Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Paraense de Medicina
versão impressa ISSN 0101-5907
Rev. Para. Med. v.21 n.4 Belém dez. 2007
Evaluation of APRI test as a liver fibrosis marker1
Avaliação do modelo APRI como marcador de fibrose hepática1
Ivanete do Socorro Abraçado AmaralI; Marina Pinto Franco DiasII; Clariana Casali Rodrigues FernandesII; Lizomar de Jesus Maués Pereira MoiaIII; Esther Castello Branco Mello MirandaI; Sâmia DemachkiIII
IHepathologist and Professor of Adults' Health (General Clinics) of State University of Pará
IIMedicine 5th-year students of State University of Pará
IIIHepathologist of Pathological Anatomy Departament of Federal University of Pará
SUMMARY
OBJECTIVE: to evaluate the performance of APRI test as an indirect marker of liver fibrosis in patients of FSCMPA with chronic viral hepatitis.
METHODS: laboratorial and histopathological (METAVIR) data of 102 patients were collected with the aim of comparing biopsy reports to test results, considering: F0-F1 as absent/ insignificant fibrosis and F2-F4 as presence of expressive fibrosis; F0-F3 and F4 as absence and presence of cirrhosis, respectively. The evaluation was based on the use of simultaneous cut-off points suggested by Wai et al. (2003) and also the development of single cut-off points, through ROC curves, for this sample.
RESULTS: the specificities found through the original cut-off points were 96% and 87% for significant fibrosis and cirrhosis, respectively. A superior performance was detected for the diagnosis of expressive fibrosis (PPV 90%) and for the exclusion of liver cirrhosis (NPV 95%). The only advantages accomplished with the use of the single cutoff points defined for this study were the classification of all patients and an increase in sensibility, which do not justify their applicability.
CONCLUSION: APRI test is able to confirm the existence of significant fibrosis and exclude cirrhosis, what makes it available, as an alternative, in clinical practice.
KEYWORDS: APRI, liver fibrosis, evaluation.
RESUMO
OBJETIVO: avaliar o desempenho do teste APRI como marcador indireto de fibrose hepática em pacientes portadores de hepatites virais crônicas da FSCMPA.
MÉTODO: foram avaliados dados laboratoriais e histopatológicos (METAVIR) de 102 pacientes a fim de comparar os resultados da biópsia com o cálculo do teste. Considerou-se: F0-F1 e F2-F4 como fibrose ausente/inexpressiva e presença de fibrose expressiva, respectivamente; F0-F3 e F4 como ausência e presença de cirrose, respectivamente. Utilizou-se os pontos de corte simultâneos sugeridos por Wai et al. (2003) e desenvolveu-se pontos de corte únicos, através de curvas ROC, para esta amostra.
RESULTADOS: as especificidades encontradas com os cortes originais foram, para fibrose significativa e cirrose, 96% e 87%, respectivamente. Evidenciou-se melhor desempenho ao confirmar diagnóstico de fibrose expressiva (VPP 90%) e em excluir cirrose hepática (VPN 95%). As únicas vantagens obtidas com os pontos definidos neste estudo foram a classificação de 100% dos pacientes e aumento da sensibilidade, o que não justifica sua aplicabilidade.
CONCLUSÃO: o teste APRI é capaz de afirmar a existência de fibrose importante e de excluir o diagnóstico de cirrose, o que viabiliza seu uso, como alternativa, na prática clínica.
DESCRITORES: APRI, fibrose hepática, avaliação.
INTRODUCTION
Liver fibrosis is a pathological process due to persistent and/or frequent hepatic damage. It is the result of the inflammatory response or the direct toxic action of a certain nocive agent, with posterior parenchymal regeneration1,2.
The fibrosis degree is related, usually, to the pathology severity. Hence, its evaluation is essential for proper patients' management and follow-up. Therefore, the exam confiability used to quantify hepatic lesion is a vital feature1,3.
Percutaneous liver biopsy is, nowadays, the gold standard method for liver diseases evaluation and evolutive analysis4,5.It is considered to be a safe procedure, however it is not free of complications. Besides, there are also limitations, related, mainly, to contraindications, risks to the patient, technique, high costs and interpretative inter/intraobserver variation 6,7,8,9,10.
For those reasons, routine laboratory tests, available in most health centers, have been used in formulas in order to develop indexes that could obtain biopsy-like results, i.e., being able to reflect metabolical, morphological and functional changes according to disease progression11.
The AST-to-platelet ratio index (APRI), created by Wai et al.3 in 2003 is one of those tests. It is applied according to the following formula:

This research attempts to demonstrate liver fibrosis predictive ability of APRI test in patients with viral chronic hepatitis from the Liver Group of Fundação Santa Casa de Misericórdia do Pará (FSCMPA) Hospital.
