Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Paraense de Medicina
Print version ISSN 0101-5907
Rev. Para. Med. vol.21 no.4 Belém Dec. 2007
Profile of hemodynamic and gasometric parameters in rabbits submitted to controlled hemorrhagic shock1
Perfil dos parâmetros hemodinâmicos e gasométricos em coelhos submetidos a choque hemorrágico controlado
Mauro José FontellesI; Raimundo Miranda de CarvalhoII; Luana Maria Relvas D'OliveiraII; André Vilarino MadeiraII; Pablo Vaz Gonçalves BorgesII; Marcelo Silveira D'OliveiraIII
IPh.D., Trauma Surgery, Professor of Human Anatomy, Universidade da Amazônia (UNAMA) and Universidade Federal do Pará (UFPA)
IIBeginning researcher, Medicine Course, Universidade Federal do Pará (UFPA)
IIIBeginning researcher, Medicine Course, Universidade Estadual do Pará (UEPA)
SUMMARY
OBJECTIVE: the aim of this study was to assess the values of hemodynamic, gasometric and electrolytic parameters, in rabbits submitted to controlled hemorrhagic shock, using an experimental model for catheterization of right carotid artery and jugular vein.
METHODS: fourteen male California rabbits were submitted to intramuscular anesthesia, and medium cervicotomy was performed for catheterization of right carotid artery and right jugular vein. The animals were bled to a mean arterial pressure of 40mmHg and were maintained at this level, by further blood withdrawal, during 20 minutes, followed by 15 minutes of resuscitation using lactated Ringer's solution and remaining shed blood volume (3:1) to mean arterial pressure equal 80mmHg, and 120 minutes of reperfusion. Arterial blood gas, serum lactate and electrolytes (sodium and potassium) samples were measured at baseline, hemorrhagic shock, at the time of resuscitation, and at the time of reperfusion (30, 60, 90 and 120min).
RESULTS: the mean of initial values of hemodynamic parameters were – MAP=82.3mmHg, RR=51.4breaths/min, HR=141.5beats/min; gasometric parameters - pH=7.358, PaCO2=46.6mmHg, PaO2=271.9mmHg, HCO3 -=25.0mmol/L, base deficit=1.5mmol/L, serum lactate=2.6mmol/L; electrolytic parameters – Na+=131.7mEq/L and K+=3.4mEq/L.
CONCLUSIONS: this study presents a reproducible model of hemorrhagic shock in Californian rabbits, which describes the progressive hemodynamic and metabolic changes that reflect the changes seen frequently in the clinical situation, besides offers a model to assess novel therapeutics interventions in a controlled setting.
KEY WORDS: Hemorrhagic shock, blood gas analysis, hemodynamic response, rabbits.
RESUMO
OBJETIVO: avaliar os valores dos parâmetros hemodinâmicos, gasométricos e eletrolíticos, em coelhos submetidos a choque hemorrágico controlado, com a utilização de um modelo experimental de cateterização da artéria carótida e veia jugular direitas.
MÉTODO: catorze coelhos Califórnia, machos, foram submetidos à anestesia intramuscular seguida de cervicotomia mediana e cateterização da artéria carótida direita e da veia jugular direita. Os animais foram sangrados até a pressão arterial média (PAM) de 40mmHg e mantidos neste nível durante 20 minutos, seguidos de 15 minutos de reposição com a utilização de solução de Ringer lactato e sangue (3:1) até a PAM atingir 80mmHg, e 120 minutos de reperfusão. Amostras dos gases arteriais, do lactato sérico e dos eletrólitos (sódio e potássio) foram coletadas no início do experimento, no choque hemorrágico, no período de reposição e na reperfusão (30, 60, 90 e 120 minutos).
RESULTADOS: a média dos valores iniciais dos parâmetros hemodinâmicos foram - PAM=82.3mmHg, FR=51.4mov/min, FC=141.5bat/min; parâmetros gasométricos - pH=7.358, PaCO2=46.6mmHg, PaO2=271.9mmHg, HCO3 -=25.0mmol/L, déficit de base=1.5mmol/L, lactato sérico=2.6mmol/L; parâmetros eletrolíticos – Na+=131.7mEq/L and K+=3.4mEq/L.
CONCLUSÕES: este estudo apresenta um modelo reprodutível de choque hemorrágico controlado em coelhos Califórnia, o qual descreve as progressivas alterações hemodinâmicas e metabólicas que refletem aquelas freqüentemente observadas em situações clínicas, além de oferecer um modelo para avaliar novas terapêuticas em estudos controlados.
