Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.2 Brasília abr./jun. 2016
http://dx.doi.org/10.5123/S1679-49742016000200004
ORIGINAL ARTICLE
Use of generic drugs in São Paulo City, Brazil, in 2003: a population-based study
1Universidade de São Paulo, Faculdade de Medicina, São Paulo-SP, Brasil
2Universidade Federal do Mato Grosso, Departamento de Estatística, Cuiabá-MT, Brasil
3Universidade Estadual de Campinas, Faculdade de Ciências Médicas, Campinas-SP, Brasil
4Universidade de São Paulo, Faculdade de Saúde Pública, São Paulo-SP, Brasil
OBJECTIVE:
to analyze the use and perception of generic drugs by people with diabetes and hypertension in São Paulo City, Brazil, considering the Brazilian Generic Drug Policy.
METHODS:
this was a cross-sectional study using data from a household health survey (ISA-Capital) in 2003; analysis was performed on knowledge regarding generic drugs and on the association between their use and sociodemographic and socio-economic characteristics.
RESULTS:
603 people with hypertension and diabetes were included in the study, low use of generic drugs was found (33.3% and 26.3, respectively) and low cost was the major reported advantage of generic drugs (71.0% and 71.1%, respectively); there was no statistically significant difference between the use of generic medication and age, sex or schooling.
CONCLUSION:
low cost and there being no difference between generic drug use and education level strengthen the importance of generic drugs for promoting equity and universal access to medication.
Key words: Generic Drug Policy; Equitable Access; Universal Access to Health Care Services; Chronic Disease; Cross-Sectional Studies
Introduction
In Brazil, every citizen is entitled to health services, having the right to receive the essential medications that are used to treat the most prevalent diseases in our population.1,2 The medicines supply, one of the Brazilian National Health System's (SUS) branches, accounts for one of the highest expenditures of the public health system, which is reflected by a raise in programs and policies that promote a more equitable access to medications by the population, from both the public and private sectors - such as the public policy of prices reduction.1-3
The access to continuous-use medication is an important strategy for controlling the prevalent diseases in Brazil.1-3 Among the aforementioned programs, we can highlight those that are related to essential medications: drugs for diabetes mellitus and hypertension, for example,2 important mortality and morbidity causes that affect men and women of all social classes.3,4 The hypertension prevalence around the world is too high, especially in Brazil, where it was estimated in 21.6%, in 2006.5 In 2010, the prevalence of diabetes in the world was 6.4%.6 In 2006, in Brazil, the prevalence of diabetes corresponded to 5.3%.5 The access to medications is one of the priority interventions to control hypertension and diabetes mellitus.3-5
There are three types of drugs: reference, similar or generic.1-3 The generic drug has a lower cost when compared to reference drugs.7 The interchangeability of a generic drug with a reference drug is proved by pharmaceutical equivalence tests and bioequivalence.7,8
In 1999, when the policy of generic drugs8 started in Brazil, health professionals, mainly physicians and pharmacists, started education campaigns in order to enhance the knowledge and the use of generic drugs. These campaigns have been intensified since the implementation of this new policy.
In the country, the generic drug policy implies financial expenditures to the health system, with impacts in the private sector, by stimulating the competition in the pharmaceutical industry,7 and in the public sector, by allowing the purchase of drugs in a lower cost, resulting in the reduction of public expenses.7,8 It is necessary to conduct studies in order to investigate if the government strategy of expanding and promoting equitable access of the population to medications - especially the essential drugs - has been effective.7,8 After all, one of the functions of the State is to ensure the equitable access to medications by the whole population.1,9,10
The objective of this study is to analyze the use and perception of generic drugs by people with diabetes and hypertension in São Paulo City, Brazil, considering the Brazilian Generic Drug Policy.
Methods
A population-based cross-sectional study was conducted, using data from São Paulo city Household Health Survey (ISA-Capital), collected through household interviews performed in 2003.
The ISA-Capital 2003 used a cluster sampling stratified in two stages. In the first stage, 60 census tracts were randomly chosen, with probability proportional to the size - expressed by the number of cities in each tract, according to the Brazilian Institute of Geography and Statistics (IBGE).11 In the second stage, a systematic sample of households was randomly chosen in each census tract, based on the list of households previously listed. The full methodology of the ISA-Capital is available at http://www.fsp.usp.br/isa-sp/.
