Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.1 Brasília jan./mar. 2016
http://dx.doi.org/10.5123/S1679-49742016000100002
ORIGINAL ARTICLE
Epidemiological characteristics of yellow fever in Brazil, 2000-2012*
Karina Ribeiro Leite Jardim Cavalcante1; Pedro Luiz Tauil2
1Ministério da Saúde, Secretaria de Vigilância em Saúde, Brasília-DF, Brasil
2Universidade de Brasília, Faculdade de Medicina, Brasília-DF, Brasil
ABSTRACT
OBJECTIVE: this study aims to describe the epidemiological characteristics of yellow fever in Brazil between the years 2000 and 2012.
METHODS: this is a descriptive ecological epidemiological study, using information from Ministry of Health databases.
RESULTS: 326 cases of yellow fever were confirmed in Brazil during this period, with 156 deaths and an average case mortality rate of 47.8%; the young male adult age group was the most affected; in epizootics, 2,856 suspected cases of yellow fever in non-human primates were reported and 31.1% of these were confirmed by laboratory tests; during the study period the area in which sylvatic transmission of the disease occurs was found to have expanded to densely population regions, such as South, Southeast and Midwest Brazil.
CONCLUSION: the risk of urban yellow fever transmission persists, as sylvatic incidence of the disease has expanded to regions with high Aedes
aegypti infestation, this being the mosquito responsible for urban transmission of the disease.
Key words: Yellow fever; Epidemiology, Descriptive; Disease Vectors; Brazil.
Introduction
The yellow fever (YF) is a short-term (maximum 12 days), acute, febrile and infectious disease, which is non-communicable. Its severity may vary. Clinical manifestations may represent evolutionary stages of disease.1 The severe form can lead to death, clinically characterized by liver and kidney failure. There is no specific etiologic treatment yet. The disease is caused by an arbovirus of the genus Flavivirus, family Flaviviridae, and it still is endemic and enzootic in many tropical regions of America and Africa and is responsible for periodic outbreaks of variable magnitude.2 In the Americas, the potential spread risk to urban areas is to be taken into account.
The YF is transmitted to people through the bite of an infected female mosquito and has a seasonal characteristic, being more frequent between the months of January and April, when environmental factors favor the increase of the vector density. Currently, there are two recognized basic cycles of circulation of YF virus: an urban, simple, human-mosquito-human type, in which Aedes aegypti is the main vector; and a sylvatic type that is complex, involving different species of mosquitoes, in America and Africa, with the inclusion of non-human primates (NHP) in viral spreadness. In the American continent, the YF is a zoonotic disease transmitted by mosquitoes of two genera, Haemagogus (H. janthinomys and H. albomaculatus) and Sabethes. NHP are the main source of infection in the sylvatic cycle, especially among monkeys of the following genus: Allouata, Cebus Atelles and Callithrix. In Africa, the sylvatic cycle involves mosquitoes of the genus Aedes (Ae. Africanus, Ae. Simpsoni, Ae. Furcifer, Ae. Luteocephalus and Ae. Taylori).3
The vectors of YF demonstrate predominantly daytime biting activity. After a period that usually ranges from nine to twelve days of infection in a viremic case, mosquitoes are capable of transmitting the disease. The incubation period in humans ranges, on average, from three to six days after the bite of the infected mosquito, and it can also reach up to 10 days.4
In 1947, the former National Service of Yellow Fever started the use of dichlorodiphenyl trichloroethane (DDT) in the attempt to fight mosquitoes. In 1950, the activities of this service reached its peak, putting 3,349 active employees in charge of 112,950 locations. In 1958, the National Department of Rural Endemic Diseases, which had already absorbed the National Yellow Fever Service, announced the eradication of Ae. aegypti in the country.5
Nevertheless, in 1967, the Ae. aegypti was again identified in Brazil in the city of Belém, capital of the state of Pará, and two years later, in 1969, in the state of Maranhão. In 1973, a final focus was eliminated and the vector was considered again eradicated from the Brazilian territory.6,7 In 1976, Ae. aegypti reappeared for the second time and reinfested the country, beginning in the city of Salvador, Bahia, due to a failure in entomological surveillance. Social and environmental changes that were considered a result of the rapid urbanization favored the settlement and spread of this mosquito in Brasil.8 Reinfestations were confirmed in the states of Rio Grande do Norte and Rio de Janeiro, and since then, the Ministry of Health has implemented programs to combat this vector and to reduce the risk of urban transmission of YF and, subsequently, to decrease the incidence of dengue, given that Ae. aegypti is also the main vector of this virus.