DESCRITORES: Choque hemorrágico, gasometria, resposta hemodinâmica, coelhos.
INTRODUCTION
Ischemia and subsequent reperfusion lead to substantial damage to cells and organs, depending on the ischemic time. On the medical practice, many causes can lead to ischemic-reperfusion lesion, but severe arterial hypotension, caused by hemorrhages, is that one which is better associated to major traumas1,2,3. Therefore, the quantification of the seriousness of tissue injury incurred following hemorrhagic shock is important in the management and prognosis of patients, as well as understanding of its physiopathology, and, for this reason, numerous recent clinical and experimental study have shown that the physiological response to hemorrhage can be unpredictable, and, traditionally, hemodynamic an metabolic parameters have long remained as the key in clinical assessment of the degree of hypovolemia or predicting outcome following hemorrhagic shock4,5. Thus, in many research laboratories, studies in small animals have been common nowadays, especially with utilization of rats, rabbits, dogs or pigs, all with purpose of better explain the evolution of different hemodynamic and metabolic parameters which occur during ischemia-reperfusion phase following the hemorrhagic shock.
OBJECTIVE
To assess the value of hemodynamic and gasometric parameters, in rabbits submitted to controlled hemorrhagic shock, using a clinically-relevant experimental model for catheterization of right carotid artery and jugular vein.
METHODS
This study was performed at the Multidisciplinary Unit of Experimental Medicine, Surgery Department, Federal University of Pará (UFPA), Brazil, and done in accordance with guidelines established by the Institutional Animal Care and Use Committee at the State University of Pará. Fourteen male Californian rabbits (2500-3000g), all proceeded from Zootecny Center of Rural Federal University of Amazon (UFRA), were used in all experiments, which were kept in, adjusted conditions of light and temperature, receiving standard diet ad libitum, except for 6 hrs before surgery when there was access to water only. All the animals were submitted to anesthesic and monitorization procedures previously to the surgery that was made for the same team, during the entire study, and were submitted to surgery in the same period of the day, avoiding any influence that the difference of the period could bring to the results. Rabbits were anesthetized with an intramuscular solution of ketamine (80mg/kg), xylazine (10mg/kg) and atropine (0.05 mg/kg). Oxygen was managed in the concentration of 2 L/min, using a plastic mask that was adapted to the face of the animal, protecting it partially. The characterization of the anesthesic plan was evaluated using parameters, such as: mustache and ears movements after stimulation (sedation); absence of tail and legs' retraction after digital pressure (surgical anesthesia). Our preliminary studies had determined these procedures settings to maintain physiological blood gas concentration prior to the onset of hemorrhage.
After anesthesic induction, it was made depilation of the neck, followed by apprehension of members in extension with plastic wires. Using aseptic technique, the blood vessels were then dissected with placement of a polyethylene arterial catheter in the right carotid artery (4mm gauge) for continuous blood pressure monitoring, arterial blood gas samples and blood withdrawal, and a venous catheter in the right jugular vein (4mm gauge) for venous blood gas samples and volemic infusion. Each catheter was initially flushed with 0.1 mL of heparin (100 U/mL), but the rabbits were not heparinized. Following a 5 min stabilization period, baseline (BL) measurements of mean arterial pressure (MAP), heart rate (HR), respiratory rate (RR) and rectal temperature (RT) were recorded, as well as arterial and venous gasometry, electrolytes and serum lactate. The rabbits were then bled to a MAP of 40 mmHg over 15 min, and maintained at this level by further blood withdrawal, when necessary. The hemorrhagic shock was continued for 20 min, followed by 15 min of resuscitation, which consisted of returning all shed blood volume, plus lactated Ringer's solution (1:3) with the goal of maintaining the MAP over 80 mmHg. At the end of the resuscitation period, hemodynamic monitoring was continued for a total of 4 h (reperfusion period), after which the animals were sacrificed by the infusion of 3 ml of Thiopental through the catheterized right carotid artery. The blood gas samples, electrolytes and arterial serum lactate levels were drawn in seven moments: at baseline, end of hemorrhagic shock period (HS), end of volume reposition (REP) and four times during the reperfusion period (30, 60, 90 and 120 min).
Comparison of parameters between each of the seven phases of the experiment was performed using one-way analysis of variance (ANOVA) and Tukey test. Differences were considered significant at p < 0.05. All results are expressed as men ± standard error of mean (SEM).