The individuals considered in this study were those that self-reported hypertension or diabetes mellitus, aged 20 years old or more, who lived in São Paulo city, and who had taken a medicine within three days before the interview - considering here any drugs for hypertension, diabetes or for treating any other disease.
The variables of interest of the study were (i) the use of generic drugs and (ii) the perception on generic drugs by the surveyed population. The use of generic drugs was assessed through the following question:
"Are you taking any generic drug?" (Possible answers: yes; no)
The perception on generic drugs was analyzed through the following questions:
"Do you know if it is possible to replace one of the drugs you took in the last three days by a generic drug?"
"In your opinion, are there any advantages in using generic drugs? Which?"
"In your opinion, are there any disadvantages in using generic drugs? Which?"
Besides the questions related to the use of medications, hypertension and diabetes mellitus, we performed a demographic description based on sex (male; female), age group (in years: 20 to 59 [adults] 60 or more [elderly]) and education level of the family head (in years of schooling: 0 to 7; 8 or more).
For the qualitative variables, we calculated the percentage and respective 95% confidence intervals (95%CI). The Pearson chi-square test (Rao-Scott) was used to verify if there was any association between the use of medication and the sociodemographic characteristics, which was considered statistically significant when p<0.05.
The data analysis was performed with the program Stata(r), version 10.0, due to the complex design of the study sample (survey mode).
All the surveyed individuals signed a Term of Consent. The study was approved by the Ethics Committee for Analysis of Research Projects of the Hospital of the Medical School of São Paulo University, in 14 February 2001, Protocol No. 381.
Results
The analyzed sample counted with a total of 603 participants: 448 individuals self-reported hypertension and having taken medicines within three days before the interview; the other 155 self-reported diabetes mellitus and also took medicines within three days before the interview (Table 1).
Table 1 - Sociodemographic characteristics of the individuals that reported hypertension and diabetes and the use of generic drugs in São Paulo-SP, Brazil, 2003
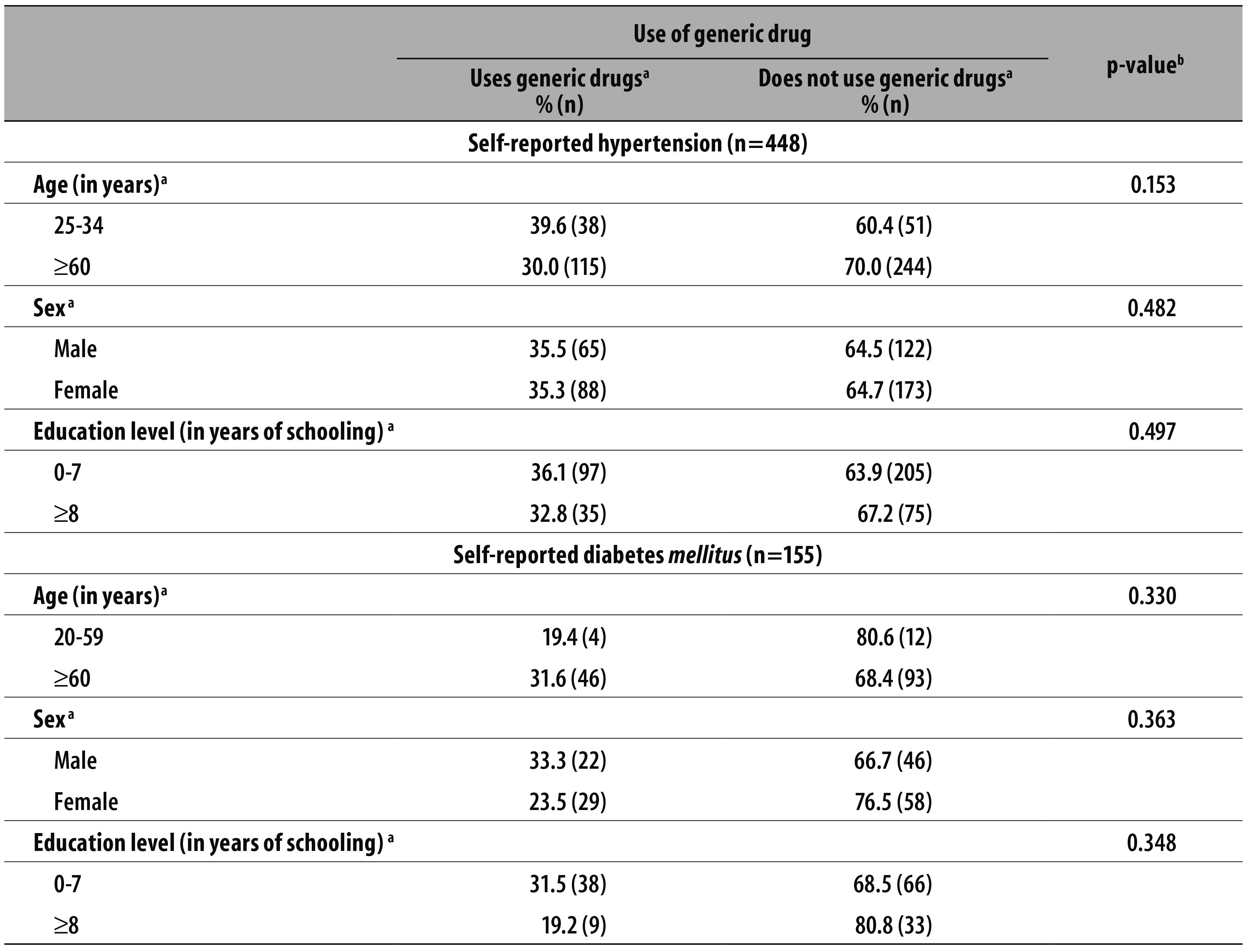