The reemergence of sylvatic transmission of YF outside the Amazon region, from 2007 on, expanded the viral circulation area in Brazil. The areas more recently attacked are in the Southeast and South regions of the country and are important objects because of the proximity to large urban centers, densely occupied by an unvaccinated population; as a consequence, there is no protection against the disease, not to mention the high infestation of Ae. aegypti, including dengue transmission in a myriad of municipalities. This fact raised the discussion about the risk of resumption of urban transmission of YF in Brazil, which was recorded for the last time in Sena Madureira, a municipality in the state of Acre, in 1942.9,4
Until 1999, the surveillance of YF was based exclusively in the event of suspected human cases. From that year on, with the observation of monkeys' deaths in several municipalities of the states of Tocantins and Goiás and the subsequent appearance of the disease in the human population, these events were seen as possible indicators of risk (sentinel event) of human cases of sylvatic transmission.
People who are at risk of getting YF in the American continent are those not vaccinated and exposed to the bites of vectors in forest areas, in the virus endemic places, especially where there is virus circulation. Forestry and rural areas most affected correspond to the hydrographic basins of the Amazon, Araguaia-Tocantins, Paraná and Orinoco in South America and the Nile and Congo rivers in Africa.10
To contribute to the improvement in the surveillance and control of yellow fever in Brazil, this study aims to describe the epidemiological characteristics of yellow fever in the country from 2000 to 2012.
Methods
This is a descriptive epidemiological study, using as source the database of the Ministry of Health on the incidence of cases and deaths related to YF in humans and in non-human primates - NHP - from 2000 to 2012. These data were provided by the Program of Surveillance, Prevention and Control of Yellow Fever of the Health Surveillance Secretariat of the Ministry of Health (SVS/MS); and the Evandro Chagas Institute, from Belém, Pará state (PA), Adolfo Lutz Institute, São Paulo state (SP) and Oswaldo Cruz Foundation (Fiocruz) Rio de Janeiro state (RJ). These are reference laboratories and are accredited by the SVS/MS to diagnose yellow fever. Whenever a suspicious case is detected in an individual, a blood sample or other tissue sample is sent to one of the aforementioned laboratories.
The notification of YF cases - as well as the epidemiological investigation - must take place within 24 hours after the suspicion. The data collection instrument, an epidemiological investigation form, available at the Information System for Notifiable Diseases (Sinan), covers the essential elements to be recorded in a routine investigation.2
The human cases were distributed by year and Federative Unit (FU) of occurrence, according to the variables 'age', 'sex', 'occupation' and 'outcome' (death, non-death). The age range, median and standard deviation were calculated. The annual fatality rates for Brazil from 2000 to 2012, and for FU were calculated, and the main occupational activities were described. There was a great diversity in the records for occupations. Thus, the item 'rural worker' was grouped according to the following occupations: agriculturist; cattleman; peasant; farmer; cowboy; rural worker; fisherman; and prospector.
The number of human cases was calculated on a monthly basis, in order to verify the existence of seasonality of the disease during the studied period.
NHP deaths data were collected from reports provided by the reference laboratories and by the Program of Surveillance, Prevention and Control of Yellow Fever.
