freitas Silva, Manoel C. de Souza, Roberto D. Naiff and Maricleide Naiff, Antonio Julio Monteiro, Deocleciano G. Primo, José Aprígio N. de Lima, João B. P. da Luz, António F. P. Martins, Nonato B. Pires, Iorlando da Rocha Barata and João A. Brandão.
REFERENCES
1 Barrai A, Badaró R, Barral-Netto M, Grimaldi Jr G, Momen H, Carvalho EM. Isolation of Leishmania mexicana amazonensis from the bone marrow in a case of American visceral leishmaniasis. Am J Trop Med Hyg. 1986 Jul;35(4):732-4. [ Links ]
2 Belli A, Garcia D, Palacios X, Rodriguez B, Valle S, Videa E, et al. Widespread atypical cutaneous leishmaniasis caused by Leishmania (L.) chagasi in Nicaragua. Am J Trop Med Hyg. 1999 Sep;61(3): 380-5. [ Links ]
3 Biagi FF. Algunos comentarios sobra las leishmaniasis y sus agentes etiológicos, Leishmania tropica mexicana, nueva subspecie. Medicina, Mexico. 1953;33:401-6.
4 Braga RR, Lainson R, Ishikawa EAY, Shaw JJ. Leishmania utingensis n.sp., a parasite from the sand fly Lutzomyia (Viannamyia) tuberculata in Amazonian Brazil. Parasite. 2003;10:111-8.
5 Brandão Filho SP, Brito ME, Carvalho FG, Ishikawa EA, Cupolillo E, Floeter-Winter L, et al. Wild and synanthropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco State, Brazil. Trans R Soc Trop Med Hyg. 2003 May-Jun;97(3):291-6.
6 Carini A, Paranhos U. Identification de l'ulcera de Baurú avec le bouton d'Orient. Bull Soc Path Exot. 1909;2:225-6.
7 Christensen HA, Herrer A, Telford SR. Enzootic cutaneous leishmaniasis in eastern Panama. II. Entomological investigations. Ann Trop Med Parasitol. 1972 Mar;66(1):55-66.
8 Christensen HA, Herrer A, Telford SR. Leishmania braziliensis from Lutzomyia panamensis in Panama. J Parasitol. 1969;55:1090-1.
9 Convit J, Lapenta P. Sobre un caso de leishmaniose tegumentaria de forma diseminada. Rev Policlin Caracas. 1946;18:153-8.
10 Corredor A, Gallego JF, Tesh RB, Morales A, Carrasquilla CF, Young DG, et al. Didelphis marsupialis, an apparent wild reservoir of Leishmania donovani chagasi in Colombia, South America. Am J Trop Med Hyg. 1989;40(5):480-6.
11 Cox FE. Classification and introduction to the parasitic protozoa. In: Cox FE, Wakelin D, Gillespie SH, Despommier DD, editors. Topley & Wilson's Microbiology and Microbial Infections, Parasitology. 10th ed. London: Hodder Arnold Press; 2005. p.186-99.
12 Cunha AM, Chagas E. Nova espécie de protozoário do género Leishmania pathogenico para o homem. Leishmania chagasi n.sp. Nota Prévia. O Hospital, Rio de Janeiro. 1937;11:3-9.
13 Dahroug MA, Almeida AB, Sousa VR, Dutra V, Turbino NC, Nakazato L, et al. Leishmania (Leishmania) chagasi in captive wild felids in Brazil. Trans R Soc Trop Med Hyg. 2010 Jan;104(1):73-4. [ Links ]
14 Deane LM, Grimaldi G. Leishmaniasis in Brazil. In: Chang KP, Bray RS, editors. Leishmaniasis. New York: Elsevier; 1985. p. 247-75.
