Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.1 Brasília jan./mar. 2016
http://dx.doi.org/10.5123/S1679-49742016000100005
ORIGINAL ARTICLE
Analysis of adverse events following immunization in Minas Gerais, Brazil, 2011: a cross-sectional study*
Suelem Santos Silva1; Valéria Conceição de Oliveira2; Helen Cristiny Teodoro Couto Ribeiro2; Tamara Gabriela Silva Alves2; Ricardo Bezerra Cavalcante2; Eliete Albano de Azevedo Guimarães2
1Governo do Estado de Minas Gerais, Secretaria de Estado de Saúde, Divinópolis-MG, Brasil
2Universidade Federal de São João Del Rei, Faculdade de Enfermagem, Divinópolis-MG, Brasil
ABSTRACT
OBJECTIVE: to analyze the main adverse
events occurring following immunization in Minas Gerais State, Brazil, in 2011.
METHODS: this is a cross-sectional study using secondary data from the
Brazilian Information System on Adverse Events Following Immunization.
RESULTS: 1,449 adverse events were found; more than half occurred in children under one year of age (56.1%; OR=1.39; 95%CI:1.08;1.78); the highest
rates per 100,000 doses administered were found in the Southern Triangle (36.3), Northwest (25.7) and Southern (22.7) areas of the state; tetravalent vaccine
had the highest reactogenicity (46.1%) and hypotonic-hyporesponsive episodes were the most frequent event (15.9%); events were significantly associated
with inactivated vaccines (OR=4.08; 95%CI:3.51;4.75); most of the reported events were closed as 'undefined' (58.9%).
CONCLUSION: events were found in all regions of Minas Gerais state and
were most frequent following administration of inactivated vaccines and among
children under one year old.
Key words: Vaccination/adverse effects; Health Information Systems; Health Evaluation.
Introduction
Adverse events following immunization (AEFI) are undesirable clinical situations that occur in individuals who have received some type of immunobiological.1 Although most AEFI are considered mild, such as local reaction and fever, they can also be moderate or severe, leading to hospitalization and to disabilities, and even to death.2 Since vaccine-induced deaths are a very rare occurrence, events classified as moderate and severe are the primary focus of AEFI surveillance.2
In 2009, a research conducted in Brazil showed that mild events represented the majority of AEFI in children vaccinated against H1N1, not requiring health treatment and disappearing within two days at the most.3 A study carried out in Cuba between 2006 and 2007 also detected the predominance of mild events, with signs and symptoms in the first 48 hours following immunization and ceasing spontaneously.4
The majority of rare AEFI - seizures, thrombocytopenia, hypotonic-hyporesponsive episode - are self-limited and do not lead to long-term issues;5 they may also result from programmatic errors, during the preparation or administration of a vaccine, caused by some procedural mistake on the part of the professionals in charge.5
In spite of being widely tested, such as clinical efficacy and seroconversion tests, and of being submitted to many quality control processes before being commercialized, immunobiologicals can still cause adverse events.6 Many AEFI are nothing more than temporal associations, and are not related to the application of the vaccines; however, when adverse events do occur, their associated factors, which may be linked to the vaccine, the vaccinated, or even the management technique of those occurrences, must be investigated.1
Health care managers and professionals must pay close attention not only to the prevention of vaccine-preventable diseases, but likewise to vaccine safety and possible AEFI. Immunobiologicals are a part of the field of Health in need of constant evaluation, monitoring, and research on the eventual risks posed by their use.1
Monitoring the safety of immunobiologicals is the main action necessary to secure levels of vaccine reliability and population adherence, as well as the continuous maintenance and reduction of controlled illnesses. In that sense, it is necessary to have surveillance systems for AEFI that provide quality data on those effects and the most vulnerable population subgroups.7 Acknowledging this need, in 2000 the Ministry of Health implemented the Brazilian Information System on Adverse Events Following Immunization (SI-EAVP), with the goal of maximizing the analyses of AEFI cases and, particularly, promoting the consolidation of data throughout the country. SI-EAPV is fed by the information provided through the adverse events notification form standardized by the Ministry.1
Understanding AEFI and their occurrence is of great importance for decision-making in related health care services and practice, and thus, the objective of this study was to analyze the main adverse events following vaccination notified in the Brazilian State of Minas Gerais, in 2011.
