INTRODUCTION
In the Brazilian backlands, many rural communities maintain their traditional lifestyle. The Chapada das Mesas National Park is an environmental protection area in Maranhão State, within the Cerrado biome1, and families residing around the Park maintain their cultural traditions as defined by the National Policy for Sustainable Development of Traditional Peoples and Communities2,3. The communities surrounding the Park are characterized by the sustainable use of natural resources and by maintaining their cultural traditions through their customs and beliefs4. These populations practice subsistence agriculture, characterized by family gardens of beans, corn, cassava, and bananas, and palm fruit extraction, such as buriti (Mauritia flexuosa) and bacuri (Platonia insignis)5. They also raise animals, such as chickens and pigs. Nevertheless, this population has very few sources of income, and 75% of the families survive with less than the Brazilian minimum wage, with a large proportion of them benefiting from governmental income transfer programs, which characterizes an economic poverty scenario5. The sanitation systems are very incipient6, and waste management has been a challenge, which favors the spread of parasitic diseases. For many reasons, open defecation is still practiced6, leading to significant environmental contamination with the infective stages of intestinal parasites. The transmission of waterborne diseases in these communities is also favored by the consumption of water collected directly from rivers, not subjected to any treatment or purification system6.
Intestinal parasitism encompasses a set of infections caused by distinct organisms, which can be helminths or protozoa7. Among gut parasites, soil-transmitted helminths (STH) spend a phase of their life cycle in the soil, which is mandatory for the maturation of the infective stages. These parasites exist in the soil as rhabditoid and filariform larvae for hookworms (Necator americanus and Ancylostoma duodenale) and embryonated eggs for Ascaris lumbricoides and Trichuris trichiura8. These organisms, therefore, have different transmission routes, being percutaneous for hookworms and oral for A. lumbricoides and T. trichiura, leading to different dissemination dynamics. Parasitic gut protozoa are transmitted orally through the ingestion of infective cysts. Among them, Giardia duodenalis and Entamoeba histolytica are the most important due to their pathogenic potential, including malabsorption, diarrhea, and dysentery9,10. Some protozoa are considered human gut commensals, such as Entamoeba coli, Endolimax nana, and Iodamoeba bütschlii11. Percutaneous transmission organisms may have a more spatially restricted cycle in the peri-domestic environment, associated with soil contamination caused by local open defecation and poor sanitation12. Orally transmitted parasites may be disseminated over longer distances by contaminated water and food due to the resistance and longevity of their eggs and cysts.
Control strategies have shaped the etiological profile of intestinal parasitism in recent decades. Mass drug administration (MDA) with anthelmintics has reduced the prevalence and parasitic load of STH, leading to an increase in the participation of protozoa in the species composition. This shift occurs because anthelmintic drugs, such as 400 mg single-dose albendazole, do not target protozoa13,14. Additionally, standard Kato-Katz smears routinely utilized in population surveys are unable to detect the cysts of gut parasitic protozoa, further exacerbating the neglect of these infections.
Maranhão State, in northeastern Brazil, has one of the highest prevalence of STH in the country, with 15.8% for hookworm, 17.5% for A. lumbricoides, and 5.8% for T. trichiura in children aged 7 to 17 years15. Hookworm infection and ascariasis presented prevalence rates of 14.3% and 9.3%, respectively, in studies conducted with school-aged children living in peri-urban communities in Maranhão16. These data suggest that rural and peri-urban communities in the State are particularly vulnerable to intestinal parasitism. Previous studies carried out in communities around the Chapada das Mesas National Park4,5,6 motivated the investigation of the epidemiological aspects of intestinal parasitism in the region to describe the socio-ecological determinants of this group of infections. The etiological profile of gut parasitic infections and the parasite load of STH are important for planning improvements in control strategies. The present study aimed to estimate prevalence and describe socio-ecological factors associated with intestinal parasitism in rural and peri-urban communities surrounding an environmental protection area in Maranhão State, Brazil.
