INTRODUCTION
In the last few years, many advances have been made towards a better understanding of the pathogenicity of mollicutes (or mycoplasmas), especially regarding its interactions with the immune system, the demonstration of its ability to invade different cellular types, and the possibility that at least a few species may play a role in the development of AIDS1. These findings and the complete genome sequencing of several species have focused on increasing attention on mollicutes, the smallest microorganisms capable of self-replication.
The most pathogenic species in laboratory rodents is Mycoplasma pulmonis. The main disease caused by M. pulmonis is murine respiratory mycoplasmosis, the expression of which is influenced by a wide range of factors. It may cause significant morbidity and mortality, particularly in laboratory animals destined for long term experiments, as well as in animals suffering from a subclinical infection that may recrudesce by a laboratory manipulation2. Furthermore, the most important consequence of M. pulmonis infection, beyond its direct pathogenic effects to the animal, can be the consequence of its presence in the analysis of experimental data. For instance, it may affect ciliary cell function, cellular kinetics3, neurogenic inflammation4,5, natural killer cell activity6,7, local and systemic immune response2,8, and induction of the production of several cytokines9. Immune system activation by mollicutes induces the liberation of preinflammatory substances and polyclonal proliferation of B and T lymphocytes10. Experimental M. pulmonis infection is also considered the best model for studying human disease caused by M. pneumoniae11.
M. pulmonis has been isolated from the urogenital tract of rats and mice2. Another important consequence of this infection is a reduction of breeding rates, which may decrease by up to 50%12. M. pulmonis might be also associated with arthritis in these animals, although a less suppurative disease than that caused by M. arthritidis, the most frequent agent at that site2. However, this disease may last for months, with immune complexes being detected in the joint tissues by immunofluorescence13,14.
The efforts to reduce M. pulmonis infection in laboratory rats have obtained a certain level of success, but have not been able to eliminate it. Prevalence studies from the 1980s still demonstrated rates of 5-20%, even in well-controlled facilities2. In spite of a lack of high scale studies in recent years, some groups have demonstrated that M. pulmonis infection still persists. In the early 1990s, a significant mollicute infection rate was found in rodents of an animal facility in São Paulo State, Brazil, that did not have any microbiological barriers15. More recently, in another animal facility in the same country, a M. pulmonis infection rate of 65% was found in laboratory rats, with colonisation of the personnel who was taking care of the facility16. More recently, our group has described a M. pulmonis infection rate of 75% in Wistar rats were bred at the University of Blumenau (FURB) facility17.
In spite of the fact that mycoplasma infection is a well-known issue, and that the routine examinations and preventive measures have been well standardised internationally for many years, it is essential that laboratories be assured about the health of the animals used in experimental research. Unfortunately, the certification of animals and accreditation of facilities is not a reality in many countries. Therefore, the aim of this research was to evaluate the effect of M. pulmonis infection in rats were bred at the animal facility of our institution regarding the levels of inflammatory proteins and peripheral blood cell types, as this information is unavailable in many research institutions that use this animal source.