15 Decker-Jackson JE, Schrot JR, Levin GV. Identification of Leishmania spp by radiorespirometry. J Protozool.1977;24(3):463-70. [ Links ]
16 Decker-Jackson JE, Tang DB. Identification of Leishmania spp by radiorespirometry. II. A statistical method of data analysis to evaluate the reproductibility and sensitivity of the technique. In: Chance ML, Walton BC, editors. Biochemical Characterization of Leishmania. Proccedings of a Workshop Pan Am Health Organization; 1980 Dec 19; Washington (DC): Switzerland.UNDP/World Bank/WHO Special Programme for Research. Training in Tropical Diseases; 1980. p. 205-45.
17 Feliciangeli MD, Rodriguez N, De Guglielmo Z, Rodriguez A. The re-emergence of American visceral leishmaniasis in an old focus in Venezuela. II. Vectors and parasites. Parasite. 1999 Jun;6(2):113-20.
18 Figueiredo FB, Gremião ID, Pereira SA, Fedulo LP, Menezes RC, Balthazar DA, et al. First report of natural infection of a bush dog (Speothos venaticus) with Leishmania (Leishmania) chagasi in Brazil. Trans R Soc Trop Med Hyg. 2008 Feb; 102(2):200-1. [ Links ]
19 Floch H. Leishmania tropica guyanensis n.sp. agent de la leishmaniose tégumentaire des Guyanes et l'Amérique Centrale. Arch Inst Pasteur Guyane Franc Terit Inini. 1954;328:1-4.
20 Forattini OP. Sobre os reservatórios naturais da leishmaniose tegumentar americana. Rev Inst Med Trop Sao Paulo. 1960;2:195-203. [ Links ]
21 Forattini OP, Pattoli DB, Rabello EX, Ferreira OA. Infecções naturais de mamíferos silvestres em área endémica de leishmaniose tegumentar do Estado de São Paulo, Brasil. Rev Saude Publica. 1972;6(3):255-61. [ Links ]
22 Forattini OP, Pattoli DB, Rabello EX, Ferreira OA. Nota sobre infecção natural de Oryzomys capito laticeps em foco enzoótico de leishmaniose tegumentar no Estado de São Paulo, Brasil. Rev Saude Publica.1973;7:181-4.
23 Garnham PCC. Cutaneous leishmaniasis in the New World, with special reference to Leishmania mexicana. Sci Rep Inst Sup Sanit. 1962;2:76-82.
24 Gentile BF, Le Pont F, Pajot FX, Besnard R. Dermal leishmaniasis in French Guiana: the sloth (Choloepus didactylus) as a reservoir host. Trans R Soc Trop Med Hyg. 1981;75(4):612-3. [ Links ]
25 Gil LH, Basano SA, Souza AA, Silva MG, Barata I, Ishikawa EA, et al. Recent observations on the sand fly (Diptera: Psychodidae) fauna of the State of Rondônia, western Amazónia, Brazil: the importance of Psychodopygus davisi as a vector of zoonotic cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2003 Sep;98(6):751-5. [ Links ]
26 Grimaldi G, Kreutzer RD, Hashiguchi Y, Gomez EA, Mimory T, Tesh RB. Description of Leishmania equatorensis sp.n (Kinetoplastida: Trypanosomatidae), a new parasite infecting arboreal mammals in Ecuador. Mem Inst Oswaldo Cruz. 1992;87(2):221-8. [ Links ]
27 Herrer A. Leishmania hertigi sp.n., from the tropical porcupine, Coendou rothschildi Thomas. J Parasitol. 1971 Jun;57(3):626-9. [ Links ]
28 Herrer A. Lutzomyia peruensis (Shannon, 1929), posible vector natural de la uta (leishmaniose tegumentaria). Rev Inst Trop Sao Paulo. 1982;24:168-72.