a) The answer 'Does not know or did not reply' was excluded
b) Pearson chi-square test. Significance level p<0,05.
The percentage of individuals who reported hypertension or diabetes, took a medicine within three days before the interview and used to take generic drugs corresponded to 33.3% (95%CI 25.2;42.4) (n=135) with hypertension, and 26.3% (95%CI 17.0;38.3) (n=49) with diabetes (data not presented in the tables).
In table 1, the sociodemographic characteristics of the surveyed individuals who declared they used generic drugs were described. Although there was no statistically significant association between this use and the analyzed categories - sex, age and education level -, the percentage of individuals who said they used generic drugs was low; never higher than 40.0% (Table 1).
The perception of the population towards generic drugs is presented in Table 2. More than 90.0% of those who reported hypertension (91.2% - 95%CI 85.6;98.4) and of those who reported diabetes (90.9% - 95%CI 79.6;97.3) mentioned that there are advantages in using generic drugs. Of those who reported hypertension, 22.7% did not know that it was possible to replace a reference drug by a generic drug; among those who reported diabetes, 22.2% ignored this possibility (Table 2).
Table 2 - Perception on generic drugs by the individuals who reported hypertension and diabetes in São Paulo-SP, Brazil, 2003

Among the advantages in using generic drugs pointed by the surveyed individuals, most of them mentioned the low cost: 71.0% of the individuals with hypertension and 71.1% of those with diabetes. Other mentioned advantages were "a higher amount of options", it is easier to find the drug, and the generics "being good" (data not presented in the tables).
However, 65.9% (n=265) of those who reported hypertension and 59.4% (n=87) of those who reported diabetes mentioned some disadvantages for using generic drugs, such as "not being as good as the reference drug" (10.4% of individuals with hypertension and 7.2% of self-reported diabetics) (data not presented in the tables).
Discussion
This study proved the low use of generic drugs and showed that a great proportion of the surveyed individuals did not know that it was possible to replace the reference drug by its generic. There was no statistically significant difference in the use of generic drugs according to age, sex and education level. Most of the surveyed individuals identified advantages in using generic drugs, mainly their low cost.