OBJECTIVE
To evaluate APRI test as a liver fibrosis marker in viral chronic hepatitis, using biochemical, hematological and histopathological data of patients registered at the Liver Group of FSCMPA Hospital.
METHODS
This study was a retrospective analysis previously approved by FSCMPA's Research Ethics Committee. Laboratorial and histopathological data of 102 patients with chronic viral hepatitis were evaluated with the aim of comparing biopsy reports to APRI results. Patients who had been through hepatitis specific treatment were excluded, as well as those which laboratorial and histopathological data were more than four months apart. The METAVIR grading was used, considering: F0-F1 as absent/insignificant fibrosis and F2-F4 as presence of significant fibrosis; F0-F3 and F4 as absence and presence of cirrhosis. Descriptive statistics and correlation tests were elaborated by BioStat 4.0 software (P"0,05=significance). The evaluation of APRI was based on the use of simultaneous cut-off points suggested by Wai et al. (2003)3 and also the development, for this sample, of single cut-off points through receiver operating characteristic (ROC) curves constructed by MedCalc 9.3.7 software.
RESULTS

DISCUSSION
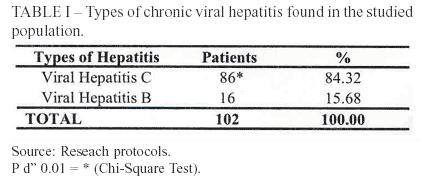
Models based on simple laboratorial tests have been developed with the objective of validating noninvasive methods that could, eventually, substitute liver biopsy1. This research studied patients with chronic viral hepatitis, being hepatitis C the most prevalent one (84%). The remaining cases were all type B, without any association with type D.
The staging frequency of liver fibrosis after biopsy, according to METAVIR score, showed a prevalence of F1 stage (42%), followed by F2 (28%), F3 and F4 (11% each)
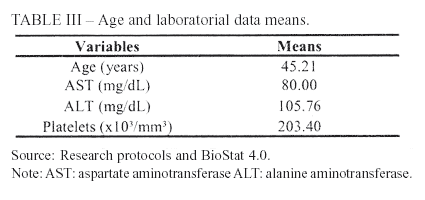
The studied patients presented an AST mean value of 80mg/dL. Wai et al. (2006)7, evaluating only hepatitis B patients, obtained means of 92 e 112mg/dL for training and validation sets, respectively. The mean value of ALT was 106mg/dL, which is compatible to Iacobellis et al. (2005),14 result of 112mg/dL.
Concerning the platelet count, the mean was 203,000/mm3. This value is similar to most recent studies that aimed the validation of noninvasive tests for liver fibrosis evaluation7,15,16,17.
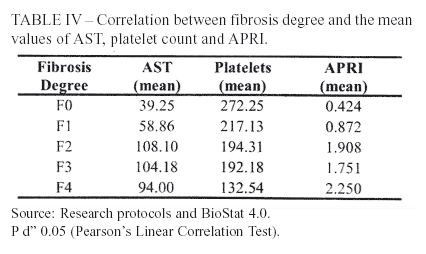
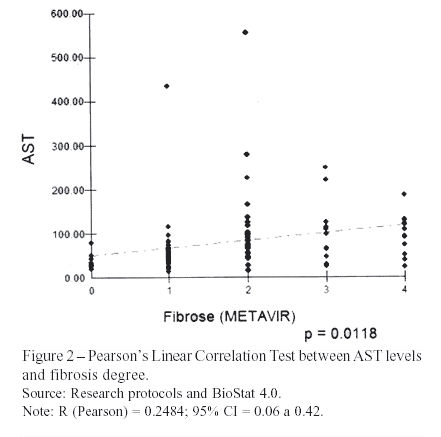
The elevation of AST according to fibrosis progression is due to its clearance deficit and, also, increased mitochondrial damage18,19,20. The patients of this research presented this trend, shown by Pearson's Linear Correlation test – r = 0.25; P = 0.0118. However, the correlation coefficient was less significant than the one achieved by the original study of Wai et al. (2003)3 – r = 0.50; P < 0.01.
Nonetheless, the mean values of AST according to fibrosis degree increased until F2 and slightly decreased at F3 and F4 stages. This feature could be related to the smaller amount of parenchyma in precirrhotic (F3) and cirrhotic patients, turning not possible the damage of a great number of hepatocytes 21.