29 Herrer A, Christensen HA, Beumer RJ. Reservoir hosts of cutaneous leishmaniasis among Panamanian forest mammals. Am J Trop Med Hyg. 1973 Sep;22(5):585-91. [ Links ]
30 Herrer A, Telford SR, Christensen HA. Enzootic cutaneous leishmaniasis in eastern Panama. I. Investigation of the infection among forest mammals. Ann Trop Med Parasitol. 1971 Sep;65(3):349-58. [ Links ]
31 Ishikawa EA, Ready PD, Souza AA, Day JC, Rangel EF, Davies CR, et al. A mitochondrial DNA phylogeny indicates close relationships between populations of Lutzomyia whitmani (Diptera: Psychodidae: Phlebotominae) from the rain-forest regions of Amazonia and northeast Brazil. Mem Inst Oswaldo Cruz. 1999 May-Jun;94(3):339-45. [ Links ]
32 Jackson PD, Stiteler JM, Wohlhieter JA, Reed SG, Badaro R, Inverso J, et al. Characterization of Leishmania responsible for visceral disease in Brazil by restriction endonuclease digestion and hybridization of kinetoplastic DNA. In: 11th International Congress of Tropical Medicine and Malaria; 2004 Sep 16-22; Calgary, Canada. 1984. p. 68.
33 Jackson PD, Wohlhieter JA, Hockmeyer WT. Leishmania characterization by restriction endonuclease digestion of kinetoplastic DNA [abstracts]. In: 5th International Congress of Parasitology; 1982 Aug 7-14; Toronto, Canada, 1982. p. 342.
34 Johnson PT, McConnell E, Hertig M. Natural infections of leptomonad flagellates in Panamanian phlebotomus sandflies. Exp Parasitol. 1963 Aug;14:107-22. [ Links ]
35 Killick-Kendrick R, Lainson R, Rioux J-A, Saf'janova VM. The taxonomy of Leishmania-like parasites of reptiles. In: Rioux J-A, editor. Leishmania, Taxonomie et phylogenese: applications eco-epidemiologiques. Colloque International CNRS/INSERM; 1984 Jul 2-6; Montpellier, Montpellie:IMEE 1984; France. p. 143-148.
36 Kreutzer RD, Corredor A, Grimaldi Jr G, Grogl M, Rowton ED, Young DG, et al. Characterization of Leishmania colombiensis (Kinetoplastida:Trypanosomatidae), a new parasite infecting humans, animals and phlebotomine sand flies in Colombia and Panama. Am J Trop Med Hyg. 1991 Jun;44(6):662-75. [ Links ]
37 Lainson R. Demographic changes and their influence on the epidemiology of the American leishmaniases. In: Service MW, editors. Demography and Vector-Borne Diseases. Florida: CRC Press Inc; 1989. p. 85-106.
38 Lainson R, Braga RR, Souza AA, Povoa MM, Ishikawa EA, Silveira FT. Leishmania (Viannia) shawi sp.n., a parasite of monkeys, sloths and procyonids in Amazonian Brazil. Ann Parasitol Hum Comp. 1989;64(3):200-7. [ Links ]
39 Lainson R, Rangel EF. Ecologia das leishmanioses: Lutzomyia longipalpis e a eco-epidemiologia da leishmaniose visceral americana (LVA) no Brasil. In: Rangel EF, Lainson R, editors. Flebotomineos do Brasil. Rio de Janeiro: Fiocruz; 2003. p. 311-36. [ Links ]
40 Lainson R, Ready PD, Shaw JJ. Leishmania in phlebotomid sandflies. VII. On the taxonomic status of Leishmania peruviana, causative agent of Peruvian 'uta', as indicated by its development in the sandfly, Lutzomyia longipalpis. Proc R Soc Lond B Biol Sci. 1979 Dec;206(1164):307-18. [ Links ]
41 Lainson R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. London: Academic Press; 1987. p. 12-120. [ Links ]
42 Lainson R, Shaw JJ. Leishmanias and leishmaniasis of the New World, with particular reference to Brazil. Bull Pan Am Health Org. 1973;7(4):1-19.
43 Lainson R, Shaw JJ. Leishmanias of neotropical porcupines: Leishmania hertigi deanei nov. subsp. Acta Amaz. 1977;7(1):51-7.