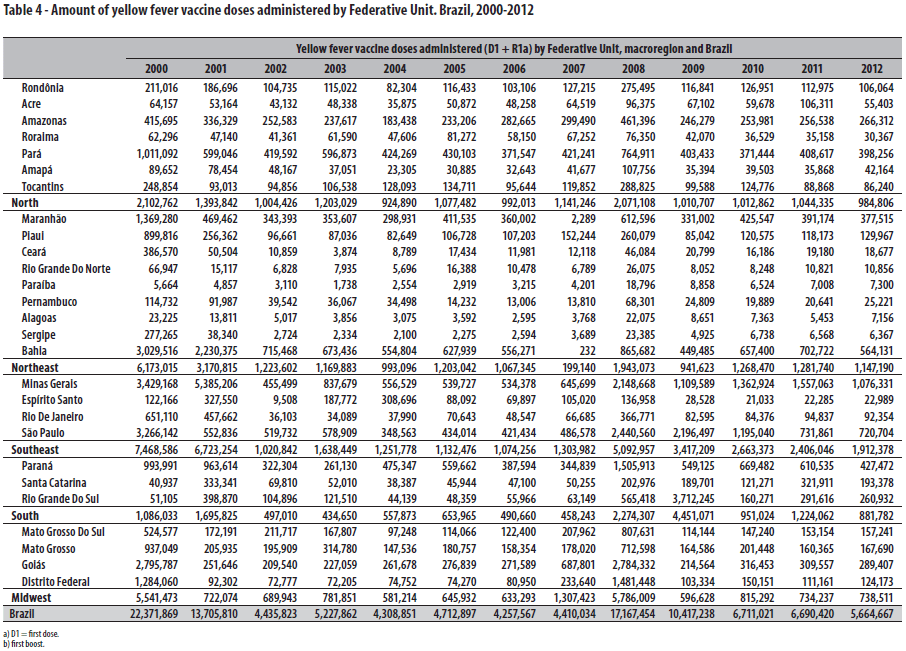
The data on doses of vaccines administered by FU, provided by the National Immunization Program (PNI/SVS/MS) have been consulted.
Taking into account that this is secondary data, with no identification of names of the affected people, the study was exempted from evaluation by the Research Ethics Committee, in accordance with the Resolution of the National Health Council (CNS) No. 466, dated December 12, 2012.
Results
From 2000 to 2012, 326 confirmed cases of YF were recorded, all caused by the sylvatic transmission cycle, and there were 156 deaths in the country, resulting in an average fatality rate of 47.8%. The year 2000 was the one with the highest number of cases and deaths (Table 1).
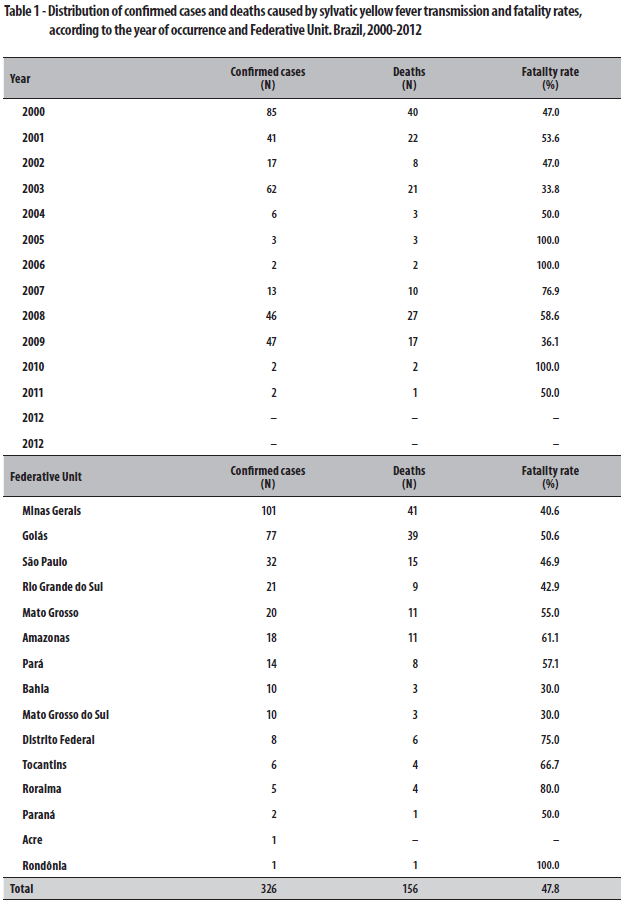
The distribution of YF cases by Federative Unit showed that the state of Minas Gerais was the most affected in the aforementioned period, with 101 confirmed cases and a fatality rate of 40.6%, followed by the state of Goiás, with 77 cases and a fatality rate of 50.6% (Table 1). Of all 326 confirmed cases in the country, 268 (86.7%) involved men, with a fatality rate of 49.6%; higher than that recorded among women: 39.7%. There was a proportion of 4.62 sick men for each sick woman. A similar phenomenon happened concerning deaths ratio: for each woman death there were 5.78 men deaths.