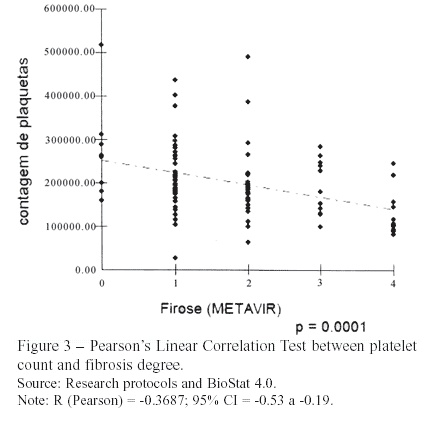
Thrombocytopenia is one of the most frequent hematological disorders expected in patients with chronic liver disease, due, primarily, to decreased hepatic thrombopoietin production and destruction of platelets by splenomegaly11,22,23. Consequently, the evaluated patients showed a progressive reduction of platelet count as fibrosis advanced. Pearson's Linear Correlation (r = -0.37, P <0.01) indicated this characteristic. Wai et al. (2003)3 obtained analogues results (r = -0.46; P < 0.01).
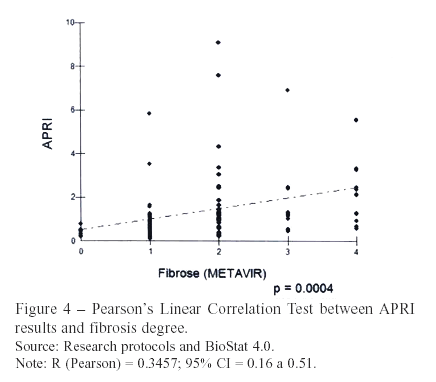
Using the Pearson's Linear Correlation test for APRI values and fibrosis stages (r = 0.35; P <0.01), elevated values of the index were associated to advanced fibrosis. Wai et al. (2003)3 accomplished a better coefficient (r = 0.60; P <0.01).
Platelet count, therefore, showed the most satisfactory correlation coefficient with fibrosis, when compared to AST or APRI. Differently, Wai et al. (2003)3 developed APRI and reached the best coefficient with the index.
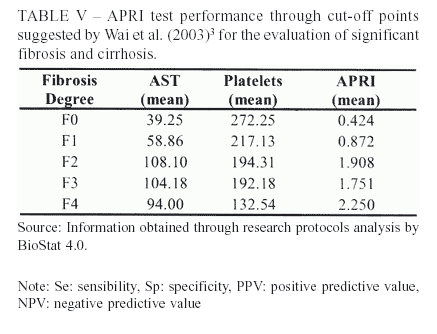
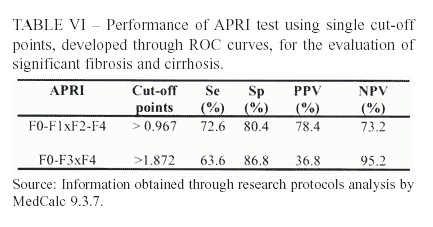
At Wai et al. (2003)3 study, for the determination of significant fibrosis, APRI d" 0.5 showed sensibility (Se) of 91% and > 1.5 showed specificity (Sp) of 95%. The present survey reached similar Sp (96.1%), while Se was lower (41.2%).
The original study presented positive predictive value (PPV) and negative predictive value (NPV) for significant fibrosis of 88% and 85%, respectively. In the actual study, only NPV achieved a non-satisfactory percentage (60%).
Wai et al. (2003)3 also used APRI to evaluate the presence of liver cirrhosis. For this quest, two distinct cut-off points were defined: d" 1.0 with 89% of Se and > 2.0 with 93% of Sp. The present research, after using the same points, obtained a modest Se (57.1%), but a satisfactory Sp of 86.8%.
Both studies, the present one and Wai et al. (2003)3, reached excellent NPV's for cirrhosis, with levels superior to 90%. On the other hand, the PPV shown by the actual survey was unsatisfactory (36.8%) as the original study was a little superior (57%).
The results of APRI evaluation after using the preestablished cut-off points of 2003, in spite of presenting different values from the original study, reaffirm that the index has a good capacity to confirm the diagnosis of significant fibrosis, besides being essential to highlight its competence to exclude patients without cirrhosis.
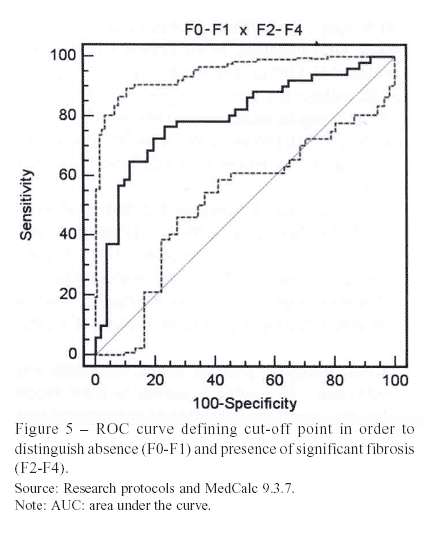
Aiming to classify 100% of patients, single cutoff points were developed through ROC curves. The point established for significant fibrosis was > 0.967.
