Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.2 n.2 Ananindeua jun. 2011
http://dx.doi.org/10.5123/S2176-62232011000200010
CASE REPORT | RELATO DE CASO | RELATO DE CASO
Molecular characterization of human parvovirus B19 associated with neuromyelitis
Caracterização molecular do parvovirus humano B19 associado com neuromielite
Caracterización molecular del parvovirus B19 humano asociado a la neuromielitis
Maria Isabel de OliveiraI; Ana Maria Sardinha AfonsoII; Cristina Adelaide FigueiredoI; Suely Pires CurtiI; Giselle Burlamaqui KlautauII; Maria Anice Mureb SallumIII
ICentro de Virologia, Instituto Adolfo Lutz, São Paulo, São Paulo, Brasil
II Departamento de Medicina da Irmandade da Santa Casa de Misericórdia de São Paulo, São Paulo, Brasil
IIIFaculdade de Saúde Pública, Universidade de São Paulo, São Paulo, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
INTRODUCTION: Human parvovirus B19 (B19V) is associated with a wide range of clinical symptoms. An acute infection can lead to anemia, erythema infectiosum, or hydrops fetalis, as well as arthritis and other clinical manifestations. Among other diseases associated with B19V, several demyelinating disorders may occur as a result of B19V infection. Phylogenetic analysis of partial erythrovirus sequences resulted in three possible B19V genotype groups. Our study involved analysis of samples from a patient with neuromyelitis that enabled the molecular characterization of human parvovirus B19.
OBJECTIVE: To study B19V infection associated with a neurological manifestation and to perform phylogenetic analysis.
METHODS: Serum and cerebrospinal fluid samples were tested for B19V infection. The serological assay for B19V was a commercial IgG and IgM enzyme immunoassay kit. Nucleic acid detection was performed using a PCR assay. The phylogenetic analyses were performed using PAUP and other software programs.
RESULTS: Our study reports that the topology of viral isolate sequences from a patient with neuromyelitis fell within a clade consisting of B19V genotype 1.
CONCLUSION: This result indicates that this virus genotype group has been circulating in Sao Paulo, Brazil. This report represents a rare case in which B19V might be the responsible agent for central nervous system infection.
Keywords: Human parvovirus B19; Neurologic manifestations; Parvoviridae infections; Sequence analysis, DNA.
RESUMO
INTRODUÇÃO: O parvovírus B19 humano (B19V) é associado a vários sintomas clínicos. Uma infecção aguda pode levar à ocorrência de anemia, eritema infeccioso ou hidropsia fetal, bem como artrite e outras manifestações clínicas. Infecções por B19V podem também ocasionar várias doenças desmielinizantes. A análise filogenética das sequências parciais de eritrovírus resultou em três possíveis grupos de genótipos de B19V. Este estudo consistiu na análise de amostras de uma paciente com neuromielite, o que possibilitou a caracterização molecular do parvovírus B19 humano.
OBJETIVO: Estudar a infecção por B19V associada a uma manifestação neurológica e realizar sua análise filogenética.
MÉTODOS: Amostras de soro e de líquido cefalorraquidiano foram testadas para a observação de infecção por B19V. O teste sorológico utilizado para a pesquisa de B19V foi um kit comercial de ensaio imunoenzimático para IgG e IgM. A detecção de ácido nucleico foi realizada utilizando-se um teste de PCR. As análises filogenéticas foram realizadas utilizando PAUP e outros programas.
RESULTADOS: Este artigo registra que a topologia de sequências de isolados virais de uma paciente com neuromielite foram inseridas em um clado constituído de grupos dentro do genótipo 1 de B19V.
CONCLUSÃO: Esses achados indicam que este genótipo do vírus tem se mantido circulante em São Paulo, Brasil. Este relato representa um caso raro no qual o B19V pode ter sido o agente responsável pela infecção no sistema nervoso central.
Palavras-chave: Parvovírus B19 Humano; Manifestações Neurológicas; Infecções por Parvoviridae; Análise de Sequência de DNA.
RESUMEN
INTRODUCCIÓN: El parvovirus B19 humano (B19V) está asociado a varios síntomas clínicos. Una infección aguda puede conducir a anemia, eritema infeccioso o hidropesía fetal, así como a artritis y a otras manifestaciones clínicas. Infecciones por B19V pueden también ocasionar varias enfermedades desmielinizantes. El análisis filogenético de las secuencias parciales de eritrovirus resultó en tres posibles grupos de genotipos de B19V. Este estudio consistió en el análisis de muestras de una paciente con neuromielitis, lo que posibilitó la caracterización molecular del parvovirus B19 humano.
OBJETIVO: Estudiar la infección por B19V asociada a una manifestación neurológica y realizar su análisis filogenético.
MÉTODOS: Se analizaron muestras de suero y de líquido cefalorraquídeo para la observación de infección por B19V. La prueba serológica utilizada para la investigación de B19V fue un kit comercial de ensayo inmunoenzimático para IgG e IgM. La detección de ácido nucleico se realizó utilizando una prueba de PCR. Los análisis filogenéticos se realizaron utilizando PAUP y otros programas.
RESULTADOS: Este artículo registra que la topología de secuencias de aislados virales de una paciente con neuromielitis fueron insertas en un caldo constituido de grupos dentro del genotipo 1 de B19V.
CONCLUSIÓN: Estos hallazgos indican que este genotipo del virus se ha mantenido en circulación en São Paulo, Brasil. Este relato representa un caso raro en el cual, el B19V puede haber sido el agente responsable por la infección en el sistema nervioso central.
Palabras clave: Parvovirus B19 Humano; Manifestaciones Neurológicas; Infecciones por Parvoviridae; Análisis de Secuencia de ADN.
INTRODUCTION
Human parvovirus B19 (B19V) is classified in the Erythrovirus genus of the Parvoviridae family1. It may be associated with various pathological conditions, such as anemia, erythema infectiosum, hydrops fetalis and arthritis. The immunological response to the virus will vary greatly as a result of an individual's hematological status. The following are examples of conditions that may affect the im mu ne response to infection: aplastic crisis, thrombocytopenia, hepatitis pancytopenia, renal disease, myocarditis and mysositis2,3.
Generally, viral transmission takes place via an aerosol route through respiratory secretions. Viremia occurs one week after exposure and usually lasts approximately five days, with virus titers peaking within the first two days. The course of infection is generally the same amongst individuals, which is likely a contributing factor in the lack of genetic diversity among B19V strains3.
A phylogenetic analysis has been conducted using partial sequences of B19V available in GenBank, and three genotype groups were proposed. Genotype 1 corresponds to B19V, and genotypes 2 and 3 are associated with V9 viruses4,5,6,7.
In addition to the disorders mentioned above, a number of demyelinating infections have been reported in association with B19V. Identification of the particular infectious agent involved is difficult. For this reason, B19V involvement in many of these disorders has not been clinically validated to date5.
Our study reports the case of a patient with neuromyelitis in which it was possible to characterize the genotype of the human parvovirus B19 that caused the infection.
MATERIALS AND METHODS
A previously healthy 27-year-old female was admitted to the Hospital Irmandade da Santa Casa de Misericórdia (ISCMSP) in Sao Paulo State, Brazil, with dizziness for 2 days, cough, expectoration, nausea, vomiting and desquamative lesions in the left cervical region, but the patient had no signs of secondary infection. Two days after admission, her symptoms had evolved to mental confusion, unresponsiveness to verbal commands or painful stimuli, tachypnea and urinary retention. An orotracheal intubation was performed. During the neurological examination, signs of meningeal irritation were not detected. The results of magnetic resonance imaging (MRI) of the brain were normal. Laboratory investigation showed normal results for blood counts (Hb 14.00 g/dL; Hct 44%; 5,000 leukocytes/mm3; platelets 183,000/mm3), serum biochemistry and prothrombin time. The cerebrospinal fluid (CSF) was clear, glucose was 60 mg/dL, protein was 98 mg/100 mL, white blood cells were 2 leukocytes/mm3; and direct staining for bacteria and fungi produced negative results. Several image tests were performed and the following hypotheses were suggested following clinical evaluation: demyelinating disease, myelopathy, neuromyelitis, auto-immune disorder, aseptic meningoencephalitis or infection.
A screening for virus infection was initiated with the collection of blood and CSF and the utilization of serological assays and nucleic acid detection by PCR. The samples were analyzed at Instituto Adolfo Lutz, Sao Paulo, Brazil, to detect measles, rubella, cytomegalovirus (CMV), human immunodeficiency virus (HIV), syphilis, hepatitis, human T lymphotropic virus (HTLV), Epstein-Barr virus (EBV), Herpes simplex 1 and 2 and varicella zoster infection. Results of these serological and PCR assays showed no sign of recent infection with these viruses. Sera and CSF samples were then tested for B19V infection. Antibodies were detected using a commercial IgG and IgM enzyme immunoassay kit (Biotrin®, Dublin, Ireland) that was specific for identifying VP2. DNA was amplified from sera and CSF with QIAmp® DNA minikit (Courtabocuf, France) Quiagen. The kits were used according to the manufacturers' recommendations. Sequences were amplified using oligonucleotide primers specific for the conserved region of the NS1 gene, which encodes the nonstructural protein of B19V (positions 1267 to 1691) described by Sanabani et al. For phylogenetic studies, amplified DNA fragments were purified with the Purilink PCR Purification Kit (Invitrogen TM Life Technologies, Carlsbad, USA) and sequenced using ABI 3100 Prism Big Dye ( Foster City, USA) Applied Biosystems, Inc.