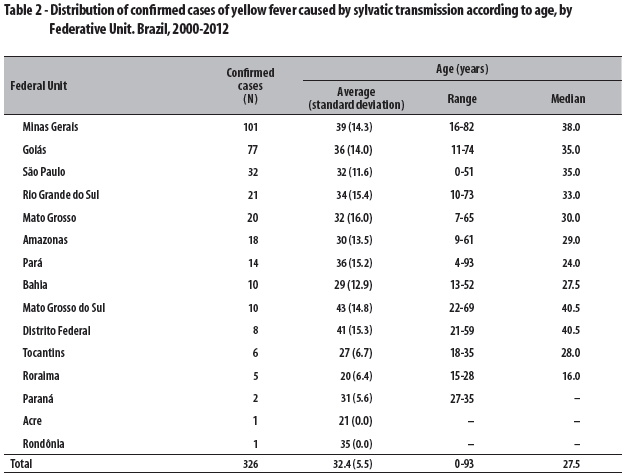
With regard to occupation, 45% of the people affected by YF were rural workers. According to age group, it was observed that the group of young adults was the most affected. The average age was 32 years old, with a range from zero to 93 years old. The state with the highest average age was Mato Grosso do Sul: 43 years old. (Table 2).
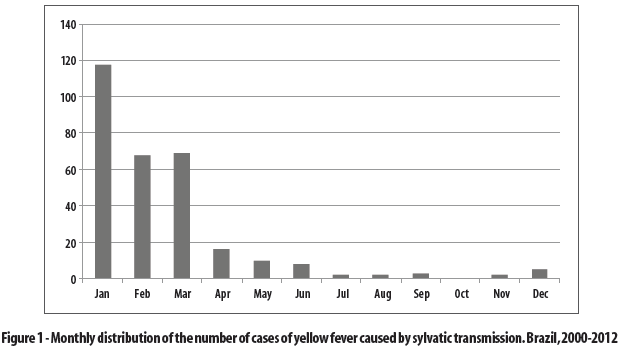
With regard to seasonality of the YF, 95% of the cases were registered from January to June (Figure 1).
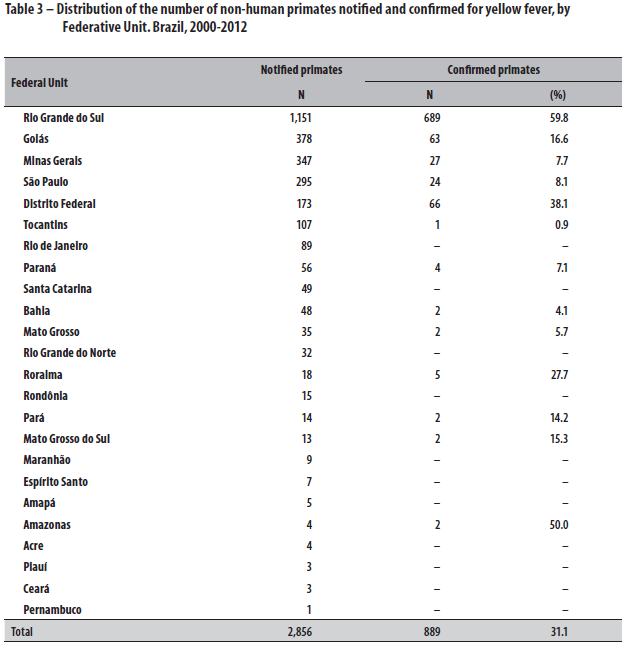
In epizootic terms, there were a total of 2,856 non-human primates notified with suspected YF, of which 889 cases (31.1%) were confirmed by laboratory tests. Among all the Federative Units, Rio Grande do Sul recorded the highest number of NHP with positive laboratory test: 77.5% of NHP reported in the whole country and with results that confirmed yellow fever (Table 3).