MATERIALS AND METHODS
SETTING
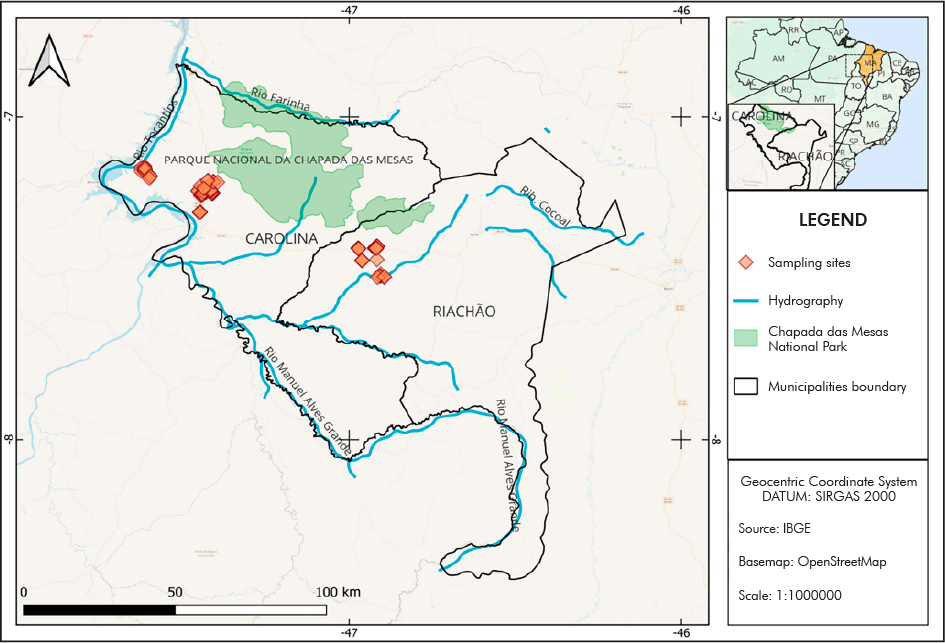
The study was carried out in two traditional communities living around the Chapada das Mesas National Park, established by law in 20051: the community of Canto Grande (population of 1,544 inhabitants; located at 7º06'30"S/48º13'33"W), in Carolina City; and the community of Alto Bonito (population of 2,148 inhabitants; located at 7º23'30"S/46º37'21"W), in Riachão City. Canto Grande is predominantly composed of rural communities, and Alto Bonito, of peri-urban neighborhoods (Figure 1).
STUDY DESIGN, SAMPLING STRATEGY, AND STATISTICAL ANALYSES
A cross-sectional survey was conducted in July 2022. Among the 335 subjects visited in Canto Grande, 262 (78.2%) returned a fecal sample. In Alto Bonito, from 287 people invited to participate in the study, 207 (72.1%) returned a fecal sample. In both communities, socio-ecological and demographic data were obtained through interviews carried out by researchers during home visits. These data included income, sanitation and housing characteristics, practice of open defecation, and source of drinking water. Residents were selected with the support of Community Health Agents (CHA). The sample size followed the parameters: margin of error of 5%, confidence level of 95%, and expected frequency of 20% for infection with gut parasites.
The results were presented as frequencies of infection by different parasite species, which were compared using prevalence ratios (PR) and respective 95% confidence intervals (CI). Statistical significance was assessed using Fisher's test. The houses visited were georeferenced using GPS, and QGIS17 was used for spatial analysis. The base map was acquired from IBGE (Brazilian Institute of Geography and Statistics), and the coordinates of the sampling sites were recorded in the SAD 69 datum geodetic coordinate system. For exploration and modeling, the maps were analyzed for spatial point patterns using the kernel method. A 2,000 m search radius was selected, and raster output maps were generated with a pixel size of 10 x 10 m. Density values on the kernel density map were scaled to visually represent individual kernels.
PARASITOLOGICAL ANALYSES
The Lutz technique was used to detect helminth eggs and protozoan cysts. For STH quantitative analysis, the Kato-Katz technique was used (Helm Test® kit, LabHouse, Belo Horizonte, Minas Gerais, Brazil). Infection intensity was determined as follows: (i) hookworm infection - light, with a parasitic load of ≤ 1,999 eggs per gram of feces (epg); moderate, with a load between 2,000 and 3,999 epg; and heavy, with ≥ 4,000 epg; and (ii) ascariasis - light, ranged of 0 to 4,999 epg; moderate, from 5,000 to 49,999 epg; and heavy, with ≥ 50,000 epg18.
ETHICS
This study received approval from the Ethics Committee for Research with Humans at the Oswaldo Cruz Institute, Oswaldo Cruz Foundation (IOC/Fiocruz), under CAAE No. 40483120.7.0000.5248 (23 December 2020), and authorization from the Chico Mendes Institute for Biodiversity Conservation (SISBIO/ICMBio), under No. 79178-1 (9 June 2022).