Methods
This is a cross-sectional study, carried out in Minas Gerais State, based on data for the year 2011 from the SI-EAVP database, which includes all notified cases in the state. The Brazilian National Immunization Program Information System (SI-PNI) database was also used to find the number of immunobiological doses administered in the analyzed period.
Minas Gerais State comprises 853 municipalities, distributed over a territory of 586,520.368 km2, with an estimated population of 20,734,097 inhabitants in 2014.8 The state was divided into 13 expanded health regions, i.e., macroregions considered as territorial basis for health care planning, given their demographic, socioeconomic, geographic, sanitary, and epidemiological characteristics, service availability and relationships between municipalities. The expanded regions are: South; Central-South; Jequitinhonha; East; North; Northwest; Eastern South; Northeast; Southern Triangle; and Northern Triangle.9
The surveillance process for adverse events comprised the notification and the investigation of events at the municipality in which they occurred, through the mandatory filling out of the forms recommended by the Ordinance No. 104 of the Ministry of Health, dated January 25, 2011.10 Each municipality forwarded the forms to the regional health management/administrative offices, where the data is consolidated and passed on to the Immunization Coordination of Minas Gerais State's Health Department (SES/MG), who is responsible for analyzing the event notifications, approving immunobiologicals, and processing and analyzing the data from SI-EAPV. Alter data consolidation, the Immunization Coordination of SES/MG forwarded the data to federal authorities.
The response variable was the presence of AEFI. The analyzed variables were:
a) sex (male; female);
b) age (in years: <1; 1 to <2; 2 to <10; 10 to <20; 20 to <60; and ≥60);
c) region where the AEFI occurred (classified by expanded health regions: South; Central-South; Jequitinhonha; East; North; Northwest; Eastern South; Northeast; Southern Triangle; and Northern Triangle);
d) types of immunobiologicals (Bacillus Calmette-Guerin [BCG]; adult diphtheria and tetanus vaccine [DT] yellow fever vaccine [YF]; hepatitis B vaccine [HB]; meningococcal C conjugate vaccine [MncC]; pneumococcal conjugate vaccine, 10-valent [Pn10]; pneumococcal vaccine, 23-valent [Pn23] influenza vaccine; measles, mumps and rubella virus vaccine [MMR]; diphtheria, tetanus, pertussis and Haemophilus influenzae type b vaccine [tetravalent]; oral poliovirus vaccine [OPV]; and oral human rotavirus vaccine (OHRV);
e) types of AEFI (ulcer larger than 1 cm following BCG; cold local abscess; hot local abscess; arthralgia; headache; headache and vomiting; persistent weeping; afebrile seizure; febrile seizure; difficulty walking; pain, redness and warmth; acute encephalopathy; induration; hypotonic-hyporesponsive episode; generalized rash; fever with temperature higher than or equal to 39.5oC; fever with temperature lower than 39.5oC; intussusception; non-suppurative lymphadenitis, lymph node larger than 3 cm; suppurative lymphadenitis; suppurative lymphadenomegaly, lymph node larger than 3 cm; non-suppurative lymphadenomegaly; myalgia; myelitis; lump; other local reactions, other severe and/or unusual events; single thrombocytopenic purpura; paresthesia; polyradiculitis (GBS); keloid; hypersensitivity reaction after 2 hours; hypersensitivity reaction within 2 hours; and generalized urticaria);
f) clinical evolution of the notified cases (cured without sequelae; cured with sequelae; death; ignored; no evolution);
g) notified cases outcomes (confirmed; undefined; discarded);
h) course followed (contraindication of subsequent doses of the vaccine that caused the AEFI; maintenance of the vaccine schedule of the immunobiological that caused the AEFI; contraindication of subsequent doses; and maintenance of the vaccine schedule, with supervision).
The variables "presence of adverse events in previous doses", "pre-existing illnesses", "family history" and "hospitalization", whose fields were over 20% incomplete, were considered of bad quality,11 and were, thus, not included in this study.
For the characterization of the studied population, the frequency of the variables were distributed, and the AEFI incidence rate (IR), calculated (IR = number of events caused by an immunobiological in a given place and time period/total number of doses administered in that same place and time period x 100,000), by the state's expanded health regions, and type of immunobiological.
The magnitude of the association between the presence of AEFI and the co-variables "age group" and "type of immunobiological" was estimated through the odds ratio (OR) and 95°% confidence intervals (95%CI). BioEstat 5.0 and Microsoft Office Excel® were the softwares used for tabulation and data analysis.