MATERIALS AND METHODS
ANIMALS AND SAMPLES
In this study, 20 Wistar rats were bred at the animal facility of our institution were evaluated, equally distributed between males and females. This is a conventional animal facility, where the animals are bred in a clean area in polypropylene boxes with chrome-plated top cover, and feeder and drinker, but without microbiological barriers. Staff working with animals use equipments for individual safety when at work. Water and shavings are sterilised by autoclaving before entering into the clean area, and the shavings are disinfected with sodium hypochloride before being discarded. The animal facility supplies animals to meet teaching and research demand for students and professors of the health and biological areas. Periodical (annually) routine examinations for infectious diseases, as well as prophylaxis for parasites (semi-annually), are performed. The animal facility breeds rats and mice. For the experiments, animals were transferred to the local facility at the research laboratory or sampling. For mycoplasma detection, bronchoalveolar aspirates were obtained with 2 mL of culture media after euthanasia with thiopental (100 mg/kg), according to recommended standard procedures18. For the evaluation of the inflammatory parameters, blood was obtained by cardiac puncture and 0.5 mL were dispensed in microtubes containaing 50 µL of a ethylenediaminetetraacetic acid (EDTA) solution for white and red blood cell counts, and the remaining sample was dispensed in a dry microtube without anticoagulant for C-reactive protein (CRP) and alpha-1 acid glycoprotein (A1GP) determination. After coagulation of the samples without EDTA for 30 min at 37° C, they were subjected to centrifugation at 2,500 rpm for 15 min, and then serum was separated to a new tube and maintained at -20° C until analysis. The study was approved by the Committee of Ethics in Animal Research of the University of Blumenau under protocol no. 018/09 in August 26, 2009, and the animals were handled according to the Position Statements of American Association Laboratory Animal Service. It is worth noticing that from 2013 on it is recommended that in animals subjected to intraperitoneal anesthesia with barbiturates, when not possible intravenously, it must be mixed with lidocaine (10 mg/mL) or another local anesthetic19.
MYCOPLASMA CULTURE
A 1 mL aliquot of the aspirates was immediately subjected to culture at 37° C in a final volume of 2 mL of modified SP-4 liquid medium and plates with solid SP-4 medium (PPLO broth base [Difco], Tryptone [Oxoid], Peptone [Difco], L-arginine chloridrate, 1% phenol red, VX supplement, 200 µg/mL penicillin, 20% foetal calf serum, CRML 1066, 20% yeast extract [Difco], and dextrose). Solid medium was prepared by adding 1% Agar Noble (Difco). Samples were incubated for up to 30 days in aerobic conditions and observed daily for growth20.
DNA EXTRACTION AND PURIFICATION
DNA from 1 mL aliquots of the bronchoalveolar samples was extracted by conventional lysis and phenol/chloroform method. Samples in 1.5 mL microtubes were centrifuged for 15 min at 12,000 g. The pellet was resuspended in 0.5 mL of lysis buffer (10 mM Tris, 1 mM EDTA, 0.5% Triton X-100, pH 8.0) with 200 µg/mL Proteinase K, and incubated at 56º C for 1 h, followed by 10 min of incubation at 100º C for Proteinase K inactivation. After cellular lysis, 500 µL of tris-buffered phenol (pH 8.0) was added for extraction, tubes were mixed in vortex for 1 min and centrifuged at 12,000 g for 5 min. This procedure was repeated once. After elimination of the organic phase (lower), 500 µL of tris-buffered (pH 8.0) phenol/chloroform/isoamyl alcohol 25:24:1 was added and tubes were mixed in a vortex for 1 min. and centrifuged at 12,000 g for 5 min. The aqueous (upper) phase was transferred to a new tube and 50 µL of 3M sodium acetate and 1 mL of ethanol PA was added, before the tubes were incubated at -20° C for 16 h for DNA precipitation. Tubes were then centrifuged at 12,000 g for 30 min, the pellet was washed with 1 mL of 70% ice cold ethanol, and the tubes were centrifuged again for 15 min at 12,000 g. Tubes were left to air dry and 200 µL of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) was added to resuspend the DNA; samples were stored at -20º C until analysis17.
POLYMERASE CHAIN REACTION (PCR) FOR M. pulmonis
M. pulmonis DNA amplification was performed with 5 µL of purified samples in 25 µL reactions, containing 20 µM of each primer: sense (5'-AGCGTTTGCTTCACTTTGAA-3') and antisense (5'-GGGCATTTCCTCCCTAAGCT-3')21, 3.0 µM MgCl2, 200 µM of each dNTP (dATP, dCTP, dGTP and dTTP) with 1.0 U of Taq DNA Polymerase in the reaction buffer supplied by the manufacturer (Invitrogen, Carlsbad, California, USA). The reaction involved an initial denaturation step for 5 min at 95º C followed by 30 amplification cycles for 1 min at 95º C, 1 min at 53º C and 1 min at 72º C, with a final extension step for 7 min at 72º C. The PCR products (266 bp) were detected by 1% agarose gel electrophoresis stained with ethidium bromide under UV light22. As a negative control, H2O was used and the UABCTIP M. pulmonis strain was used as a positive control (Institute Pasteur, Paris, France).