Although the majority of the population surveyed has seen advantages in using generic drugs, their use in 2003, after five years of the implementation of the Brazilian Generic Drug Policy, was found to be low for both the population of patients with hypertension and the patients with self-reported diabetes. Almost one decade later, a study conducted by Blatt et al12 in Tubarão, Santa Catarina State, in 2012, showed that 77.8% of the local population frequently used generic drugs, showing a higher use of generics than the identified in the present study. According to a research conducted in Rio Branco, Acre State, in 2006, only 27.9% of the surveyed individuals considered the education campaigns on generic drugs to be regular, good or great.13 Although the population knowledge on generic drugs has been growing since a specific policy was implemented in the country, more information on the quality of generic drugs is still necessary.
Besides having campaigns to increase their use, it is also necessary to provide more generic drugs in the public and private sectors, especially in the public sector. Miranda et al,14 in a study published in 2009, called the attention to the lack of generic drugs in the National List of Generic Drugs (Rename). The Rename aims at guiding the supply of drugs by SUS, especially those destined to the treatment of the most prevalent diseases in Brazil, such as diabetes and hypertension.15 According to Miranda et al, Rename's drugs should be the basis of the production of generic drugs in Brazil, in order to answer to the priority needs of the population, besides being always available to the individuals who need them;1,15 however, a study conducted by Oliveira, Assis and Barboni,16 in 2010, revealed low availability of Rename drugs in Brazilian cities.
Besides the low availability of generic drugs in the public sector,14 the study of Bevilacqua, Farias and Blatt, published in 2011,17 focusing on a medium size city of Santa Catarina State, when comparing the prices of generic and similar drugs purchased by SUS, showed a lower price for similar drugs. This finding shows that the Brazilian Generic Drug Policy is an important element for the management of Pharmaceutical Care, but it is still far from the real need of the public health.
The main disadvantage highlighted by the population in this present study was that the generic drug was "not as good as the reference drug". The Brazilian Generic Drug Policy should overcome gaps of the health-industrial complex in the country, among them, the local business-based innovation.18 After the inclusion of the generic drugs, an improve in the quality of the medicines in Brazil could be observed, for both the reference and the similar drugs.7 After the Resolutions of the National Health Surveillance Agency's Board (Anvisa) - RDC No. 13,319 and No. 13,420 -, dated 29 May, 2003, the tests of bioequivalence and relative bioavailability on similar drugs became mandatory, requiring from them the same quality applied to the generic drugs. Despite the advances in specific Legislation, according to Gadelha,21 the Brazilian health-industrial complex, which includes the local pharmaceutical industry is not standardized. The big contrast in the quality of the pharmaceutical industrial park, for both generic and similar drugs, is one of the problems faced in the Brazilian market, due to the need imposed to the public sector of getting good quality at a low cost, among its criteria to purchase medicines.22
The surveyed population pointed that the main advantage in using generic drugs is their lower cost. The low cost of generic drugs is the main reason for replacing a reference drug by a generic one.7,23 This study reinforces the importance of the generic drug to promoting universal access to medication, whose recent expansion happens not only due to the generic drug itself, but also to other Public Health policies and programs.7,24,25 For the users, one of the effects of the generic drug policy in Brazil8 is the option of buying a drug which is equivalent in quality to the reference drug, but cheaper; this is an important resource for increasing access to medication, although this access is still lower than the expected. A study developed by Barros et al26 in Brazil, in the period 2003-2008, found that hypertension and diabetes are more prevalent in the groups of individuals with lower education level and who do not have health insurance. The aforementioned study conducted by Miranda et al,14 based on the five Brazilian regions, reminds that the low availability of generic drugs at SUS jeopardizes mainly individuals with lower income and who depend on the public system for health care.
There was no statistically significant difference when comparing the use of generic drugs and the population's education level. When we use the education level as an indicator,27 it is possible to conclude that all the people of all social classes use generic drugs and, consequently, the generic drug policy can contribute to achieving equitable and universal access to these products. This is an important characteristic of the Generic Drug Policy in Brazil, a country where the economic, social and health care inequality requires the formulation of equitable public policies.9,10 If equity, one of the main principles of SUS, has not been fully achieved yet,28 the National Drug Policy1 gave a great step towards the reorganization of the Pharmaceutical Care, whose accomplishment aims at the timely access to adequate amounts of medication, with the correct provision to control high impact diseases, such as the hypertension and diabetes mellitus.