44 Lainson R, Shaw JJ. Leishmaniasis in Brazil. I. Observations on enzootic rodent leishmaniasis-incrimination of Lutzomyia flaviscutellata (Mangabeira) as the vector in the lower Amazonian basin. Trans R Soc Trop Med Hyg. 1968;62(3):385-95. [ Links ]
45 Lainson R, Shaw JJ. Leishmaniasis in Brazil. V. Studies on the epidemiology of cutaneous leishmaniasis in Mato Grosso State, and observations on two distinct strains of Leishmania isolated form man and forest animals. Trans R Soc Trop Med Hyg. 1970;64(5):654-67. [ Links ]
46 Lainson R, Shaw JJ. Leishmaniasis of the New World: taxonomic problems. Brit Med Bull. 1972 Jan;28(1):44-8. [ Links ]
47 Lainson R, Shaw JJ. New World Leishmaniasis. In: Cox FEG, Wakelin D, Gillespie SH, Despommier DD, editors. Topley & Wilson's Microbiology and Microbial Infections: parasitology. 10th ed. London: Hodder Arnold ASM Press; 2005. p. 313-49. [ Links ]
48 Lainson R, Shaw JJ. New World leishmaniasis - the Neotropical Leishmania species. In: Cox FE, Kreier JP, Wakelin D, editors. Topley & Wilson's Microbiology and Microbial Infections. 9th ed. London: Hodder Headline Group; 1998. p. 241-66. [ Links ]
49 Lainson R, Shaw JJ. Some reservoir-hosts of Leishmania in wild animals of Mato Grosso State, Brazil. Two distinct strains of parasites isolated from man and rodents. Trans R Soc Trop Med Hyg. 1969;63(3):408-9. [ Links ]
50 Lainson R, Shaw JJ. Taxonomy of the New World Leishmania species. Trans R Soc Trop Med Hyg. 1972;66(6):943-4.
51 Lainson R, Shaw JJ. The role of animals in the epidemiology of South American leishmaniasis. In: Lumsden WHR, Evans DA, editors. Biology of the Kinetoplastida. London: Academic Press; 1979. p. 1-116. [ Links ]
52 Lainson R, Shaw JJ, Lins ZC. Leishmaniasis in Brazil: IV The fox, Cerdocyon thous (L) as a reservoir of Leishmania donovani in Para State, Brazil. Trans R Soc Trop Med Hyg. 1969;63(6):741-5. [ Links ]
53 Lainson R, Shaw JJ, Póvoa M. The importance of edentates (sloths and anteaters) as primary reservoirs of Leishmania braziliensis guyanensis, causative agent of "pian-bois" in North Brazil. Trans R Soc Trop Med Hyg. 1981;75(4):611-2.
54 Lainson R, Shaw JJ, Silveira FT, Braga RR. American visceral leishmaniasis: on the origin of Leishmania (Leishmania) chagasi. Trans R Soc Trop Med Hyg. 1987;81(3):517.
55 Lainson R, Shaw JJ, Ward RD, Fraiha H. Leishmaniasis in Brazil. IX. Considerations on the Leishmania braziliensis complex: importance of sandflies of the genus Psychodopygus (Mangabeira) in the transmission of L. braziliensis in north Brazil. Trans R Soc Trop Med Hyg. 1973;67(2):184-96. [ Links ]
56 Lainson R, Strangways-Dixon J. Dermal leishmaniasis in British Honduras: some host-reservoirs of Leishmania braziliensis mexicana. A preliminary note. Brit Med J. 1962;1:1596-8. [ Links ]
57 Lainson R, Strangways-Dixon J. The epidemiology of dermal leishmaniasis in British Honduras. Part II. Reservoir-hosts of Leishmania mexicana among the forest rodents. Trans R Soc Trop Med Hyg. 1964;58:136-53. [ Links ]
58 Lainson R, Ward RD, Shaw JJ. Leishmania in phlebotomid sandflies: VI. Importance of hindgut development in distinguishing between parasites of the Leishmania mexicana and L. braziliensis complexes. Proc R Soc Lond B Biol Sci. 1977;199:309-20. [ Links ]
59 Lima H, de Gugielmo Z, Rodríguez A, Convit J, Rodriguez N. Cotton rats (Sigmodon hispidus) and black rats (Rattus rattus) as possible reservoirs of Leishmania spp. in Lara State, Venezuela. Mem Inst Oswaldo Cruz. 2002 Mar;97(2):169-74. [ Links ]
60 Lindenberg A. L'ulcere de Bauru ou le bouton d'orient au Brésil. Bull Soc Path Exot. 1909;2:252-4.
61 Llanos-Cuentas EA, Roncal N, Villaseca P, Paz L, Ogusuku E, Perez JE, et al. Natural infections of Leishmania peruviana in animals in the Peruvian Andes. Trans R Soc Trop Med Hyg. 1999 Jan-Feb;93(1):15-20. [ Links ]
62 Lühe M. Die im Blute schmarotzenden Protozoen und ihre nachsten Verwandren. In: Mense C, Barth IA, editors. Hanbuch der Tropenkrankheiten; 1906. p.203.
63 Lukes J, Maurico IL, Schönian G, Dujardin JC, Soteriadon K, Dedet J-P, et al. Evolutionary and geographical history of the Leishmania donovani complex, with a revision of current taxonomy. Proc Natl Acad Sci USA. 2007 May;104(22):9375-80. [ Links ]
64 Luz E, Giovannoni M, Borba AM. Infecção de Lutzomyia monticola por Leishmania enriettii. Ann Fac Med Univers Fed Parana. 1967;9-10:121-B.
65 Machado MI, Milder RV, Pacheco RS, Silva M, Braga RR, Lainson R. Naturally acquired infections with Leishmania enriettii Muniz and Medina 194B in guinea-pigs from São Paulo, Brazil. Parasitology. 1994;109(Pt2):135-8.
66 Márquez M, Scorza JV. Criterios de nuliparidad y de paridad en Lutzomyia townsendi (Ortiz, 1959) del occidente de Venezuela. Mem Inst Oswaldo Cruz 1982;77(3):229-46. [ Links ]
67 Martinez E, Le Pont F, Mollinedo S, Cupolillo E. A first case of cutaneous leishmaniasis due to Leishmania (Viannia) lainsoni in Bolivia. Trans R Soc Trop Med Hyg 2001 Jul;95(4):375-7. [ Links ]
68 Martinez E, Le Pont F, Torrez M, Telleria J, Vargas F, Dujardin JC, et al. Lutzomyia nuneztovari anglesi (Le Pont and Desjeux, 19B4) as a vector of Leishmania amazonensis in a sub-Andean leishmaniasis focus of Bolivia. Am J Trop Med Hyg. 1999 Nov;61(5): B46-9.
69 Matta AA. Sur les leishmanioses tégumentaires. Classification générale des leishmanioses. Bull Soc Path Exot. 1916;9:494-503.
70 McMahon-Pratt D, Bennett E, David JR. Monoclonal antibodies that distingu ish su bspecies of Leishmania braziliensis. Am J Immunol. 1982 Sep;129(3):926-7. [ Links ]
71 Medina HSG. Estudos sôbre leishmaniose. I. Primeiros casos de leishmaniose espontânea observados em cobaios. Arq Bioi Tec. 1946;1:39-74. [ Links ]
72 Medina R, Romero J. Estudio clínico y parasitológico de una nueva cepa de leishmania. Arch Venez Med Trop Parasitol Med. 1959 Jul;3:298-326.
73 Medina R, Romero J. Leishmania pifanoi n.sp. El agente causal de la leishmaniasis tegumentaria difusa. Arch Venez Pat Trop Parasitol Med. 1962;4:349-53.
74 Migone LE. Un caso de kala-azar a Asunción (Paraguay). Bull Soc Path Exot. 1913;6:118-20.
75 Miles MA, Lainson R, Shaw JJ, Póvoa M, Souza AA. Leishmaniasis in Brazil: XV. Biochemical distinction of Leishmania mexicana amazonensis, L. braziliensis braziliensis and L. braziliensis guyanensis - aetiological agents of cutaneous leishmaniasis in the Amazon Basin of Brazil. Trans R Soc Trop Med Hyg. 1981;75(4):524-9. [ Links ]
76 Miies MA, Póvoa MM, Souza AA, Lainson R, Shaw JJ. Some methods for the enzymic characterization of Latin-American Leishmania with particular reference to Leishmania mexicana amazonensis and subspecies of Leishmania hertigi. Trans R Soc Trop Med Hyg. 1980;74(2):243-52. [ Links ]
77 Nery-Guimarães F, Costa O. Observações sôbre o comportamento da "Leishmania" produtora de infecção natural em "Oryzomys goeldi" na Amazônia. (Segunda nota). O Hospital, Rio de Janeiro. 1964;66:287-92.
78 Noyes HA. Implications of a Neotropical origin of the genus Leishmania. Mem Inst Oswaldo Cruz. 1998 Sep;93(5):657-62. [ Links ]
79 Noyes HA, Arana BA, Chance ML, Maignon R. The Leishmania hertigi (Kinetoplastida; Trypanosomatidae) complex and the lizard Leishmania: their classification and evidence for a neotropical origin of the Leishmania-Endotrypanum clade. J Eukaryot Microbiol. 1997 Sep-Oct;44(5):511-7. [ Links ]
80 Penna HA. Leishmaniose visceral no Brasil. Brasil Med. 1934;48:949-50.
81 Pessôa SB. Classificação das leishmanioses e das espécies do gênero Leishmania. Arq Hig Saude Pub. 1961;26:41-50.
82 Pita-Pereira D, Souza GD, Zwetsch A, Alves CR, Britto C, Rangel EF. First report of Lutzomyia (Nyssomyia) neivai (Diptera: Psychodidae: Phlebotominae) naturally infected by Leishmania (Viannia) braziliensis in a periurban area of South Brazil using a multiplex polymerase chain reaction assay. Am J Trop Med Hyg. 2009 Apr;80(4):593-5. [ Links ]
83 Pizarro P. Relación de la conquista del Perú. Colección de libros y documentos referentes a la historia del Perú. Lima: Collection Urteaga-Romero; 1571. (1st Series) [ Links ]
84 Ponce C, Ponce E, Morrison A, Cruz A, Kreutzer R, McMahon-Pratt D, et al. Leishmania donovani chagasi: a new clinical variant of cutaneous leishmaniasis in Honduras. Lancet. 1991 Jan;337(8733):67-70. [ Links ]
85 Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009 Nov;104(7):937-54. [ Links ]
86 Rangel EF, Lainson R, Souza AA, Ready P, Azevedo ACR. Variation between geographical populations of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) sensu lato (Diptera: Psychodidae: Phlebotominae) in Brazil. Mem Inst Oswaldo Cruz. 1996 Jan-Feb;91(1):43-50. [ Links ]
87 Ready PD, Day JC, Souza AA, Rangel EF, Davies CR. Mitochondrial DNA characterization of populations of Lutzomyia whitmani (Diptera: Psychodidae) incriminated in the peri-domestic and silvatic transmission of Leishmania species in Brazil. Bull Entomol Res. 1997 Apr;87(2):187-95. [ Links ]
88 Rocha NM, Melo MN, Babá EH, Dias M, Michalick MS, Costa CA, et al. Leishmania braziliensis braziliensis isolated from Akodon arviculoides captured in Caratinga, Minas Gerais, Brazil. Trans R Soc Trop Med Hyg. 1988 Jan;82(1):68. [ Links ]
89 Ryan L, Lainson R, Shaw JJ. Leishmaniasis in Brazil. XXIV. Natural flagellate infections of sandflies (Diptera: Psychodidae) in Pará State, with particular reference to the rôle of Psychodopygus wellcomei as the vector of Leishmania braziliensis braziliensis in the Serra dos Carajás. Trans R Soc Trop Med Hyg. 1987 May-Jun;81(3):353-9. [ Links ]
90 Ryan L, Silveira FT, Lainson R, Shaw JJ. Leishmanial infections in Lutzomyia longipalpis and Lu. antunesi (Diptera: Psychodidae) on the island of Marajó, Pará State, Brazil. Trans R Soc Trop Med Hyg. 1984;78(4):547-8. [ Links ]
91 Saf'janova VM. Classification of the genus Leishmania Ross. In: The Leishmanias, Protozoology. Leningrad: Academy of Sciences; All Union Society of Protozoologists; 1982. p. 95-101.