EVALUATION OF THE INFLAMMATORY RESPONSE
- Haematological analysis: platelet, red blood cell (RBCs) and white blood cell (WBCs) counts were performed in the samples obtained with EDTA, immediately after collection. Analyses were performed with a Cell Dyn 1400 device (Abbott, USA). For differential white blood cell counts, a blood smear stained by the Giemsa method was made and observed under an optical microscope.
- CRP and A1GP: serum levels of CRP and A1GP were determined in the serum samples obtained from blood collected without EDTA. They were quantified by immunoturbidimetry using commercial kits (BioTécnica, Varginha, Minas Gerais, Brazil), according to the manufacturer's instructions, using a Siemens ADVIA 1650 automated device (Siemens AG, Germany).
Statistical analysis: statistical analysis was performed using the unpaired t-test with Welsh's correction, with the aid of the software GraphPad Prism(r) (La Jolla, California, USA); one-tailed P values < 0.05 were considered to be significant (95% CI).
RESULTS
Among the studied sample, 16/20 (80%) were positive for M. pulmonis, either by culture or PCR. Samples were considered negative only with a concordance of both methods. Two samples were negative by PCR but positive by culture, and one was negative by culture and positive by PCR. Many samples presented contamination in the culture, making it impossible to identify mycoplasma growth, but were positive by PCR (13/20). Table 1 presents the CRP and A1GP results of each sample, being positive or negative for mycoplasma.
Table 1 - CRP and A1GP results of the samples obtained from rats with (pos) or without (neg) M. pulmonis infection
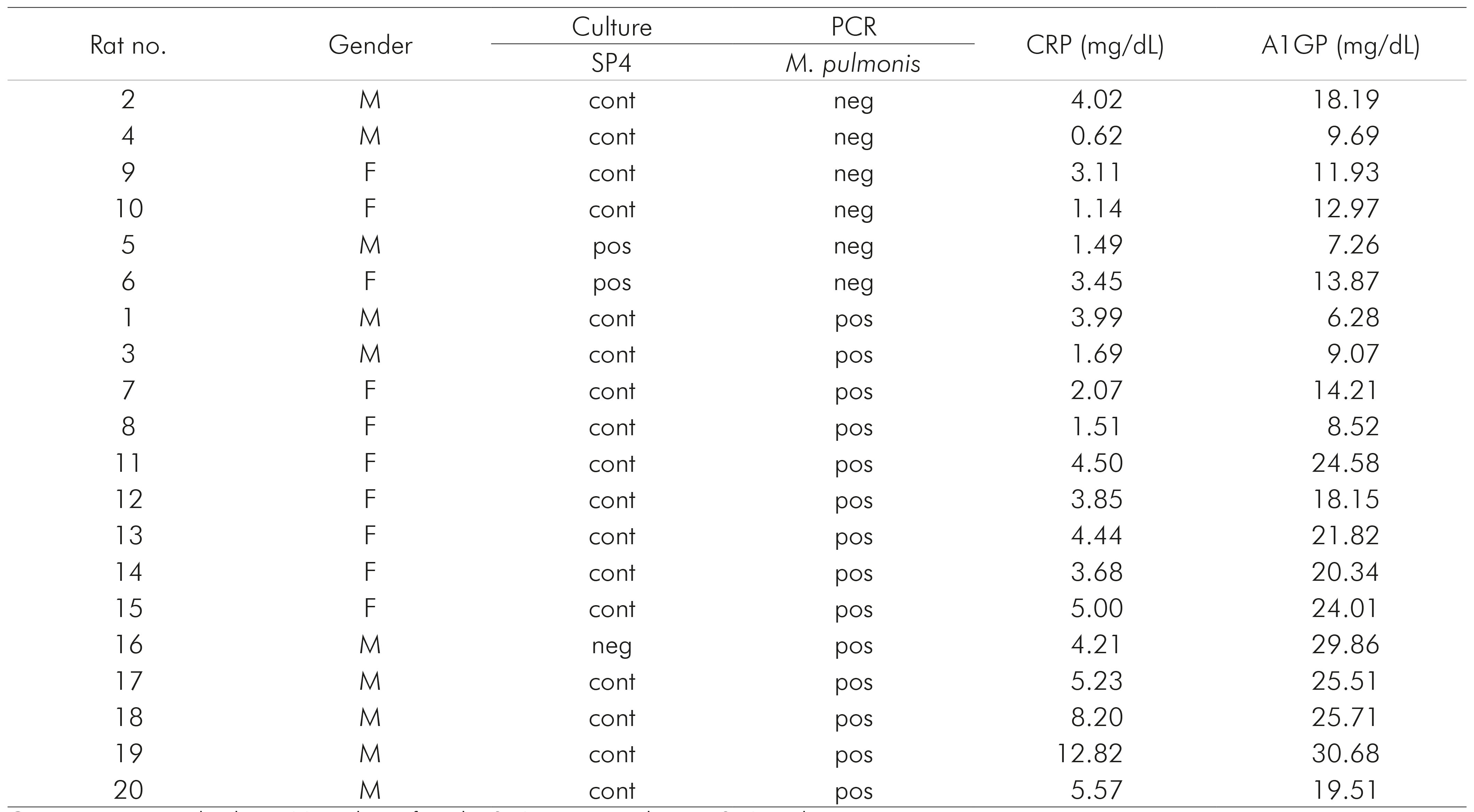
Cont: contaminated culture; M: male; F: female; SP4: positive culture in SP4 medium.