RESULTS
Among the 469 residents studied in the two communities, 42.6% were classified as living in poverty (family income less than the minimum wage), 31.6% practiced open defecation, 16.2% lived in houses with uncoated clay floors, and 4.1% drank water directly from the river (Table 1).
Table 1 - Prevalence of soil-transmitted helminthiases in different groups defined by sociodemographic variables in Canto Grande and Alto Bonito communities, Chapada das Mesas National Park, Maranhão State, Brazil, 2022
| Variables | Total samples | Hookworm infection | Ascariasis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | PR (95% CI) | p-value | N | % | PR (95% CI) | p-value | |
| Community | ||||||||||
| Alto Bonito | 207 | 44.1 | 5 | 2.4 | 1 | 6 | 2.9 | 1 | ||
| Canto Grande | 262 | 55.9 | 54 | 20.6 | 8.56 (3.49-21.02) | < 0.001 | 1 | 0.4 | 0.13 (0.02-1.08) | 0.047 |
| Age group | ||||||||||
| < 10 years | 85 | 18.1 | 9 | 10.6 | 1 | 1 | 1.2 | 1 | ||
| 10 - 17 years | 54 | 11.5 | 10 | 18.5 | 1.72 (0.75-3.97) | 0.213 | 4 | 7.4 | 6.29 (0.72-54.84) | 0.074 |
| ≥ 18 years | 330 | 70.4 | 40 | 12.1 | 1.13 (0.57-2.23) | 0.850 | 2 | 0.6 | 0.51 (0.04-5.61) | 0.498 |
| Sex | ||||||||||
| Female | 243 | 51.8 | 22 | 9.1 | 1 | - | - | 1 | ||
| Male | 226 | 48.2 | 37 | 16.4 | 1.80 (1.09-2.95) | 0.025 | 7 | 3.1 | Undefined | 0.005 |
| Income (R$) | ||||||||||
| > 1,300 BRL | 269 | 57.4 | 27 | 10.0 | 1 | 3 | 1.1 | 1 | ||
| ≤ 1,300 BRL | 200 | 42.6 | 32 | 16.0 | 1.75 (1.08-2.83) | 0.023 | 4 | 2.0 | 1.80 (0.40-7.99) | 0.464 |
| Defecation site | ||||||||||
| Latrine | 321 | 68.4 | 13 | 4.0 | 1 | 1 | 0.3 | 1 | ||
| Open (bushes) | 148 | 31.6 | 46 | 31.1 | 7.65 (4.26-13.71) | < 0.001 | 6 | 4.1 | 13.01 (1.58-107.13) | 0.004 |
| House floor | ||||||||||
| Coated | 393 | 83.8 | 29 | 7.4 | 1 | 2 | 0.5 | 1 | ||
| Clay | 76 | 16.2 | 30 | 39.5 | 5.33 (3.41-8.34) | < 0.001 | 5 | 6.6 | 12.92 (2.55-65.41) | < 0.001 |
| Drinking water source | ||||||||||
| Piped | 397 | 84.6 | 57 | 14.4 | 1 | 7 | 1.8 | 1 | ||
| Rudimentary well | 36 | 7.7 | - | - | Undefined | 0.008 | - | - | Undefined | 1.000 |
| Water truck | 17 | 3.6 | - | - | Undefined | 0.143 | - | - | Undefined | 1.000 |
| Direct from river | 19 | 4.1 | 2 | 10.5 | 0.73 (0.19-2.77) | 1.000 | - | - | Undefined | 1.000 |
Conventional sign used: - Data equal to zero, not resulting from rounding; PR: Prevalence ratio; 95% CI: Confidence intervals.
Differences were identified in the etiological profile of intestinal parasitism in the two communities. The hookworm infection prevalence was 20.6% in Canto Grande and 2.4% in Alto Bonito (PR=8.56; 95% CI=3.49-21.02; p < 0.001). The ascariasis prevalence in Canto Grande and Alto Bonito was 0.4% and 2.9%, respectively (PR=0.13; CI 95%=0.02-1.08; p=0.047). Infections with gut protozoa were more frequent in Alto Bonito, and statistically significant differences were observed for Entamoeba coli and E. nana. The factors associated with STH infections are also shown in table 1. Specifically, hookworm infection was associated with the male sex, open defecation, clay floors in homes, and lower monthly family income. Ascariasis was also associated with open defecation. Concerning intestinal protozoa, the ingestion of water directly from a river was associated with G. duodenalis infection (Table 2).