Individuals of different education levels use generic drugs, hence the deduction that the national policy for these drugs may not only contribute to the equity promotion, but also be of great importance to its equitable access and expansion to the users.
A limitation of this study is the fact that the data were collected in 2003. Since then, a series of political and economic changes have happened in Brazil, including the establishment of policies to improve the access to medication, as the implementation, in 2004, of the Popular Pharmacy Program (Programa Farmácia Popular), which has a big demand from SUS users,24 and, in 2011, of the Program Health is Priceless (Programa Saúde Não Tem Preço), responsible for providing free medicines for hypertension and diabetes control to patients.25
The sociodemographic conditions of the population with hypertension and diabetes who used generic drugs were analyzed. Some results were possibly influenced by the small number of participants: for instance, the variable education level was divided into two categories, mainly due to the small size of the population with zero to three years of schooling. It was not possible to analyze the population income due to the excess of missing data for this variable. Another limitation was the fact that the study was based in self-reported data, which were subject of no answer - 'did not know or did not answer' - and to memory bias.
In the ISA-Capital survey, the questions related to medications were exclusively about the generic drugs, without mentioning the similar drugs. It is important to highlight that the similar drug, in some cases, is cheaper than the generic drug; and is usually cheaper than the reference drug.14
This study brought information on the use of generic drugs and sociodemographic characteristics of the population with diabetes and hypertension, diseases that are priority in the Brazilian Public Health. Its results may contribute to the theoretical basis for managers and decision makers, at improving the policies that aim at promoting the equitable access to medication, and, especially, in developing education campaigns on generic drugs, directed to the general population and health professionals.
We recommend the conduction of more researches on the current use of generic drugs and the policies that aim at improving the access to drugs by the Brazilian population.
Referências
1. Ministério da Saúde (BR). Secretaria de Políticas de Saúde. Departamento de Formulação de Políticas de Saúde. Política nacional de medicamentos. Brasília: Ministério da Saúde; 2001. p. 5-35. (Série C. Projetos, Programas e Relatórios; n. 25). [ Links ]
2. Ministério da Saúde (BR). Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Assistência Farmacêutica e Insumos Estratégicos. Relação nacional de medicamentos essenciais: Rename. Brasília: Ministério da Saúde; 2010. p. 15-232. [ Links ]
3. Malta DC, Morais Neto OL, Silva Júnior JB. Apresentação do plano de ações estratégicas para o enfrentamento das doenças crônicas não transmissíveis no Brasil, 2011 a 2022. Epidemiol Serv Saude. 2011 out-dez;20(4):425-38. [ Links ]
4. Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011 Apr;377(9775):1438-47. [ Links ]
5. Schmidt MI, Duncan BB, Hoffmann JF, Moura L, Malta DC, Carvalho RMSV. Prevalência de diabetes e hipertensão no Brasil baseada em inquérito de morbidade autorreferida, Brasil, 2006. Rev Saude Publica. 2009 nov;43(2):74-82. [ Links ]
6. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010 Jan;87(1):4-14. [ Links ]
7. Dias CRC, Romano-Lieber NS. Processo da implantação da política de medicamentos genéricos no Brasil. Cad Saude Publica. 2006 ago;22(8):1661-9. [ Links ]
8. Brasil. Lei nº 9.787, de 10 de fevereiro de 1999. Altera a Lei no 6.360, de 23 de setembro de 1976, que dispõe sobre a vigilância sanitária, estabelece o medicamento genérico, dispõe sobre a utilização de nomes genéricos em produtos farmacêuticos e dá outras providências. Diário Oficial da República Federativa do Brasil, Brasília (DF),1999 fev 11; Seção 1:1 [ Links ]