CRP results were significantly higher (P = 0.0330), in M. pulmonis infected rats (mean = 4.48, SD = 0.70) than in those without mycoplasma infection (mean = 2.22, SD = 0.80). The same happened to A1GP (P = 0.0321), with a mean of 18.71 (SD = 2.005) in M. pulmonis infected rats and a mean of 13.20 (SD = 1.800) in rats without mycoplasma (Figure 1).
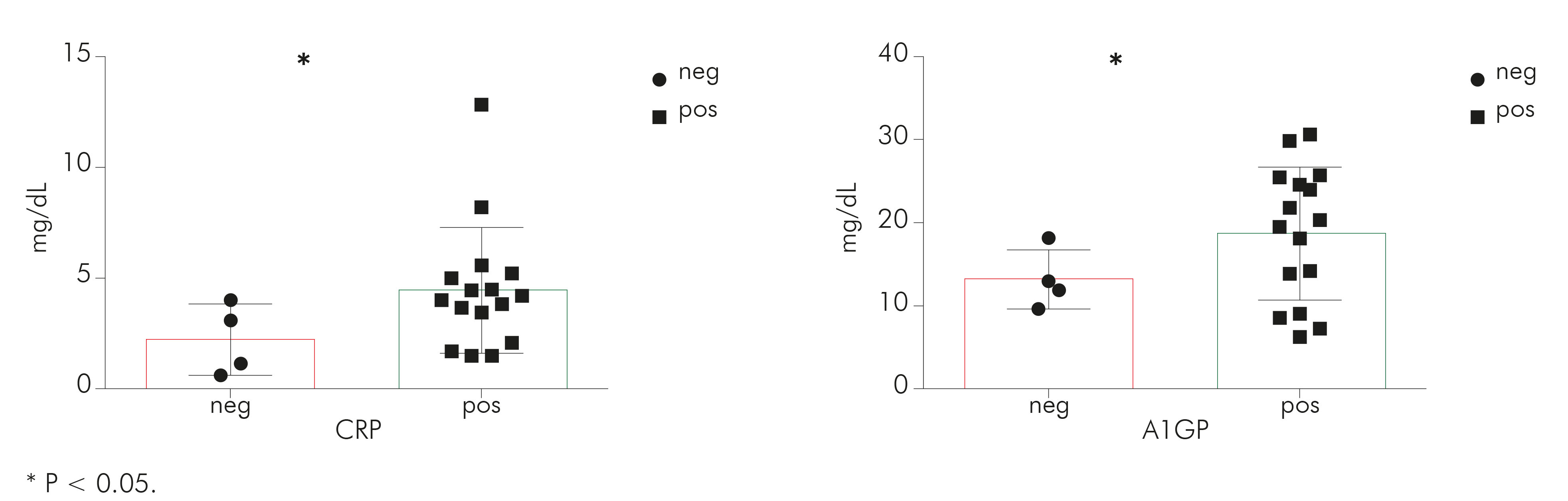
Figure 1 - Graphical representation of CRP and A1GP results of the samples obtained from rats with (pos) or without (neg) M. pulmonis infection
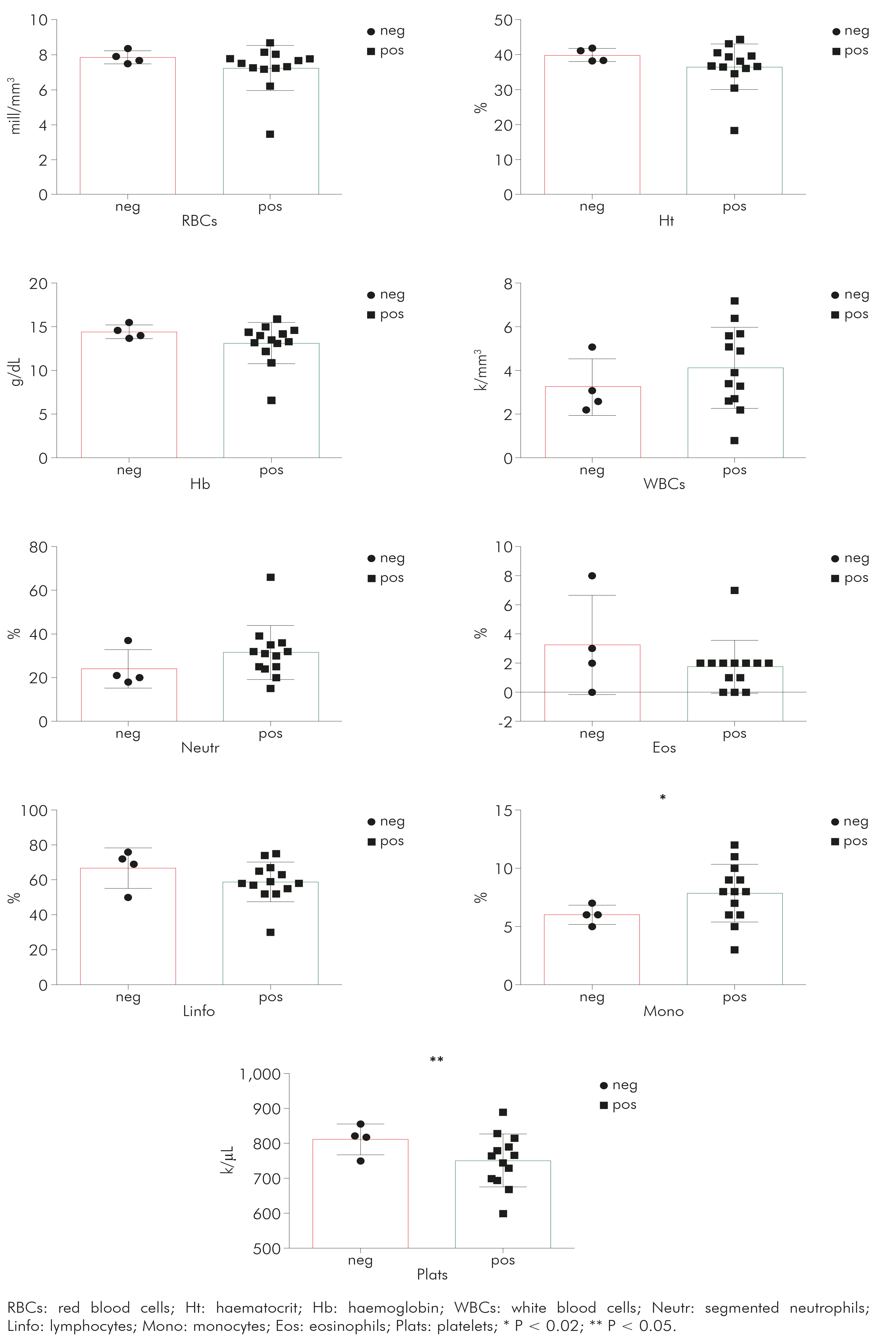
In the same manner, haematological tests provided additional evidence of inflammation in mycoplasma-infected animals. These results are summarised in table 2. Three samples of mycoplasma-infected rats had inadequate EDTA blood samples for haematological analysis.