Table 2 - Prevalence of intestinal protozoan infections in different groups defined by sociodemographic variables in Canto Grande and Alto Bonito communities, Chapada das Mesas National Park, Maranhão State, Brazil, 2022
| Variables | Total samples | Giardia duodenalis | Entamoeba histolytica/dispar | Entamoeba coli | Endolimax nana | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | PR (95% CI) | p-value | N | % | PR (95% CI) | p-value | N | % | PR (95% CI) | p-value | N | % | PR (95% CI) | p-value | |
| Community | ||||||||||||||||||
| Alto Bonito | 207 | 44.1 | 19 | 9.2 | 1 | 7 | 3.4 | 1 | 30 | 11.5 | 1 | 60 | 29.0 | 1 | ||||
| Canto Grande | 262 | 55.9 | 13 | 5.0 | 0.54 (0.27-1.07) | 0.096 | 4 | 1.5 | 0.45 (0.13-1.52) | 0.156 | 49 | 23.7 | 0.48 (0.32-0.73) | < 0.001 | 28 | 10.7 | 0.37 (0.24-0.55) | < 0.001 |
| Age group | ||||||||||||||||||
| < 10 years | 85 | 18.1 | 9 | 10.6 | 1 | 2 | 2.4 | 1 | 18 | 21.2 | 1 | 16 | 18.8 | 1 | ||||
| 10-17 years | 54 | 11.5 | 5 | 9.3 | 0.87 (0.30-2.47) | 1.000 | - | - | Undefined | 0.512 | 8 | 14.8 | 0.69 (0.32-1.49) | 0.381 | 14 | 25.9 | 1.38 (0.73-7.58) | 0.398 |
| ≥ 18 years | 330 | 70.4 | 18 | 5.5 | 0.51 (0.24-1.11) | 0.134 | 9 | 2.7 | 1.16 (0.25-5.26) | 1.000 | 53 | 16.1 | 0.75 (0.47-1.22) | 0.262 | 58 | 17.6 | 0.93 (0.56-1.54) | 0.753 |
| Sex | ||||||||||||||||||
| Female | 243 | 51.8 | 14 | 5.8 | 1 | 5 | 2.1 | 1 | 44 | 18.1 | 1 | 51 | 21.0 | 1 | ||||