The AUROC (area under the ROC curve), able to evaluate the diagnostic efficiency of the test, was 0.704, which is only a modest value, not excellent.
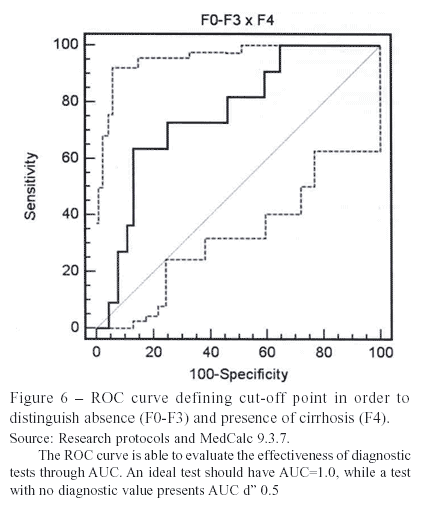
Another ROC curve was also constructed for the evaluation of cirrhosis, finding a single point of > 1.872. The AUROC was, again, modest (0.758).
However, using single cut-off points, there was no relevant variation in the main parameters, except for a slight increase in sensibility.
The comparison between the original study's Se, Sp, PPV and NPV and the ones found in the present study displayed an inferior upshot of the latter in most parameters, whether using the cut-off points of Wai et al. (2003)3 or the single points defined for the actual sample. It can be justified by the fact that patients with hepatitis B, C and co-morbidities (HIV and alcoholism) were studied in a unique group.
Wai et al. (2006)7 demonstrated, in a population of only hepatitis B patients, that APRI was not able to evaluate the presence of significant fibrosis and cirrhosis with the same effectiveness of the original study in hepatitis C patients. In the study of 2006, the AUROC for significant fibrosis was 0.63, and 0.64 for cirrhosis. However, in the study of 2003 the AUROC's were admirable (0.88 and 0.94).
Hence, the interval of until four months between liver biopsies and laboratorial results dates, adopted in the present study, might have led to a result bias, as patients with hepatitis B usually go through aminotransferases fluctuation7.
Using the single cut-off points, there was no superior performance whether for fibrosis or cirrhosis. This evidence shows that they are not likely to be used in clinical practice, as the original Wai et al. (2003)3 cut-off points are more effective.
The APRI is, therefore, able to prove the presence of significant fibrosis and the absence of cirrhosis in a great number of patients with viral chronic hepatitis. The index, however, is inadequate to evaluate distinct etiologies of liver diseases through the same cut-off point.
CONCLUSION
It was shown that APRI is able to predict the presence of significant fibrosis and cirrhosis through the simultaneous cut-off points of Wai et al. (2003)3. Although modest sensibilities were achieved, the specificities for expressive fibrosis and cirrhosis were 96% e 87%, respectively. A better performance was evidenced to confirm significant fibrosis (PPV = 90%) and to exclude liver cirrhosis (NPV = 95%).
Through the construction of ROC curves for significant fibrosis and cirrhosis, it was possible to classify the entire population of 102 patients by the following cut-off points: > 0.967 and > 1.872, respectively. Better sensibilities were found, which were not enough to justify the use of those points as the other parameters did not follow this tendency.
It's important to emphasize the need of new prospective studies, in order to validate the applicability of APRI in habitual clinical practice. But still, to evaluate significant fibrosis and the exclusion of cirrhosis, the index can be used as an alternative, mostly in patients with contraindications for liver biopsy execution.
REFERÊNCIAS
1 - COUTO, OFM. Validação e comparação de testes laboratoriais simples como preditores de fibrose hepática em portadores de hepatite C crônica. Available at: http://dspace.lcc.ufmg.br/dspace/bitstream/1843/ECJS-73AGQL/1/Osvaldo+ Fl%C3%A1vio+de+Melo+Couto.pdf. Accessed on: June 25th, 2007.
2 - LEE, KS. Hepatic fibrogenesis. Korean J. Gastroenterol. 2006; 48:297-305.
3 - WAI, CT et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatol. 2003; 38:518-26.
4 - AFDHAL, NH; NUNES, D. Evaluation of liver fibrosis: a concise review. Am. J. Gastroenterol. 2004;99:1160-74.