Table 2 Haematological parameters [median (SD)] in the blood samples obtained from rats with (pos) or without (neg) M. pulmonis infection
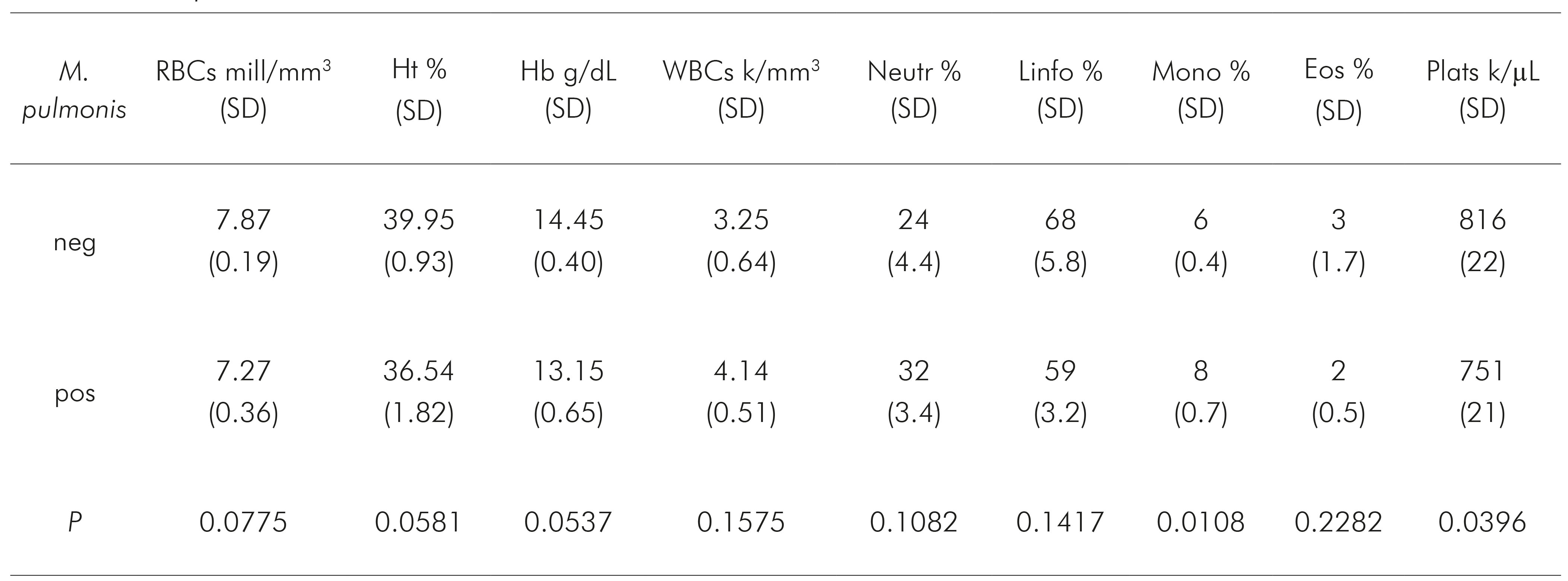
RBCs: red blood cells; Ht: haematocrit; Hb: haemoglobin; WBCs: white blood cells; Neutr: segmented neutrophils; Linfo: lymphocytes; Mono: monocytes; Eos: eosinophils; Plats: platelets.