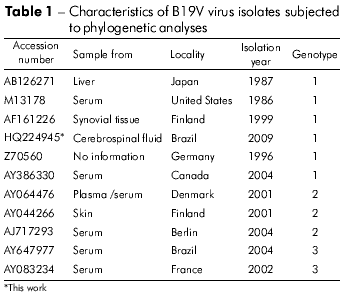
The sequence generated from the erythrovirus HPV B19 sample was subjected to Blast searches using the GenBank database. The viral isolate sequences used for phylogenetic analysis, along with isolation locations, isolation years and genotype, are presented in table 1. Alignment was performed using the prototype sequences of genotype 1 (strain Pvbua; accession number: M13178), genotype 2 (strain Lali; accession number: AY044266), and genotype 3 (strain V9; accession number: AY647977) and the CLUSTAL X program (http://www-igbmc.u-strasbg.fr/BioInfo/ClustalX/). Analysis was performed using the MEGALIGN (DNASTAR, Inc.) software. Phylogenetic analysis was performed using the PAUP version 4.0 beta 10 program for Windows, which was written by D. L. Swofford (Sinauer Associates, Sunderland, USA). The Modeltest software, version 3.6 (http://darwin.uvigo.es/11), was used for statistical analysis8.
RESULTS
Serum samples were positive for B19V-specific IgG and negative for B19V-specific IgM. CSF samples were negative for both B19V-specific IgG and IgM. B19V DNA was detected only in CSF by PCR. The sequence was deposited in GenBank (accession number: HQ224945) and was aligned to a 423 bp fragment at position 1,282 to 1,704 in the NS1 gene sequence with accession number M13178.
Comparisons between the NS1 nucleotide and amino acid sequences from the isolate in this study and those from representative groups of B19V showed a high level of identity with genotype 1. Pairwise nucleotide identity among strains ranged from 99.1% to 98.6% in genotype 1, from 87.2% to 87.7% in genotype 2 and from 88.2% to 86.8% in genotype 3 (MEGALIGN, DNASTAR, Inc.).
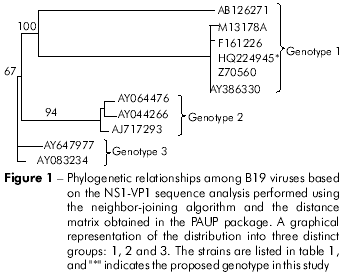
Sequence HQ224945 generated from the SP Brazil isolate was recovered in the clade formed by sequences representatives of the genotype 1 (Figure 1). Aligned sequences with the reference genotype showed that the amino acid sequence had a mutation at the A619T position (alanine changed to threonine) through the analysis of the partial NS1 region from the erythrovirus variant studied in this work.
DISCUSSION
Molecular test results for the patient described in this study suggested a B19V infection. The viral DNA detected in CSF indicated an acute viral infection that was likely the cause of the neuromyelitis. However, the precise mechanism whereby B19V affects the central nervous system (CNS) is unclear. P-antigen is the cellular receptor for B19V and it plays an important role in viral infection and replication. This antigen is present in endothelial, macrophage and microglial cells9.
The patient described in this report had a history of undetermined disease with acute neurological symptoms. Of note in the clinical history of this patient is the presence of an infectious condition preceding the neurological symptoms and diagnosis of neuromyelitis. Discovery of the etiologic agent could only be performed after a PCR assay analysis of CSF. It is unclear why patients exhibit different neurologic manifestations following infection despite having similar severe demyelinating lesions. Extensive investigation of possible causative infectious agents is required in cases without a definite etiology.
Our study of B19V infection in a patient with neuromyelitis showed that the isolate belonged to genotype 1. The study by Zakrzewska et al9 showed B19V infection in systemic sclerosis (SSc) patients, indicating that infection with genotype 1 viruses was more frequent in the skin and that there was simultaneous persistence of genotype 2, which was detected in SSc skin and bone marrow samples. Piotr2 reported genotypes 1 and 2 in immunocompromised patients. However, both genotypes were associated with high viremia in renal transplant patients treated with chemotherapy.
There is evidence that the large nonstructural protein NS1 transactivates both regulatory and structural gene expression and that the synthesis of the NS1 transcripts occurs early in the course of infection10.
In our study, we did not make an association of mutation at position 619 with a probable infection. This mutation in the NS1 gene has not been reported in any previously described virus isolate. Further studies of the clinical significance of other erythrovirus variants should be performed.