| Male | 226 | 48.2 | 18 | 8.0 | 1.38 (0.70-2.71) | 0.365 | 6 | 2.7 | 1.29 (0.39-4.16) | 0.765 | 35 | 15.5 | 0.85 (0.57-1.28) | 0.461 | 37 | 16.4 | 0.78 (0.53-1.14) | 0.236 |
| Income (R$) | ||||||||||||||||||
| ≤ 1,300 BRL | 200 | 42.6 | 15 | 7.5 | 1 | 2 | 1 | 1 | 33 | 16.6 | 1 | 38 | 19.1 | 1 | ||||
| > 1,300 BRL | 269 | 57.4 | 17 | 6.3 | 0.84 (0.43-1.64) | 0.711 | 9 | 3.3 | 3.34 (0.73-15.31) | 0.126 | 46 | 17.0 | 1.03 (0.68-1.55) | 0.901 | 50 | 18.5 | 0.97 (0.66-1.43) | 0.905 |
| Defecation site | ||||||||||||||||||
| Latrine | 321 | 68.4 | 21 | 6.5 | 1 | 11 | 3.4 | 1 | 54 | 16.8 | 1 | 61 | 19.0 | 1 | ||||
| Open (bushes) | 148 | 31.6 | 11 | 7.4 | 1.13 (0.56-2.29) | 0.698 | - | - | Undefined | 0.020 | 25 | 16.9 | 1.00 (0.92-1.09) | 0.541 | 27 | 18.2 | 0.99 (0.90-1.09) | 0.476 |
| House floor | ||||||||||||||||||
| Coated | 393 | 83.8 | 24 | 6.1 | 1 | 11 | 2.8 | 1 | 63 | 16.0 | 1 | 71 | 18.1 | 1 | ||||
| Clay | 76 | 16.2 | 8 | 10.5 | 1.72 (0.80-3.69) | 0.209 | - | - | Undefined | 0.139 | 16 | 21.1 | 1.31 (0.80-2.14) | 0.314 | 17 | 22.4 | 1.23 (0.77-1.97) | 0.421 |
| Drinking water source | ||||||||||||||||||
| Piped | 397 | 84.6 | 21 | 5.3 | 1 | 9 | 2.3 | 1 | 65 | 16.4 | 1 | 76 | 19.1 | 1 | ||||
| Rudimentary well | 36 | 7.7 | 4 | 11.1 | 1.99 (0.72-5.99) | 0.259 | 2 | 5.6 | 2.32 (0.52-10.35) | 0.248 | 9 | 25.0 | 1.44 (0.78-2.67) | 0.259 | 4 | 11.1 | 0.55 (0.22-1.42) | 0.271 |
| Water truck | 17 | 3.6 | 1 | 5.9 | 1.11 (0.16-7.79) | 0.612 | - | - | Undefined | 1.000 | 1 | 5.9 | 0.36 (0.05-2.43) | 0.494 | 1 | 5.9 | 0.30 (0.04-2.07) | 0.217 |