This study was conducted in accordance to the Resolution No. 466/2012 of the National Health Council (CNS), and approved by the Research Ethics Committee of the São João de Deus Hospital - Log No. 141/2011 - dated June 22, 2012.
Results
Throughout the time of the study, 1,458 adverse events following immunizations recommended by the National Immunization Program (PNI) were notified. Nine events had inconsistencies in the variable "age", and were excluded from the analyses. Thus, 1,449 AEFI were included in this study. 10,590,033 administered doses of immunobiologicals were identified in the PNI; of those, 1,964,955 had inconsistencies in the variable "age", and 818,650 in the variable "type of immunobiological", and were excluded from the analyses.
Of all individuals who had AEFI, 544 (53.5%) were female (data not shown in the table). Over half of AEFI occurred in the population younger than 1 year of age (56.1%), followed by adults (16.8%). Through the univariate analysis, it was found that, when compared to the elderly, children younger than 1 year of age (OR 1.39; 95%CI 1.08;1.78) and adults aged 20 years or older but younger than 60 years of age (OR: 1.99; 95%CI 1.52;2.61) were more likely to show adverse events. The occurrence of AEFI was lower among adolescents (OR=0.38; 95%CI 0.23;0.62) (Table 1).
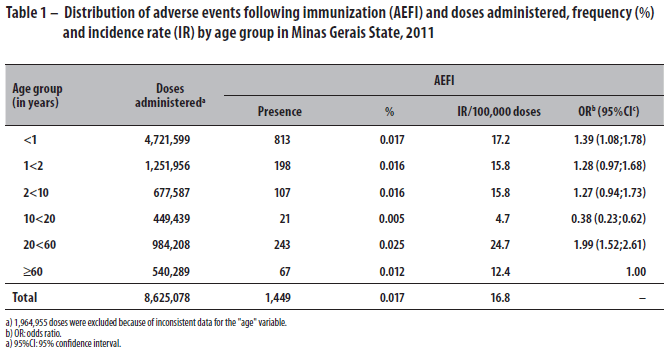
When evaluating the number of AEFI by expanded health regions, higher incidence rates were found in the Southern Triangle (IR = 36.3/100,000 doses administered), Northwest (IR = 25.7/100,000 doses administered) and South (IR = 22.7/100,000 doses administered) regions of the state (Table 2).
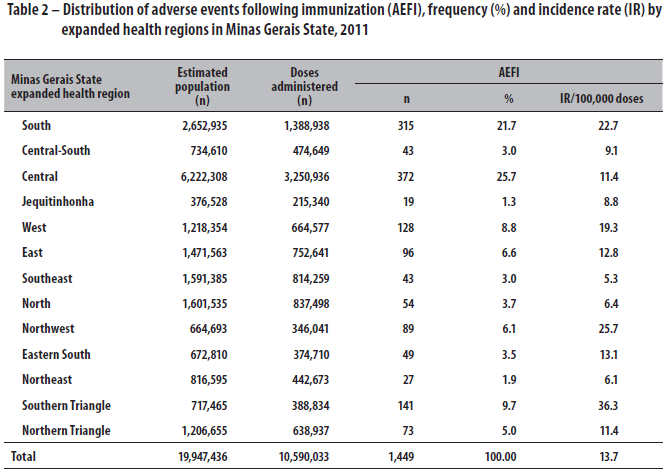
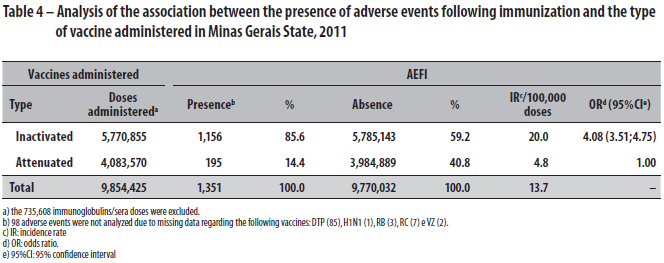
Regarding the distribution of doses administered per immunobiological (Table 3), it was observed that the tetravalent vaccine had the greatest reactogenicity (46.1%), followed by the influenza vaccine (14.3%). Those and the pneumococcal 23-valent vaccine were the immunobiologicals with the greatest risk of causing adverse events among the people vaccinated in that period. Analyzing the association between the presence of AEFI by the type of immunobiological (inactivated or attenuated vaccines), it was found that inactivated vaccines (OR=4.08; 95%CI 3.51;4.75) had greater chances of causing adverse events when compared to attenuated vaccines (Table 4).