92 Santoro F, Lemesre JL, Rizvi FS, Afchain D, Sadogirsky M. Spécificité au niveau de proteins de surface des promastigotas de Leishmania donovani (Laveran et Mesnil, 1903), Leishmania infantum Nicolle, 1908 et Leishmania chagasi Cunha et Chagas, 1937. In: Rioux JA, editor. Leishmania. Taxonomie et Phylogenese. Applications Éco-Épidémiologiques. Colloque Internat; 1984 July 2-6; Montpellier: L'Institut Méditerranéen d'Études Épidémiologiques et Écologiques; 1986. p. 71-6.
93 Santos SO, Arias J, Ribeiro AA, Paiva Hoffmann M, Freitas RA, Malacco MA. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis. Med Vet Entomol. 1998 Jul;12(3):315-7. [ Links ]
94 Santos SO, Arias JR, Hoffmann MP, Fulan MB, Ferreira WF, Pereira C, et al. The presence of Lutzomyia longipalpis in a focus of American visceral leishmaniasis where the only proven vector is Lutzomyia cruzi, Corumbá. Mato Grosso do Sul State. Rev Soc Bras Med Trop. 2003 Sep-Oct;36(5):633-4. [ Links ]
95 Shaw JJ. New World leishmaniasis: the ecology of leishmaniasis and the diversity of leishmanial species in Central and South America. In: J Farrell, editors. World Class Parasites: Leishmania. London: Kluwer Academic Publishers Boston; 2002. p. 11-31.
96 Shaw JJ, Lainson R. Leishmaniasis in Brazil: II. Observations on enzootic rodent leishmaniasis in the lower Amazon Region the feeding habits of the vector, Lutzomyia flaviscutellata in reference to man, rodents and other animals. Trans R Soc Trop Med Hyg. 1968;62(3):396-405. [ Links ]
97 Shaw JJ, Lainson R, Ryan L, Braga RR, McMahon-Pratt D, David JR. Leishmaniasis in Brazil: XXIII. The identification of Leishmania braziliensis braziliensis in wild-caught neotropical sandflies using monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81(1):69-72. [ Links ]
98 Sherlock IA, Miranda JC, Sadigursky M, Grimaldi G. Natural infection of the opossum Didelphis albiventris (Marsupialia, Didelphidae) with Leishmania donovani in Brazil. Mem Inst Oswaldo Cruz. 1984 Oct-Dec;79(4):511. [ Links ]
99 Silveira FT, Ishikawa EA, Souza AA, Lainson R. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará State, Brazil, caused by Leishmania (Viannia) lindenbergi n.sp. A new leishmanial parasite of man in the Amazon Region. Parasite. 2002 Mar;9(1):43-50. [ Links ]
100 Silveira FT, Lainson R, Corbett CEP. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil a review. Mem Inst Oswaldo Cruz. 2004 May;99(3):239-51. [ Links ]
101 Silveira FT, Lainson R, Shaw JJ, Braga RR, Ishikawa EAY, Souza AAA. Leishmaniose cutânea na Amazônia: isolamento de Leishmania (Viannia) lainsoni do roedor Agouti paca (Rodentia: Dasyproctidae) no Estado do Pará, Brasil. Rev Inst Med Trop Sao Paulo. 1991 Jan-Feb;33(1):18-22. [ Links ]
102 Silveira FT, Souza AAA, Lainson R, Shaw JJ, Braga RR, Ishikawa EAY. Cutaneous leishmaniasis in the Amazon Region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominidae) by Leishmania lainsoni in Pará State, Brazil. Mem Inst Oswaldo Cruz. 1991 Jan-Mar;86(1):127-30.