9. Silva LMV, Almeida Filho N. Eqüidade em saúde: uma análise crítica de conceitos. Cad Saude Publica. 2009;25 supl 2:217-26. [ Links ]
10. Paim JS, Silva LMV. Universalidade, integralidade, equidade e SUS. BIS Bol Inst Saude. 2010 ago;12(2):9-14. [ Links ]
11. Instituto Brasileiro de Geografia e Estatística. Cidades @: São Paulo. [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2016 [ citado 2016 fev 26]. Disponível em: Disponível em: http://www.ibge.gov.br [ Links ]
12. Blatt CR, Trauthman SC, Schmidt EH, Marchesan S, Silva LM, Martins JL. Conhecimento popular e utilização dos medicamentos genéricos na população do município de Tubarão, SC. Cienc Saude Coletiva. 2012 fev;17(1):79-87. [ Links ]
13. Faria MAS, Tavares-Neto J. Conhecimento popular sobre medicamento genérico em um distrito docente-assistencial do município de Rio Branco, Estado do Acre, Brasil. Epidemiol Serv Saude. 2006 set;15(3):37-45. [ Links ]
14. Miranda ES, Pinto CBS, Reis ALA, Emmerick ICM, Campos MR, Luiza VL, et al. Disponibilidade no setor público e preços no setor privado: um perfil de medicamentos genéricos em diferentes regiões do Brasil. Cad Saude Publica. 2009 out;25(10):2147-58. [ Links ]
15. Ministério da Saúde (BR). Secretaria de Ciência,Tecnologia e Insumos Estratégicos. Departamento de Assistência Farmacêutica e Insumos Estratégicos. Relação nacional de medicamentos essenciais: RENAME 2014. 9 ed. Brasília: Ministério da Saúde; 2015. p. 6-16. [ Links ]
16. Oliveira LCF, Assis MMA, Barboni AR. Assistência farmacêutica no Sistema Único de Saúde: da Política Nacional de Medicamentos à Atenção Básica à Saúde. Cienc Saude Coletiva. 2010 nov; 15 supl 3:3561-7. [ Links ]
17. Bevilacqua G, Farias MR, Blatt CR. Aquisição de medicamentos genéricos em município de médio porte. Rev Saude Publica. 2011 jun;45(3):583-9. [ Links ]
18. Quental C, Abreu JC, Bomtempo JV, Gadelha CAG. Medicamentos genéricos no Brasil: impactos das políticas públicas sobre a indústria nacional. Cienc Saude Coletiva. 2008 abr;13 supl:619-28. [ Links ]
19. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC nº 17, de 2 de março de 2007. Dispõe sobre o registro de medicamento Similar e dá outras providências. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2007 mar 5; Seção 1:46. [ Links ]
20. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC nº 134, de 29 de maio de 2003. Dispõe sobre a adequação de produtos já registrados. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2003 jun 2; Seção 1:26. [ Links ]
21. Gadelha CAG. Desenvolvimento, complexo industrial da saúde e política industrial. Rev Saude Publica. 2006 set;40 (N Esp):11-23. [ Links ]
22. Luiza VL, Castro CGSO, Nunes JM. Aquisição de medicamentos no setor público: o binômio qualidade - custo. Cad Saude Publica.1999 out-dez;15(4):769-76. [ Links ]
23. Rumel D, Nishioka SA, Santos AAM. Drug interchangeability:clinical approach and consumer's point of view. Rev Saude Publica. 2008 Sep;40(5):921-7. [ Links ]
24. Pinto CBS, Costa NR, Castro CGSO. Quem acessa o Programa Farmácia Popular do Brasil?: aspectos do fornecimento público de medicamentos. Cienc Saude Coletiva. 2011 jun;16(6):2963-73. [ Links ]
25. Programas do Governo. Programa Saúde Não Tem Preço do governo federal [Internet]. Programas do Governo; 2016 [ citado 2016 fev 26]. Disponível em: Disponível em: http://www.programadogoverno.org/saude-nao-tem-preco/ [ Links ]
26. Barros MBA, Francisco PMSB, Zanchetta LM, Cesar CLG. Tendências das desigualdades sociais e demográficas na prevalência de doenças crônicas no Brasil, PNAD: 2003- 2008. Cienc Saude Coletiva. 2011 set;16(9):3755-68. [ Links ]
27. Cesar CLG. Condições de vida. In: Cesar CLG, Carandina L, Alves MCP, Barros MBA, Goldbaum M. Saúde e condição de vida em São Paulo: inquérito multicêntrico de saúde no estado de São Paulo - ISA/SP. São Paulo:USP/FSP; 2005. [ Links ]
28. Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011 May;377(9779):1178-97. [ Links ]
Received: September 19, 2015; Accepted: February 05, 2016











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI