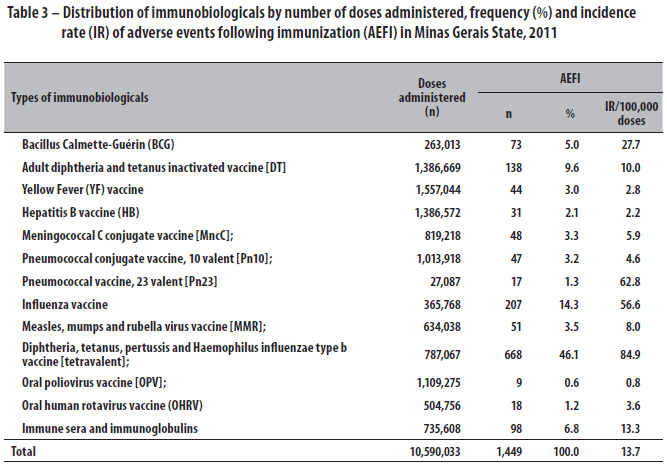
With regard to the dose related to the AEFI, 38.1% of the cases were found to be related to the first administered dose, 20.8% to the second dose, and 11.8% to the third dose. There was a lower frequency of AEFI related to the last doses of vaccination schedules and booster shots: first booster (11.3%) and second booster (4.7%) (data not shown in the table).
Table 5 shows the types of AEFI by the presence of systemic and local events. The most frequent events were hypotonic-hyporesponsive episode (HHE) (15.9%); induration (11.7%); fever with temperature lower than 39.0o C (11.7%); and pain, redness and warmth (8.6%).
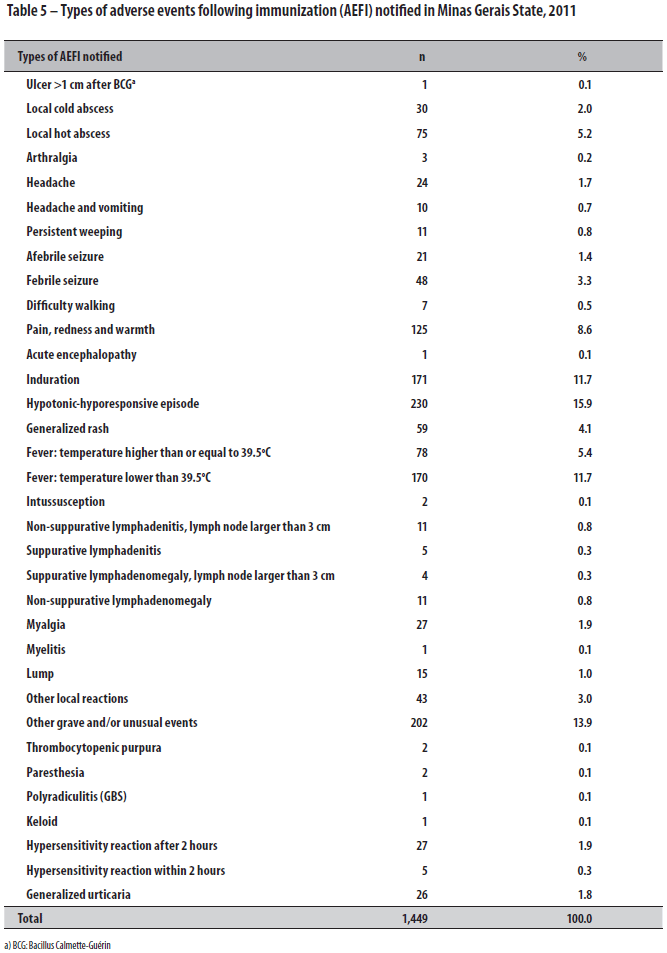
Of the 230 notified cases of hypotonic-hyporesponsive episode (HHE), 44.1% were confirmed, 55.5% were classified as undefined, and 0.4% were discarded. On the course followed after AEFI notified as HHE, the DTP vaccine with whole cells was contraindicated, the acellular DTP being recommended instead for 94.8% of the cases. In 2.2% of the cases, the DPT vaccine was contraindicated; in 0.9% the schedule was kept; and 1.2% of the cases did not have that information (data not shown in the table). There were two registered cases of intussusception associated with the immunobiological rotavirus: one was notified on the second dose, not associated with the vaccine; the other was confirmed in the first dose, administered to a child aged 5 months and 23 days (data not shown in the table).
Regarding the clinical evolution of the individuals notified as having AEFI, 99.3% were cured without sequelae, 1 individual was cured with sequelae related to intussusception, 1 died of febrile seizure, and 5 cases were classified as "unknown" and/or "without evolution". Of the notified AEFI, 40.2% were closed as confirmed, 58.9% were classified as undefined and 0.9% as discarded (data not shown in table).
The courses adopted after AEFI were as follows: in 36.1% of the cases, subsequent doses of the vaccine that caused the AEFI were contraindicated and replaced by less reactogenic vaccines; 48.0% of the cases kept the immunization schedule of the vaccines that caused the AEFI; in 4.0% of the cases, subsequent doses were contraindicated; 8.8% had this field filled as "ignored"; and in 3.1% of the cases, the immunization schedules were maintained under supervision (data not shown in table).
Discussion
In this study, no differences were observed regarding the occurrence of AEFI by sex. The occurrence of AEFI all over Minas Gerais state's expanded health regions, with variations in the incidence rates among the state's expanded health regions. An association between inactivated vaccines and the individuals' age group was found, predominantly with those under 1 year of age. The immunobiological with the highest occurrence of AEFI was the tetravalent vaccine, and the most frequent event was the hypotonic-hyporesponsive episode. However, most of the notified events were closed as undefined.
Most AEFI occurred in children under 1 year of age. These results corroborate those of other national studies;12,13 and those of a survey carried out in Cuba, where 46.8% of the adverse events following immunization were found in infants.4 Currently, there are 11 vaccines available in the public health services for children under 1 year of age, potentially impacting - beyond the immunologic immaturity of those children - the greater frequency of adverse events, contributing to the significant increase of AEFI cases in that age group.13
Regarding the incidence rate of AEFI, all expanded health regions had risks of adverse events following the application of immunobiologicals. Nevertheless, that risk was unevenly distributed among the regions. Studies carried out in Brazil have also noted the heterogeneous distribution of AEFI, with greater incidence in more developed regions.14,15
The immunobiological with the greatest incidence of adverse events was the tetravalent vaccine - a combination of the DTP and the Haemophilus influenzae b vaccines -, administered to 2, 4, and 6 month-olds to prevent morbidity and mortality by diphtheria, tetanus, pertussis and meningitis caused by H. influenzae b. The results of that investigation corroborate a study carried out in 2006, in the municipality of Teresina, Piauí State, where 63.0% of the notified events were found to be related to the tetravalent vaccine.12 In São Paulo State, between 1984 and 2001, 54,204,325 doses of the DTP vaccine were administered, with 10,051 cases of adverse events related to it, corresponding to 6,266 cases, resulting in an average of 1.6 AEFI-DTP per notified case.16
The occurrence of AEFI related to the pneumococcal 23-valent and influenza vaccines was also detected. This fact is believed to have suffered influence of the raise in the number of doses administered in the annual influenza-immunization campaigns in April and May, when the antipneumococcal vaccine is offered. As the goal of mass immunization campaigns is to vaccinate a large amount of the population in a short period, they result in safety challenges and increasing AEFI rates.5
Inactivated vaccines were associated to a greater number of adverse events when compared to attenuated vaccines. The presence of aluminum as an adjuvant in inactivated vaccines predisposes the immunized individual to more significant local adverse events, such as hyperemia, edema, and pain at the application local, besides increasing the adverse events' risk with subsequent doses,17 related to immune complex deposition.1 It is also noticeable that flaws in the vaccines preservation, such as exposure to low temperatures, may result in the inactivation of the aluminum adjuvant, by freezing.18 In this case, the efficacy of the vaccine may decrease, and the risk of adverse events following immunization, such as sterile abscesses, increase.19
With regard to the greater frequency of hypotonic-hyporesponsive episodes (HHE), similar results were likewise observed in the aforementioned studies, carried out in São Paulo State16 and in the municipality of Teresina, Piauí State.12 HHE is a severe event, of difficult clinical characterization, due to its short-lived occurrence, among other reasons. That makes it hard for the health care professional to evaluate, since they might lack the necessary training to define the event and might thus take it for a clinical manifestation of another illness.20
HHE can be associated to various vaccines; however, it is usually related to the pertussis component of the DTP vaccine.20 A survey based on data from the Vaccine Adverse Event Reporting System (VAERS), Canada's AEFI notification system, found that, in the 215 cases evaluated, the average age at the start of an HHE was four months of age, over half of the children affected were female, with an average interval of 210 minutes between the immunization and the episode. 93% of the children affected had received a vaccine with pertussis.21 According to that same study, during HHE, 90.1% of the children presented pallor and 49%, cyanosis. The author related, furthermore, the absence of relevant conclusions regarding the family history or the mother's gestational history.21
The results of the present study on the clinical evolution of AEFI show that most events evolved into cures, without sequelae or further damage to the patients, confirming literature findings which show that the benefits of immunization outweigh the risks of possible adverse effects,13 and thus, do not imply contraindication of later doses.12 Regarding the course chosen after AEFI, it was observed that in most cases, the immunization schedule was kept, reaffirming that those events are mostly benign and fleeting.13
Over half of the events notified were classified as undefined when closed, from which issues with the quality of SI-EAVP's data (such as typos, incomplete fields and flaws in the information flow) can be inferred. This does make proper decision-making somewhat harder, besides favoring skepticism towards immunization on the part of the vaccinated and even the health care professionals, who might attribute to the vaccines events that are unrelated to them, or that mean only a temporal association.2,12
The limitations of this study were, thus, notably, the under notification of adverse events and the quality of the data available at SI-EAVP. Some fields in the recommended form were in blank. Furthermore, the "closure" field was often incorrectly filled, creating the large proportion of undefined cases and compromising the full understanding of the actual situation of AEFI, since that makes it harder to distinguish them from those not associated with the vaccines. Future studies should analyze the health care personnel's knowledge of AEFI notifications, and the implementation of the SI-EAVP.
With that purpose, beyond evaluating the information system in its ability to monitor and evaluate the notified cases of AEFI, it is also necessary to verify the quality of the form used to investigate into the event, and the insufficient number of professionals trained for this activity, once it has been incorporated into the health care service routine. The notification of AEFI as an acquired practice, and the permanent health education of the relevant personnel are fundamental steps towards guaranteeing the quality and safety of administered immunobiologicals.13 In this sense, obstacles such as insufficient numbers of health care professionals trained in AEFI surveillance, and issues with information quality, can influence the handling of each case. The continuous and systematic AEFI monitoring process is the main instrument for the safety control of vaccines.12
Even though AEFI are the object of only a limited amount of the Brazilian scientific research, knowledge on this topic can be applied in the practice of health surveillance services and in the planning and executing of actions focused on the safety use of immunobiologicals. Interventions on the organization and practical activity of health care services regarding the notification of AEFI must be stimulated at state and municipal levels.
Authors' contributions
Guimarães EAA, Silva SS and Oliveira VC were responsible for the study conception and design, data analysis, and drafting of the manuscript.
Ribeiro HCTC, Alves TGS and Cavalcante RB collaborated in the data analysis and revision of the manuscript.
All authors are responsible for all aspects of the article, including the assurance of its accuracy and integrity.
References
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de vigilância epidemiológica de eventos adversos pós-vacinação. 3. ed. Brasília: Ministério da Saúde; 2014. (Série A. Normas e Manuais Técnicos).
2. Silva Júnior AM. Proposta de gestão on-line das informações de vigilância epidemiológica de eventos adversos pós-vacinação [dissertação]. Rio de Janeiro (RJ): Escola Nacional de Saúde Pública Sérgio Arouca, Fundação Oswaldo Cruz; 2010.
3. Andrade GN, Pimenta AM, Silva DA, Madeira AMF. Eventos adversos pós-vacinação contra influenza pandémica A (H1N1) 2009 em crianças. Cad Saude Publica. 2012 set; 28(9):1713-24.
4. Mato ID, Cardeso ALC, López GJ, Valdés YL. Caracterización de eventos adversos asociados a vacunas que inmunizan contra enfermedades infecciosas: años 2006-2007. Rev Cuba Farm. jul-sep 2010; 44(3):325-35.
5. Brito GS. Eventos adversos e segurança de vacinas. In: Farht CK, Carvalho ES, Weckx LY, Carvalho LHFR Succi RFCM. Imunizações: fundamentos e prática. 5. ed. São Paulo: Atheneu; 2007. p. 43-6.
6. World Health Organization. Supplementary information on vaccine safety: part 2 : background rates of adverse events following immunization [Internet]. Geneva: World Health Organization; 2000. [cited 2014 Jun 13]. Available from: https://extranet.who.int/iris/restricted/bitstream/10665/66675/1/WHO_V-B_00.36_eng.pdf
7. Freitas FRM, Waldman EA. Vigilância de eventos adversos pós-vacina DPT e preditores de gravidade: estado de São Paulo, 1984-2001. Epidemiol Serv Saude. 2007 abr-jun;16(2):136-8.
8. Instituto Brasileiro de Geografia e Estatística. População estimada de 2010 Minas Gerais [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2014. [citado 2014 nov 12]. Disponível em: http://www.ibge.gov.br/home/estatistica/populacao/censo2010/tabelas_pdf/total_populacao_minas_gerais.pdf
9. Brasil. Ministério da Saúde. Portaria n° 399, de 22 de fevereiro de 2006. Divulga o Pacto pela Saúde 2006: consolidação do SUS e aprova as diretrizes operacionais do referido Pacto. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2006 fev 23; Seção 1:43
10. Brasil. Ministério da Saúde. Portaria n° 104 de 25 de janeiro de 2011. Define as terminologias adotadas em legislação nacional, conforme o disposto no Regulamento Sanitário Internacional 2005 (RSI 2005), a relação de doenças, agravos e eventos em saúde pública de notificação compulsória em todo o território nacional e estabelece fluxo, critérios, responsabilidades e atribuições aos profissionais e serviços de saúde. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2011 jan 26; Seção 1:37.
11. Romero DE, Cunha CB. Avaliação da qualidade das variáveis epidemiológicas e demográficas do Sistema de Informações sobre Nascidos Vivos, 2002. Cad Saude Publica. 2007 mar; 23(3):701-14.
12. Araújo TME, Carvalho PMG, Vieira RDF. Análise dos eventos adversos pós-vacinais ocorridos em Teresina. Rev Bras Enferm. 2007 jul-ago;60(4):444-8.
13. Piacentini S, Contrera-Moreno L. Eventos adversos pós-vacinais no município de Campo Grande (MS, Brasil). Ciênc Saude Coletiva. 2011 jan-fev;16(2):531-6.
14. Monteiro SAMG. Avaliação dos eventos adversos pós-vacina tetravalente: Brasil, 2002-2005 [dissertação]. Cuiabá (MT): Universidade Federal de Mato Grosso; 2007.
15. Montenegro SAMG, Takano OA, Waldman EA. Avaliação do sistema brasileiro de vigilância de eventos adversos pós-vacinação. Rev Bras Epidemiol. 2011 set;14(3):361-71.
16. Freitas FRM, Sato HK, Aranda CMSS, Arantes BAF, Pacheco MA, Waldman EA. Eventos adversos pós-vacina contra a difteria, coqueluche e tétano e fatores associados à sua gravidade. Rev Saude Publica. 2007 dez;41(6):1032-41.
17. Hunter RL. Overview of vaccine adjuvants: present and future. Vaccine. 2002 May;20 Suppl 3:S7-12.
18. Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014 Sep;42(5):237-59.
19. World Health Organization. Aide-memoire for prevention of freeze damage to vaccines [Internet]. Geneva: World Health Organization; 2007. [cited 2014 May 10]. Available from: http://apps.who.int/iris/bitstream/10665/69673/1/WHO_IVB_07.09_eng.pdf
20. Waldman EA, Luhm KR, Monteiro SAMG, Freitas FRM. Vigilância de eventos adversos pós-vacinação e segurança de programas de imunização. Rev Saude Publica. 2011 fev;45(1):173-84.
21. DuVernoy TS, Braun MM. Hypotonic-hyporesponsive episodes reported to the Vaccine Adverse Event Reporting System (VAERS), 1996-1998. Pediatrics. 2000 Oct;106(4):E52.
 Correspondence:
Correspondence:
Eliete Albano de Azevedo Guimarães -
Rua Sebastião Gonçalves Coelho, No. 400,
Bairro Chanadour, Divinópolis-MG,
Brasil. CEP: 35501-296
E-mail: elietealbano@ufsj.edu.br
Received on: 10/07/2014
Approved on: 28/10/2015
*This research was funded with resources from the National Council for Scientific and Technological Development / Ministry of Science, Technology and Innovation - CNPq/MCTI: Notice No. 004/2012/PROPE - undergrad research grant for Tamara Gabriela Silva Alves.











 texto em
texto em 