| Direct from river | 19 | 4.1 | 6 | 31.6 | 5.97 (2.73-13.0) | 0.001 | - | - | Undefined | 1.000 | 4 | 21.1 | 1.28 (0.52-3.15) | 0.535 | 7 | 36.8 | 1.92 (1.03-3.58) | 0.075 |
Conventional sign used: - Data equal to zero, not resulting from rounding; PR: Prevalence ratio; 95% CI: Confidence intervals.
Of 59 hookworm-positive subjects, 50 had their infection intensity assessed by Kato-Katz smears. The parasitic loads detected ranged from 24 epg to 16,728 epg, with an average of 1,304 epg (standard deviation = 417 epg). It was observed that 86.0% (n = 43) of hookworm-positive subjects had light, 4.0% (n = 2) had moderate, and 10.0% (n = 5) had heavy hookworm infection intensity (Figure 2). From seven A. lumbricoides infections identified, the parasitic load of three could be determined with two moderate and one light infection detected.
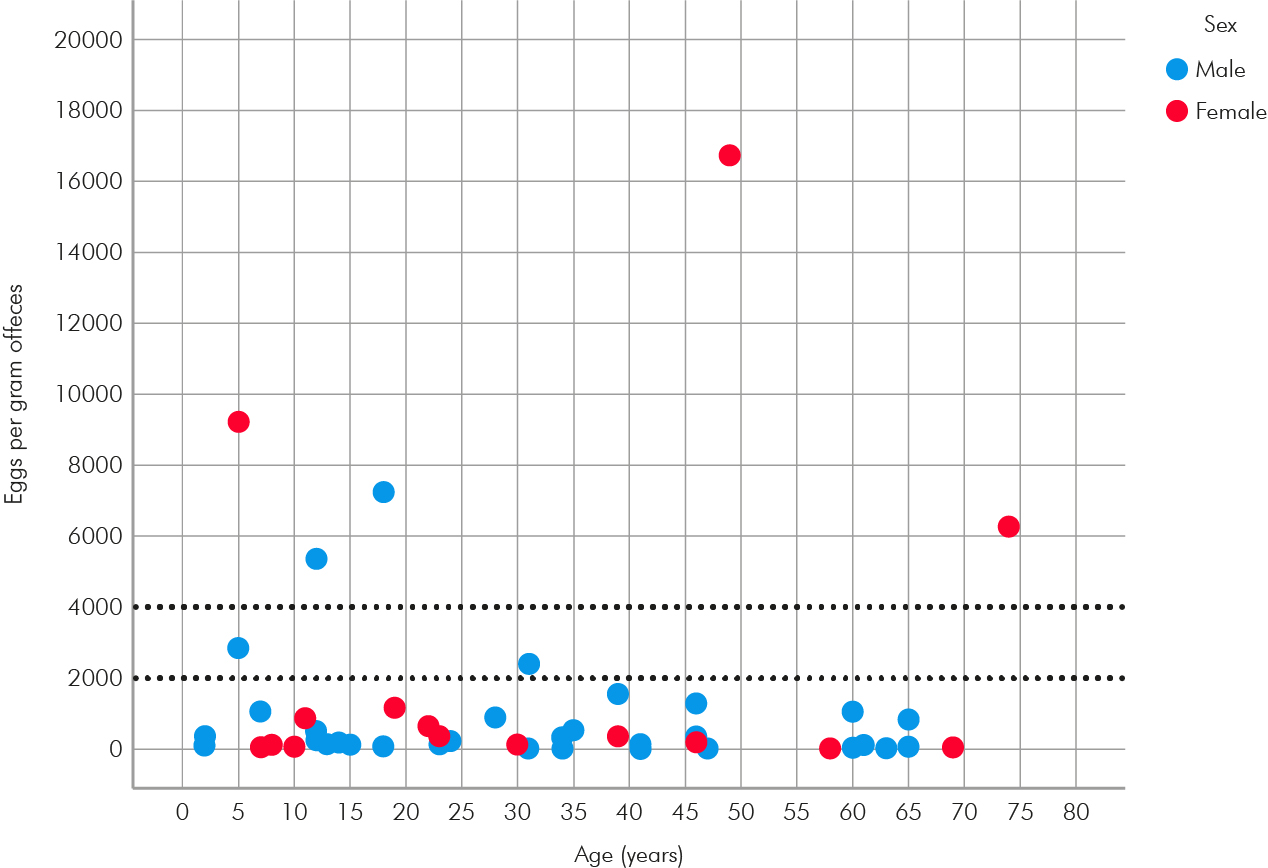
Dotted lines delimit heavy (≥ 4,000 epg), moderate (2,000 to 3,999 epg), and light (≤ 1,999 epg) parasitic loads.
Figure 2 - Scatter diagram demonstrating the parasite loads of hookworm infections in relation to age and sex of infected residents in Canto Grande and Alto Bonito communities, Chapada das Mesas National Park, Maranhão State, Brazil, 2022.
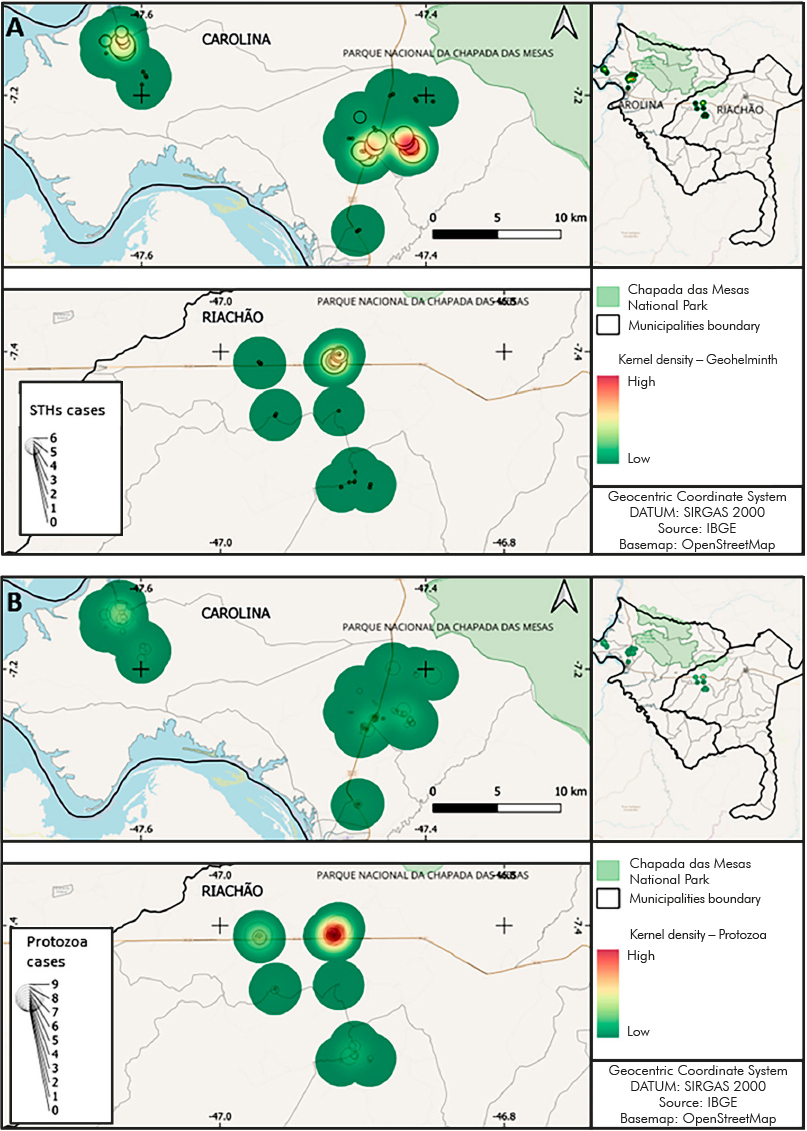
Figure 3 shows the spatial distribution of intestinal parasites in the studied communities. It was observed that the STH hotspots were in the Canto Grande (Carolina) community, while the protozoa hotspots were concentrated in Alto Bonito (Riachão).