The sequence generated in this study had the same reproducibility described in previous studies that used a small fragment of the NS1 gene to identify three distinct genotypic groups3. To date, only a limited number of studies have been conducted to detect virus in cerebrospinal fluid. There are reports showing the circulation of these genotypic groups in Brazil but not in association with infections of the CNS3,11,12,13.
CONCLUSION
An accumulation of similar cases is required to elucidate the role the virus plays in the development of neuromyelitis. The patient described in this case report recovered without sequelae and represents a rare case of B19V as a possible cause of CNS infection.
ACKNOWLEDGMENTS
The present work was supported by Instituto Adolfo Lutz. The authors gratefully acknowledge the contribution of study participants and the clinical team: Jonas Gordilho Souza, Zied Rasslan, Carlos Albert Lima and Luiz Claudio Rodrigues Marrochi of the Irmandade da Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil.
REFERENCES
1 Siegl G, Bates RC, Berns KI, Carter BJ, Kelly DC, Kurstak E, et al. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985 Jul;23(2):61-73.
2 Piotr G, Kalińska A, Kara M, Wieczorek R, Ejduk A, Sulkowska E, et al. Identification and characterization of acute infection with parvovirus B19 genotype 2 in immunocompromised patients in Poland. J Med Virol. 2011 Jan;83(1):142-9. Doi:10.1002/jmv.21947 [Link]
3 Sanabani S, Neto WK, Pereira J, Sabino EC. Sequence Variability of Human Erythroviruses Present in Bone Marrow of Brazilian Patients with Various Parvovirus B19-Related Hematological Symptoms. J Clin Microbiol. 2006 Feb;44(2):604-6. Doi:10.1128/JCM.44.2.604–606.2006 [Link]
4 Hemauer A, von Poblotzki A, Gigler A, Cassinotti P, Siegl G, Wolf H, et al. Sequence variability among different parvovirus B 19 isolates. J Gen Virol. 1996 Aug;77:1781-5. [Link]
5 Kerr JR, Barah F, Chiswick ML, McDonnell GV, Smith J, Chapman MD, et al. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. J Neurol Neurosurg Psychiatry. 2002 Dec;73(6):739-46. [Link]
6 Servant A, Laperche S, Lallemand F, Marinho V, De Saint Maur G, Meritet JF, et al. Genetic diversity within human erytroviruses: identification of three genotypes. J Virol. 2002 Sep;76(18):9124-34. Doi:10.1128/JVI.76.18.9124–9134.2002 [Link]
7 Sevant-Delmas A, Laperche S, Mercier M, Lefrère JJ. Genetic diversity of human Erytroviruses. Pathol Biol. 2009 Mar;57(2):167-74.
8 Posada D, Crandall KA. Model Test: testing the model of DNA substitution. Bioinformatic. 1998 Oct;14(9):817-8. [Link]
9 Zakrzewska K, Corcioli F, Carlsen KM, Giuggioli D, Fanci R, Rinieri A, et al. Human parvovirus B19 (B19V) infection in systemic sclerosis patients. Intervirology. 2009 Aug;52(5):279-82. [Link]
10 Doerig C, Hirt B, Antonietti JP, Beard P. Nonstrutural protein of parvovirus B19 and minute virus of mice controls transcription. J Virol. 1990 Jan;64(1):387-96. [Link]
11 Freitas RB, Melo FL, Oliveira DS, Romano CM, Freitas MR, Macêdo O, et al. Molecular characterization of human erythrovirus B19 strains obtained from patients with several clinical presentations in the Amazon region of Brazil. J Clin Virol. 2008 Sep;43(1):60-5. [Link]
12 Keller LW, Barbosa ML, Melo FL, Pereira LM, David-Neto E, Lanhez LE, et al. Phylogenetic analysis of a near-full-length sequence of an erythrovirus genotype 3 strain isolated in Brazil. Arch Virol. 2009;154(10):1685-7. Doi:10.1007/s00705-009-0482-x [Link]
13 Mendonça MC, Ferreira AM, Santos MG, Oviedo EC, Bello MS, Siqueira MM, et al. Genotyping of human parvovirus B19 in clinical samples from Brazil and Paraguay using heteroduplex mobility assay, single-stranded conformation polymorphism and nucleotide sequencing. Mem Inst Oswaldo Cruz. 2011 Jun;106(4):502-4. [Link]
 Correspondence / Correspondência / Correspondencia:
Correspondence / Correspondência / Correspondencia:
Maria Isabel de Oliveira
Instituto Adolfo Lutz, Centro de Virologia
Av. Dr. Arnaldo, n° 355
CEP: 01246-902
São Paulo - São Paulo – Brasil
E-mail:olive40@uol.com.br
Received / Recebido em / Recibido en: 28/6/2011
Accepted / Aceito em / Aceito en: 30/10/2011












 Curriculum ScienTI
Curriculum ScienTI