It was possible to observe (Figure 2) that monocytes (P = 0.0180) presented higher and platelets (P = 0.0396) had lower levels in mycoplasma-infected rats than in those non-infected animals. For RBCs (P = 0.0775), Ht (P = 0.0581) and Hb (P = 0.0537) showed borderline results, demonstrating a tendency towards anaemia in the infected group, which is compatible with chronic infection. For the other parameters, there was no significant difference (Figure 2).
DISCUSSION
As previously mentioned, mycoplasma infection in laboratory animals may cause important morbidity and mortality, especially in those used for long duration studies, or in those with a subclinical infection that may be complicated by experimental procedures2. However, the most important impact might be its interference in interpreting experimental results. For instance, if a studied group presents a higher level of inflammation or immune system activation, one may not be certain whether this finding is due to the intervention being studied, or the eventual presence of mollicute infections. On the other hand, control groups, if also infected, may bring an important bias to the result.
In this direction, the aim of this study was to evaluate the level and effect of M. pulmonis infection in rats bred by the animal facility of the University of Blumenau, and discuss the impact of the use of these animals in scientific experimentation.
It was observed a level of 80% of M. pulmonis infection. Around the world, similar rates have been found as well, for instance in Taiwan, with a 40% M. pulmonis infection rate23. In Western Europe facilities, there were better sanitary conditions than might be expected, in which rates can be as low as 3%24,25. The level of M. pulmonis infection found in the animal facility of our institution makes the use of these animals in experimental models that evaluate immunity and/or infections by other microorganisms simply impossible.
With this study, we not only had information about the level of mycoplasma infection in laboratory rodents in our reality, which was not available before, but also observed a significant activation of the inflammatory response, and therefore an immune modulation, in the infected animals group. This information is not only important to researches that might be using this kind of animal for experimentation, but may also serve as an alert for researchers and those responsible for animal breeding to check the practices and quality of the facility, in case it was not available, as in ours. This is essential to assure the supply of high quality rodents for research.
This high prevalence of M. pulmonis in rats bred at one of the most important facilities of our State constitutes a severe issue, requiring prophylactic and preventive measures to be applied. The use of molecular methods to monitor facilities may constitute an expressive advantage, but, as it was observed, some samples may present inhibitory substances in the DNA sample, being positive only by culture, in spite of the fact that the later method might be more subject to contamination. It is also worth noticing that the inflammatory parameters evaluated are acute-phase proteins, thus, they indicate the presence of an inflammatory process in progress in respiratory tract. One should also consider that the observed results may not be exclusively due to M. pulmonis infection, but also to other recognized respiratory agents such as the cilia-associated respiratory bacillus, a frequent pathogen co-infecting rats along with M. pulmonis. Evaluations of an animal facility for experimental purposes should include screening for these organisms as well.
CONCLUSION
It is considered that, before developing experimentations evaluating the immune modulation or effects of other infectious diseases models, researchers must verify the conditions of the animal facility to offer the required animals, if this is not automatically provided. More than that, it would be advisable that the animal facility could ship a certificate of quality along with the animals, indicating the management type applied to breeding, and every sanitary test executed. As a further step, it was observed that an organised and directed effort for the accreditation of facilities in every country would be of great interest for the scientific community, for example, through the Association for Assessment and Accreditation of Laboratory Animal Care International, the Federation of Laboratory Animal Science Associations, among other entities.