103 Souza A, Ishikawa E, Braga R, Silveira F, Lainson R, Shaw JJ. Psychodopygus complexus, a new vector of Leishmania braziliensis to humans in Pará State, Brazil. Trans R Soc Trop Med Hyg. 1996 Mar-Apr;90(2):112-3. [ Links ]
104 Souza AAA, Silveira FT, Lainson R, Barata IR, Silva MGS, Lima JAN, et al. Fauna flebotomínica da Serra dos Carajás, Estado do Pará, Brasil, e sua possível implicação na transmissão da leishmaniose tegumentar americana. Rev Pan-Amaz Saude. 2010;1(1):45-51. [ Links ]
105 Splendore A. Buba-blastomicosi-leishmanosi. Nota sopra alcune affezioni framboesiche osservate in Brasile. Arch für Schiffs-und Tropenhyg. 1911;15:105-15.
106 The thirtieth annual report of the work and operation of the Gorgas Memorial Laboratory, covering the fiscal year ended; 1957 June 30. Washington: United States Government Printing Office; 1957.
107 The twenty-ninth annual report of the work and operation of the Gorgas Memorial Laboratory, covering the fiscal year ended; 1956 June 30. Washington: United States Government Printing Office; 1957.
108 Travi BL, Jaramillo C, Montoya J, Segura I, Zea A, Gonçalves A, et al. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am J Trop Med Hyg. 1994 May;50(5):557-65. [ Links ]
109 Travi BL, Vélez ID, Brutus L, Segura I, Jaramillo C, Montoya J. Lutzomyia evansi, an alternative vector of Leishmania chagasi in a Colombian focus of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):676-7. [ Links ]
110 Velez IR. Uta et espundia. Bull Soc Path Exot. 1913;6:545.
111 Vianna G. Sobre uma nova especie de Leishmania (Nota Preliminar). Bras Med. 1911;25:411.
112 Ward RD, Lainson R, Shaw JJ. Experimental transmissions of Leishmania mexicana amazonensis Lainson & Shaw, between hamsters by the bite of Lutzomyia flaviscutellata (Mangabeira). Trans R Soc Trop Med Hyg. 1977;71(3):265-6.
113 Ward RD, Lainson R, Shaw JJ. Further evidence of the rôle of Lutzomyia flaviscutellata (Mangabeira) as the vector of Leishmania mexicana amazonensis in Brazil. Trans R Soc Trop Med Hyg. 1973;67(4):608-9. [ Links ]
114 Ward RD, Shaw JJ, Lainson R, Fraiha H. Leishmaniasis in Brazil: VIII. Observations on the phlebotomine fauna of an area highly endemic for cutaneous leishmaniasis, in the Serra dos Carajas, Pará State. Trans R Soc Trop Med Hyg. 1973;67(2):174-83. [ Links ]
115 Wright JH. Protozoa in a case of tropical ulcer ("Delhi sore"). J Med Res. 1903 Dec;10(3):472-82. [ Links ]
116 Zeledón R, Hidalgo H, Viquez A, Urbina A. Atypical cutaneous leishmaniasis in a semi-arid region of north-west Costa Rica. Trans R Soc Trop Med Hyg. 1989 Nov-Dec;83(6):786. [ Links ]
117 Zuckerman A, Lainson R. Leishmania. In: Kreier JP, editor. Parasitic protozoa. London: Academic Press; 1977. p. 57-133. [ Links ]
 Correspondência / Correspondence / Correspondencia:
Correspondência / Correspondence / Correspondencia:
Instituto Evandro Chagas
Seção de Parasitologia
Av. Almirante Barroso, 492
CEP: 66090-000
Belém-Pará-Brasil
E-mail: ralphlainson@iec.pa.gov.br
Recebido em / Received / Recibido en: 16/4/2010
Aceito em / Accepted / Aceito en: 28/5/2010











 texto em
texto em 