5 - ISHAK, K et al. Histological grading and staging of chronic hepatitis. J. Hepatol.1995;22:696-9.
6 - TONIUTTO, P et al. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J. Gastroenterol. Hepatol.1997;22:1904-8.
7 - AI, C.T. et al. Noninvasive model for predicting histology in patients with chronic hepatitis B. Liver Int.2006;26:666-72.
8 - AL-MOHRI, H et al. Validation of a simple model for predicting liver fibrosis in HIV/hepatits C virus-coinfected patients. HIV Med.2005;6:375-8.
9 - THULUVATH, PJ et al. Noninvasive markers of fibrosis for longitudinal assessment of fibrosis in chronic liver disease: Are they ready for prime time? Am. J. Gastroenterol.2005;100:1981-3.
10- POYNARD, T et al. Biomarkers as non-invasive assessment of hepatic fibrosis in chronic hepatitis C. J. Gastroenterol. Hepatol.2004;19:236-45.
11- TESTA, R et al. Noninvasive ratio index to evaluate fibrosis staging in chronic hepatitis C: role of platelet count/spleen diameter ratio index. J. Intern. Med.2006;260:142-50.
12- KELLEHER, TB; AFDHAL, N. Assessment of liver fibrosis in co-infected patients. J. Hepatol.2006;44:126-31.
13- PARISE, ER et al. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int.2006;26:1095-9.
14- IACOBELLIS, A et al. External validation of biochemical indices for noninvasive evaluation of liver fibrosis in HCV chronic hepatitis. Am. J. Gastroenterol.2005;100:868-73.
15- BOURLIERE, M et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J. Viral Hept.2006;13:659-70.
16- MAOR, Y et al. Non-invasive biomarkers of liver fibrosis in haemophilia patients with hepatitis C: can you avoid liver biopsy? Haemophilia. 2006;12:372-9.
17- KELLEHER, TB et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J. Hepatol.2005;43:78-84.
18- OKUDA, M et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroentol.2002;122:366-75.
19- HORIUCHI, S; KAMIMOTO, Y; MORINO, Y Hepatic clearance of rat liver aspartate aminotransferase isozymes: evidence for endocytotic uptake via different binding sites on sinusoidal liver cells. Hepatol.1985;5:376-82 apud TONIUTTO, P et al. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J. Gastroenterol. Hepatol.2007;22:1904-8.
20- NALPAS, B et al. Serum activity of mitochondrial aspartate aminotransferase: a sensitive marker of alcoholism with or without alcoholic hepatitis. Hepatol., v. 4, p. 431-434, 1984 apud WAI, CT et al. Noninvasive model for predicting histology in patients with chronic hepatitis B. Liver Int.2006;26:666-72.
21- AMARAL, ISA. Co-infecção vírus da imunodeficiência humana e vírus da hepatite C (HIV/HCV): aspectos epidemiológicos, clínicos e laboratoriais de uma população atendida em um serviço de hepatopatias na cidade de Belém-Pará. [THESIS–MASTERS]. Belém (PA): Federal University of Pará – Health Sciences Center; 2006.
22- ADINOLFI, LE et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br. J. Haematol.2001;113:590-5.
23- ASTER, RH. Pooling of platelets in the spleen: role in the pathogenesis of 'hypersplenic' thrombocytopenia. J. Clin. Invest., v.45, p. 645-657, 1966 apud TONIUTTO, P et al. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J. Gastroenterol. Hepatol.2007;22:1904-8.
 Address mail:
Address mail:
Clariana Casali Rodrigues Fernandes
Avenida José Bonifácio 902, apto 702. São Braz, Belém -PA.
CEP: 66063-010 - Brazil
Telephone: +55 91 3249-9145.
email:clarianacasali@gmail.com
Ivanete do Socorro Abraçado Amaral
Avenida Gentil Bittencourt 1990, apto 1102. São Braz, Belém –PA
CEP: 66063-090 - Brazil
Telephone: +55 91 3272-0626
email:ivaneteabracado@hotmail.com
Lizomar de Jesus Maués Pereira Moia
Travessa Bom Jardim 658. Jurunas, Belém – PA
CEP: 66025-180 - Brazil
Telephone: +55 91 8844-4844
email: lizmoia@hotmail.com
Marina Pinto Franco Dias
Avenida Conselheiro Furtado 2818, apto 7000. São Braz, Belém -PA.
CEP: 66063-060 - Brazil
Telephone: +55 91 3229-0347.
email:marinafdias@gmail.com
Recebido em 07.08.2007
Aprovado em 12.12.2007
1Study performed at Fundação Santa Casa de Misericórdia do Pará Hospital