DISCUSSION
The present study assessed the epidemiology of intestinal parasitism in rural communities living around an environmental protection area of Maranhão State, northeastern Brazil. As assessed by the socio-ecological data survey, almost half of the population lives in poverty and almost 1/3 practices open defecation, which characterizes a favorable scenario to the spread of parasitic diseases. The main finding is the persistence of STH transmission foci, mainly hookworms, significantly associated with open defecation in the peri-domestic environment. Therefore, differences in the vulnerability to intestinal parasitism could be observed on the micro-regional scale. Studies in traditional communities carried out in other countries have also demonstrated the vulnerability of these populations to intestinal parasitic infections and increased risk in demographic concentration and poverty situations19,20. The prevalence of hookworm infection was significantly higher in the Canto Grande community, which is predominantly rural and, as observed in fieldwork, has poorer sanitation conditions. On the other hand, ascariasis and giardiasis were significantly more frequent in Alto Bonito, which points to different transmission dynamics for parasites transmitted percutaneously and orally.
The transmission of hookworms is intrinsically related to soil contamination in peri-domestic environments with fecal matter. Previous studies conducted in the communities of Chapada das Mesas National Park demonstrated that most people practice open defecation6. It can be inferred that low sanitation, associated with poverty, allows the maintenance of hookworm transmission in the region. The association between open defecation and STH has been demonstrated in several regions, including Ethiopia21,22, India23, Mozambique24, and Central America25. Effective strategies to reduce the proportion of the population that practices open defecation are a major challenge in the region, where people maintain ancestral habits regarding how they live and interact with the environment. In this sense, the study data, which have a micro-regional scope, can be extrapolated to different communities in similar socio-ecological contexts and where open defecation is still practiced. Solutions to interrupt the transmission of intestinal parasites in traditional rural communities require more comprehensive approaches, focusing on health education, awareness of the relationship with the environment, and more efficient intervention strategies in the context of primary health care. The development of latrines adapted to rural communities in northeastern Brazil to improve sanitation and reduce environmental contamination with fecal matter remains a difficult task26,27,28,29,30.
In the present study, although most of the STH infections were of light intensity, individuals with moderate and heavy parasitic loads of hookworms were identified, which demonstrates that these nematodes are a health problem in these communities and a potential cause of iron-deficiency anemia. It is important to note that the single-dose albendazole mass treatment strategy implemented in Brazil targets school-aged children, so infections in adults and preschoolers may feed the cycles of STH transmission in the studied communities.
The factors associated with hookworm infection were not related to infections by intestinal protozoa. Thus, a more uniform distribution was observed for these organisms, and the water consumption directly from a river was significantly associated with giardiasis. Previous studies in these communities have shown that the majority of residents of the National Park drink water collected directly from rivers, which are vulnerable to pollution from garbage and solid waste, in a scenario conducive to the transmission of waterborne diseases. Improving the management of drinking water supplies and food hygiene can influence intestinal protozoa transmission in the region. These organisms, which are not the target of any specific policy, have not been detected due to the unreliability of the most common diagnostic techniques and are more difficult to treat since single-dose drugs, such as secnidazole, are not yet universally available. Chronic giardiasis, apparently asymptomatic and thus often undiagnosed, impacts the physical development of children given its association with protein-caloric malnutrition31,32. Data from this study reinforces that intestinal parasitism should be considered a heterogeneous group of infections and that the simplification of the phenomenon, which tends to contribute to the development of unified control strategies, should be avoided.
CONCLUSION
The present study concludes that STH and intestinal protozoa persist in traditional communities in Brazil, where improving sanitation systems is a challenge. Traditional ways of living and interacting with the environment can be associated with the transmission of parasitic diseases perpetuation in a poverty scenario, lack of health education, absence of health promotion programs, and inefficient primary health care systems.