RESULTS
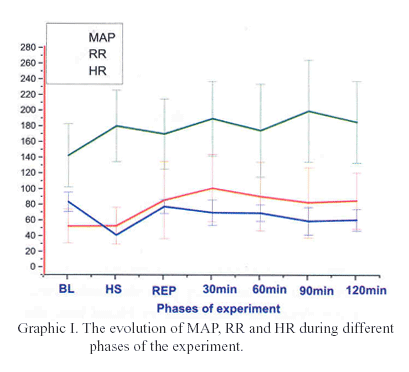
The hemodynamic parameters variation during each phase of the experiment is presented on table I. It was observed statistical significant difference related to MAP when compared BL to 90 min and 120 min (p<0.01); related to RR when compared 30 min to BL and HS (p<0.05).
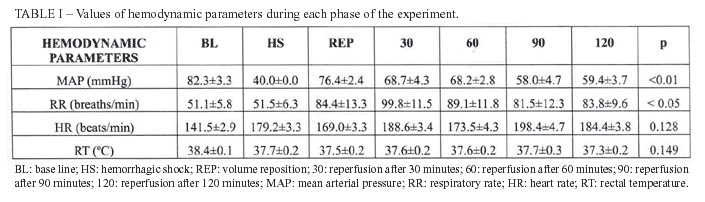
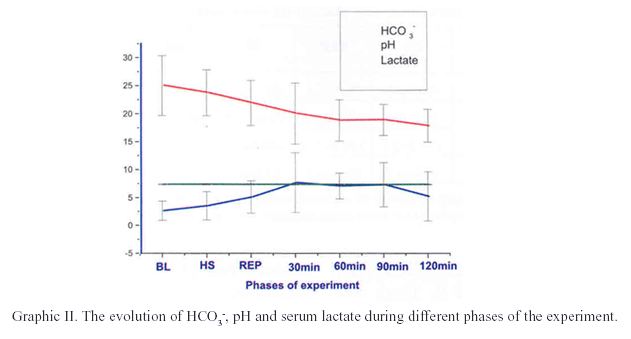
The arterial gasometric data, lactate, sodium and potassium are expressed in table II. There was statistical difference related to bicarbonate when compared BL to 60 min, 90 min and 120 min (p<0.01), as well as when compared HS to 60 min and 120 min (p<0.05). The difference was also found and it was related to Base Deficit when compared BL to 60 min, 90 min and 120 min (p<0.05); HS to 60 min, 90 min and 120 min (p<0.05). The serum lactate analysis showed p<0.01 when compared BL to 30min, and p<0.05 when compared BL to 90 min and 120 min.
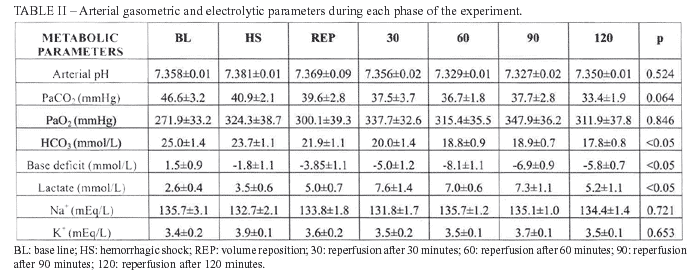
The figures I and II show the evolution of the hemodynamic and metabolic parameters during different phases of the experiment.
DISCUSSION
Hemorrhagic shock remains a major cause of morbidity and mortality in surgical patients and trauma victims, mainly due to ischemia-reperfusion lesions followed by multiple organ dysfunctions.
Consequently to a considerable blood loss, occurs a compensatory redistribution of the blood volume, from less noble tissues (skin, muscles and splanchnic organs) to brain, heart and lungs that could be affected by subsequent reperfusion. This way, patients victims of severe trauma can develop an important systemic repercussion, the systemic inflammatory response syndrome (SIRS), with subsequently respiratory and infection complications, heart and kidney dysfunctions, culminating in multiple organs and systems failure, specially lung and heart6,7,8.
Thus, recent studies have suggested that changes in hemodynamics and metabolic parameters may be more accurate measures of circulating blood volume and subsequent prognosis in hemorrhagic shock. On the other hand, several trials affirm that there are no universally accepted, clinically useful indicators of tissue hypoperfusion or hypoxia; however, numerous clinical studies have show that physiological response to hemorrhage can be unpredictable4,5. For this reason, for a long time ago, the experimental models using different animal species have been utilized as a method to study the ischemia-reperfusion repercussion following hemorrhagic shock. These models, despite several limitations, remain essential in the development of new approaches for hemorrhagic shock treatment, because they provide fundamental information about the magnitude of blood loss, the repercussion of the different periods of hemorrhagic shock period, the evaluation of hemodynamic and gasometric parameters, the effect of the anesthesic method, aggressive fluid resuscitation, the pharmacokinetics, toxicity and mechanism of drug action, and other major uncontrolled factors, such as the presence of drugs or alcohol, which cannot be duplicated by other methods9. For instance, Girisgin et al10, using a experimental model in rabbits, studied importance of early and effective fluid resuscitation in hypovolemic shock treatment with the utilization of fluid replacement via the rectum, as a possible life-saving method in situations where veins cannot be accessed quickly. Lhuillier et al11, also with a rabbit model, evaluate the nitric oxide involvement in the regulation of hepatic microcirculation under physiological conditions and in hemorrhagic shock, and concluded that it plays a important role in the autoregulation of liver microcirculation during the hemorrhagic shock. In the same manner, Komori et al12, studied the effects of hydroxyethyl starch on the microcirculation, hemodynamics, and colloidal osmotic pressure in a rabbit model of hemorrhagic shock, and verified that intravenous infusion of this solution effectively maintains these parameters during acute severe hemorrhage.
Thus, our study consists in the development of experimental model to perfect the surgical technique for the vascular catheterization with subsequent clinical and laboratorial analyses in Californian rabbits, inasmuch as its consolidation is essential, because the procedure needs to be done carefully and it comes only with practice. The basic supposition of this present experimental study is that if all animals received the same hemorrhagic shock model, in the same location, the same duration of hemorrhagic hypotension, and the same aggressive fluid resuscitation, then injury severity was proportional to the amount blood loss. The reliability of data interpretation depends on the validity of that assumption.
In relation to hemodynamic parameters, the MAP analysis showed that it doesn't return to the initial level (82.30 mmHg) after 120 minutes of reperfusion, evolving with lower values than normal during the experiment, thus demonstrating hemodynamic stabilization instead of the normalization. The concept of compensated shock has been used for situations of ongoing inadequate tissue perfusion despite normalization of blood pressure, heart rate and urine output13. In consequence to a severe and prolonged hypovolemia, the neuroendocrine responses that are characteristics of compensated shock begin to fail and a decompensate state develops, which is characterized by failure of the microcirculation with progressive peripheral vasodilatation, absence or few reactivity to vasopressor drugs and capillary leak. According to Kazuo et al14, with an experimental model in Lewis rats, they demonstrated that although transient hyperemia may be observed after short-term ischemia, the final tissue blood flow after ischemia-reperfusion does not reach preischemic levels. This state frequently occurs in patients that suffered multiple traumas.
In hemorrhagic shock there is the loss of metabolic balance, culminating in the production of metabolic acidosis. The plasmatic catecholamine levels are elevated soon after injury, and a direct relationship between the severity of injury and the plasma catecholamine changes can be observed. These events are later followed by compensatory mechanisms that attempt to restore the initial homeostasis. Thus, the elevation of RR occurs, aiming the reduction of CO2 retention and the elimination of H+, leading to an arterial pH normalization8. In this experiment, the fall of RR was present in the beginning due to the anesthesia. On the other hand, there was progressive elevation during the hemorrhagic shock period, with tendency to stabilization in the subsequently stages, according to what was expected. The elevation of HR is one of the primordial compensatory mechanisms to correct the decrease of tissue perfusion. It generates the increase of cardiac output, elevating blood pressure15. In our study, it was observed the elevation of HR along the period of hemorrhagic shock, however no returning to initial values after the intravenous fluid resuscitation. The maintenance of these mechanisms and the large blood volume loss keep HR elevated, aiming to maintain the tissue perfusion. In the literature, it was found this progressive elevation during the experiment, supporting this observation15,16.
One other factor is the anesthesia; anesthetics, e.g., the ketamine and xylazine used in the present study, may affect the vascular response and blood flow after ischemia, which would thus not reflect the physiological response of each vasculature. However, in the clinical setting, ischemia-reperfusion following hemorrhagic shock is usually studied in surgical procedures occurring under anesthesia.
In relation to gasometric parameters, the hemorrhagic shock promotes a deoxygenated environment leading to a production of organic acids with metabolic acidosis15. In this study, it was ratified by the presence of arterial pH reduction during shock period. The results suggest that the recovery to initial level is a delayed effect because, after 120 minutes, it didn't happen. On the other hand, there was a progressive elevation of arterial pH during this stage, without, however, to reach the normal levels. This tendency was observed in other studies, where the values of arterial pH increased after 60 minutes of reperfusion17.
In compensatory homeostatic situations, the accumulation of CO2 stimulates the elevation of H+ concentration, dislocating the pH for acidosis limits. In shock, the elimination of this gas by respiration reduces its partial pressure, conducting pH to normal values15. This event was very evident in the results of the study, showing the reduction of PCO2 just like it was expected. In the literature, it was found a study that observed the elevation of PCO2 during the progression of the experiment, however it was used, for volemic reposition, NaCl 7,5% associated to dextran 6%, a different solution from the one used in this study18. In addition, the elevation of PaO2 is a sign of compensation due to inappropriate perfusion. It leads to better use of residual oxygen by the tissues that need this supply to generate aerobic metabolism and energy15.
In relation to electrolytic measures, there is a fall of the concentration of Na+ during the blinding process and shock because to influx of sodium due to acidosis. On the other hand, during the reperfusion process occur the reverse mechanism with inversion of flows, and the use of a fluid containing sodium and potassium ions contributes to this increase. In the literature, this sequence was observed, supporting the decrease followed by the increase after the establishment of the reperfusion18. During the period of hemorrhagic shock, potassium tends to leave the cell, elevating its serum concentration, but it tends to decrease in reperfusion because of the inverse mechanism. The elevation of its serum concentration was observed in other study in the literature4,5,17,19. The increased blood levels of these ions may have a deleterious effect on the functions of central vital organs, and an early buffering in treatment of severe hemorrhagic shock is therefore of major importance20.
Thus, there are several mechanisms to neutralize the acidosis, and the most important and efficient is the serum bicarbonate15. In our study, is possible to observe the consumption of bicarbonate in the beginning of the compensatory processes, because of the acidosis that was established in shock period. Another studied parameter was the base deficit, which is defined as the amount of base required to tritrate one liter of blood to a normal pH at normal physiological values of PaO2, PaCO2, and temperature. In this study, it was observed the negative progression of the bases' turnover during the stages of the experiment. The analysis in the consumption of bases during shock and reperfusion periods is an indicator that brings confidence to analyze the process of installation and reversion of metabolic acidosis. On the literature, it was observed that the consumption of bases was increased during the period of shock when rats were submitted to controlled hemorrhagic shock, confirming the tendency to correct the metabolic acidosis21.
Several laboratory and clinical studies have related the serum lactate measurements as a reliable and sensitive method for quantification the hypoperfusion as the result of blood loss, being considered as an excellent indicator of the progress of the homeostatic balance loss, indicating the aggravation of hemorrhagic shock state and the adequacy of resuscitation.4,22,23,24. In our study, the progressive elevation of serum lactate induced by hemorrhagic shock was reflected by deteriorating blood pH, in addition to worsening arterial base deficit. The author concluded that metabolic parameters such as the arterial measurements of serum bicarbonate, base deficit and lactate were more accurate indicators of degree of shock insult than hemodynamic parameters.
Anyway, this study reflects differing responses of the animals during hemorrhagic shock state, and extends its observations for other investigators, aiming better comprehension about this dangerous clinical situation.
CONCLUSION
This study presents a reproducible model of hemorrhagic shock in Californian rabbits, which describes the progressive hemodynamic and metabolic changes that reflect the changes seen frequently in the clinical situation, besides offers a model to assess novel therapeutics interventions in a controlled setting.
REFERENCES
1. Demetriade SD, Murra YJ, Charalambidesk, et al. Trauma fatalities: time and location of hospital deaths. J Am Col. Surg, 2004; 198:20-6.
2. Melniker LA, Leibner E, McKenney MG, Lopez P, William M, Briggs WM, et al. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: the first sonography outcomes assessment program trial. Ann Emerg Med, 2006; 483(3):227-35.
3. Audonnet-Blaise S. Resuscitation of severe but brief haemorrhagic shock with PFC in rabbits restores skeletal muscle oxygen delivery and does not alter skeletal muscle metabolism. Resuscitation, 2006; 70:124-32.
4. Shah NS, Kelly E, Billiar TR, Marshall HM, Harbrecht BG, Udekwu AO, Peitzman AB. Utility of clinical parameter of tissue oxygenation in a quantitative model of irreversible hemorrhagic shock. Shock, 1998; 10(5):343-7.
5. Moomey CB, Melton SH, Croce MA, Fabian TC. Prognostic value of blood lactate, base deficit, and oxygen-derived variables in an LD50 model of penetrating trauma. Crit Care Med, 1999; 27(1):154-61.
6. Neff MJ. The epidemiology and definition of the acute respiratory distress syndrome. Respir Care Clin N Am 2003; 9: 273-82.
7. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med, 2003; 348:138-50.
8. Moore FA, Moore EE, Haenel JB. Post injury multiple organ failure. In: Feliciano DV, Moore EE, Mattox KL. Trauma. 8a ed. Connecticut: Appleton; 2003. p. 1427-60.
9. Garrido AG, Figueiredo LFP, Silva MR. Experimental models of sepsis and septic shock:na overview. Acta Cir bras, 2004; 19(2):82-8.
10. Girisgin AS, Acar F, Cander B, Gul M, Kocak S, Bodur S. Fluid replacement via the rectum for treatment of hypovolaemic shock in an animal model. Emerg Méd J, 2006; 23(11):862-4.
11. Lhuillier F, Robert MO, Crova P, Goudable J, Arnal F, Cespuglio R, Annat G, Viale JP. Nitric oxide and liver microcirculation during autoregulation and haemorrhagic shock in rabbit model. Br J Anaesth, 2006; 97(2):137-46.
12. Komori M, Takada K, Tomizawa Y, Uezono S, Nishiyama K, Ozaki M. Effects of colloid resuscitation on peripheral microcirculation, hemodynamics, and colloidal osmotic pressure during acute severe hemorrhage in rabbits. Shock, 2005; 23(4):377-82.
13. Holm C, Melcer B, Hörbrand F, Donnersmarck GH, Mühlbauer W. The relationship between oxygen delivery and oxygen consumption during fluid resuscitation of burn-related shock. J Burn Care Rehabil, 2002; 21(2):147-54.
14. Kazuo H, Nishida T, Seiyama A, Ueshima S, Hamada E, Ito T, Matsuda H. Recovery of blood flow and oxygen transport after temporary ischemia of rat liver. Am J Physiol, 1998; 275(1):243-49.
15. Guyton AC. Tratado de fisiologia médica. 10a ed. Rio de Janeiro (RJ): Guanabara Koogan; 2002.
16. Melleti JFA, Braz JRC, Modolo NSP. Comportamento hemodinâmico e metabólico do choque hemorrágico: estudo experimental no cão. Rev Bras Anestesiol, 2003; 53(5):623-32.
17. Hirano ES, Mantovani M, Morandin RC. Isquemia e reperfusão hepática total em condições de normalidade e sob estado de choque hemorrágico controlado: efeitos no seqüestro de neutrófilos no rim de rato. Acta Cir Bras, 2005; 20(4):292-9.
18. Melleti JFA, Braz JRC, Modolo NSP. Efeitos hemodinâmicos e metabólicos imediatos determinados pelas soluções de cloreto de sódio a 7,5% e de sua associação ao dextran 70 a 6% na reanimação do choque hemorrágico: estudo experimental em cães. Rev Bras Anestesiol, 2006; 56(5):478-94.
19. Hirano ES, Mantovani M, Morandin RC. Isquemia e reperfusão hepática total em condições de normalidade e sob estado de choque hemorrágico controlado: efeitos no seqüestro de neutrófilos no rim de rato. Acta Cir Brás, 2005; 20(4):292-9.
20. Haljamäe H. The pathophysiology of shock. Acta Anaesthesiol Scand, 1993; 37(supl 98):3-6.
21. Hirano ES, Mantovani M, Morandin RC, Fontelles MJP. Modelo experimental de choque hemorrágico. Acta Cir Bras, 2003; 18(5):465-70.
22. Muir WW. Shock. Compend. Contin. Educ Pract Vet, 1998; 20(5):549-66.
23. Abramson D, Scalea TM, Hitchcock R, et al. Lactate clearance and survival following injury. J Trauma, 1993; 35:584-8.
24. Davis JW. The relationship of base déficit to lactate in porcine hemorrhagic shock. J Trauma, 1994; 36:168-72.
 Adress for reprint requests:
Adress for reprint requests:
Mauro José Fontelles. E.mail:mikefox@uol.com.br
Rua Antônio Barreto, 983/1502 – Umarizal
Belém – Pará – Brasil
CEP 66055-050 Fone:
(91) 32251850
Recebido em 06.08.2007
Aprovado em 12.12.2007
1From the Multidisciplinary Unit of Experimental Medicine, Surgery Department, Universidade Federal do Pará (UFPA), Brazil.