According to the Ministry of Health, 110,081,513 doses of vaccines against YF were administered in Brazil, from 2000 to 2012, immunizing the population in all Federative Units (Table 4). The observation of epizootics served as a risk predictor element of occurrence of YF in humans (Ordinance No. 5 issued by SVS/MS, dated, February 21, 2006) and triggered a series of actions, including dynamic revision of the transmission areas and adequacy to vaccination strategies each time the local vaccination coverage was expanded.
Discussion
Ninety-five percent of cases of YF presented in this study were confirmed by laboratory tests. The most affected group was the young adult male rural workers. Rural areas are considered risk for the spreading of this virus.
The year 2000 was the one with the highest number of confirmed cases in the country, during the studied period. A large number of cases in densely populated areas has been identified, such as in the regions South, Southeast and Midwest, areas with high density of infestation of the urban vector, Aedes aegypti. This fact is preocupying, given that it indicates the possibility of an increase of risk of re-urbanization of the disease transmission, since the sylvatic transmission seems to be migrating to densely populated areas, where the mosquito vector of urban cycle, the Ae. Aegypti, is abundant. The reasons for this geographic expansion, for now, are not fully known.
The incidence of sylvatic YF proved to be seasonal, coinciding with the rainy season in the endemic area, when there is increased density of vectors. In Brazil, this period ranges from January to June. Over the years, the incidence of YF has shown a cyclical trend, with an increase within every five to seven years. This fact is explained by the higher viral circulation, due to the accumulation of susceptible monkeys.1
The fatality rate of YF in Brazil is very high, and is indeed, too relevant. Besides, the virulence of the infectious agent, the delay in identifying the disease and the absence of effectively ethiologic treatment contribute to the high fatality rate.1
The occurrence of cases and deaths was higher among men, probably due to the work in rural areas and, consequently, a greater exposure to infection. According to the results of the present study, as noted above,11 the most affected group by YF showed a similar profile, mostly represented by male young adults, with an average age of 32 years old and usually rural workers. It is the population under greater exposure to environments where the viruses are circulating.
The Epizootics Surveillance System in non-human primates was released in 1999, alter an intense transmission period in the Midwest region of the country, where the occurrence of animal diseases in NHP preceded and followed the occurrence of human cases of sylvatic transmission. Since then, the Ministry of Health started to encourage regional initiatives to detect virus circulation in its enzootic cycle.12
The main prevention measure of YF in humans is vaccination. Since 1998, the Ministry of Health has intensified the implementation of the YF vaccine, including it in the vaccination calendar. The vaccine is produced in Brazil and prepared with live attenuated virus; generally, there are a few reactions, respecting contraindications, and it has been used for more than 60 years, proving to be the most effective method to prevent yellow fever. By the time of the conclusion of the present study, there was no record in the database on the vaccination status of the vast majority of cases occurred during the studied period. The administration of the vaccine aims at protecting the population by fostering the development of protective antibodies13 and to establish an epidemiological barrier to the spread of the sylvatic virus in urban areas, where the Ae. aegypti is present.14
In the country, the areas considered at higher risk for YF include the North and Midwest regions, the states of Minas Gerais and Maranhão and part of Bahia, Piauí, São Paulo, Rio Grande do Sul, Santa Catarina and Paraná.2 Considering the risk of yellow fever virus ciruclation, Brazil is divided into two main areas. The first area, target of vaccine recommendation presents the highest risk for the disease. The second, an area with no vaccine recommendation represents a smaller risk for the disease.2
After the occurrence of recent serious events -even lethal - ascribed to the YF vaccine, there is no agreement on the vaccination of the population that lives in areas infested by Ae. aegypti and (or) by Ae. albopictus. Specialists who are not in favor of the geographical expansion of immunization coverage take into account relevant facts, such as the occurrence of deaths associated with the vaccine in the states of São Paulo, Minas Gerais, Rio Grande do Sul and Goiás. In the literature, there have been reports of death associated with the vaccine in the United States and Australia. The factors that lead some people to present serious adverse events associated with the vaccine are not fully known.3
There are also favorable views on expanding the present area of vaccination coverage, depending on the sylvatic YF transmission detection in regions of the states of Bahia and Sao Paulo (2000) and Minas Gerais (2001). Those areas are infested with Ae. aegypti and have had no cases of the sylvatic form of the disease for years. In 2001, in the western region of Rio Grande do Sul state, there was viral circulation with the death of monkeys confirmed in by laboratory tests, in a place where there had also been no record of animal diseases by YF for more than 20 years.3
A limitation of this study lies in the use of secondary data, which can lead to a possible underreporting of cases. The low number of cases of sylvatic yellow fever reduces the risk of reintroduction of the urban form of the disease. However, when visiting cities with high infestation by Ae. aegypti, people from endemic areas in the early stages of the disease and during the period of transmissibility, bring risk to urban transmission of yellow fever in Brazil.
Authors' contributions
Cavalcante KRLJ contributed to the analysis, interpretation of data and writing of the manuscript.
Tauil PL contributed in the conception and design of the study and on the relevant critical review of the intellectual content of the manuscript.
Both authors approved the final version of the manuscript and are responsible for all aspects of the study, ensuring its accuracy and integrity.
References
1. Lopes AC. Tratado de clínica médica. 2.ed. 3841-3849. São Paulo: Roca; 2009. v. 3.
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância em Saúde. Manual de vigilância de epizootias de primatas não humanos. Brasília: Ministério da Saúde; 2005. p.25-26. (Serie A. Normas e Manuais Técnicos).
3. Tauil PL. Aspectos críticos do controle da febre amarela no Brasil. Rev Saude Publica. 2010;44(3):555-8.
4. Coura JR. Dinâmica das doenças infecciosas e parasitárias. 2. ed. Rio de Janeiro: Guanabara Koogan; 2013. v. 2 p. 1788- 1798
5. Franco O. História da febre amarela no Brasil. Rio de Janeiro: Superintendência de Campanhas de Saúde Pública, Ministério da Saúde; 1969.
6. Ministério da Saúde (BR). Fundação Nacional de Saúde. Plano de intensificação das ações de controle da dengue (PNCD): instituído em 24 de julho de 2002. Brasília: Ministério da Saúde; 2002.
7. Nobre A, Antezana D, Tauil PL. Febre amarela e dengue no Brasil: epidemiologia e controle. Rev Soc Bras Med Trop. 1994; 27 supl 3:59-66.
8. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Análise de Situação de Saúde. Dados e indicadores selecionados. Brasília: Ministério da Saúde; 2003.
9. Romano APM, Ramos DG, Araujo FAA, Siqueira GAM, Ribeiro MPD, Leal SG, et al. Febre amarela no Brasil: recomendações para a vigilância, prevenção e controle. Epidemiol Serv Saude. 2011 jan-mar;20(1):101-6.
10. Vasconcelos PFC. Febre Amarela. Rev Soc Bras Med Trop. 2003 mar-abr;36(2):275-93
11. Vasconcelos PFC. Febre amarela: reflexões sobre a doença, as perspectivas para o século XXI e o risco de reurbanização. Rev Bras Epidemiol. 2002;5(3):244-58
12. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Guia de vigilância de epizootias em primatas não humanos e entomologia aplicada à vigilância da febre amarela. 2. ed. Brasília: Ministério da Saúde; 2014.
13. Ministério da Saúde (BR). Superintendência de Campanhas de Saúde Publica. Manual de vacinação anti-amarílica: instruções para vacinadores. Brasília: Ministério da Saúde; 1987.
 Correspondence:
Correspondence:
Karina Ribeiro Leite Jardim Cavalcante -
Ministério da Saúde, Secretaria de Vigilância em Saúde,
Coordenação-Geral de Laboratórios, SCS,
Quadra 4 Bloco A, Edifício Principal, 3° andar,
Brasília-DF, Brasil. CEP: 70304-000
E-mail: karina.cavalcante@saude.gov.br
Received on 20/01/2015
Approved on 18/09/2015
*Part of the master thesis of Karina Ribeiro Leite Jardim Cavalcante, submitted to the Master Program in Public Health of the University of Brasília/Ministry of Health, July, 2014.











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI
