Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.3 Brasília jul./set. 2016
http://dx.doi.org/10.5123/S1679-49742016000300023
ECONOMIC EVALUATION
Outcomes in health economic evaluation studies
1Universidade de Sorocaba, Programa de Pós-Graduação em Ciências Farmacêuticas, Sorocaba-SP, Brasil
2Universidade de Brasília, Faculdade de Ceilândia, Brasília-DF, Brasil
3Universidade de Brasília, Faculdade de Medicina, Brasília-DF, Brasil
Health economic evaluation studies are conducted in many different ways, depending on the health outcomes selected.1 In the present paper, we discuss the main types of outcomes used, presenting their characteristics, how to measure them and the scientific evidences that they support. We also discuss some types of complementary analyses used to test the robustness and reliability of the findings.
Clinical outcomes
Clinical outcomes are the signs and symptoms that result of a condition or its clinical management, and are usually measured in a medical appointment.2 Some outcomes are simple to be measured, such as relevant sequelae and death. Others depend on the knowledge on the natural history of the disease, such as diabetic retinopathy. There are also those outcomes resulting from the interventions, such as adverse reactions.
In some cases, health economic evaluation studies prioritize only the outcomes used in clinical trials or those used in reports of the official sanitary department. This choice leads to selective reporting bias, meaning that only the results that worked are enhanced.3 Moreover, the use of surrogate outcomes should be avoided - e.g., biochemical and physiological parameters that are not necessarily related with important results for the patients.4 When using surrogate outcomes, it is important to justify its usage through relevant clinical evidence.
One option to enhance the statistical power of clinical researches is the use of combined outcomes.5 Many clinical results are grouped in one outcome, such as the presence of one or more comorbidities.
Utility outcomes
The utility is expressed by the preference of the individual for a given health state.6 The quality-adjusted life years - QALY and the disability-adjusted life years - DALY are commonly used.
QALY tends to be the most common indicator. It is obtained by applying techniques or tools that measure the impact of the condition in various dimensions.7 Among the most common dimensions, we can mention mobility, anxiety and depression, self-care, pain and illness. In Brazil, two tools are validated: the EQ-5D (EuroQol five dimensions questionnaire) and the SF-6D (Short-form 6 dimensions). Indirect techniques on QALY measurement include the time-trade-off, the standard gamble and the visual analog scale.6 These strategies use scales or scenario simulation.
DALY describes the quantity of years lost due to disablement and death.7 Based on the life expectance, the unproductivity years are reduced as a consequence of a damage and the years of life lost.
Regardless of the method for utility measurement, this measurement is recommended to be performed in representative sample of individuals with interest clinical condition. Besides, the utility is influenced by factors intrinsic to the context under investigation, such as the values and perceptions of the society toward adverse situations. Thus, using utility data from other countries may compromise the robustness of the study.
Monetary outcomes
Cost-benefit analyses measure the outcomes in monetary units.1 This strategy is particularly useful when it is necessary to compare interventions of distinct areas, for instance, between vaccination campaign and acquisition of Intensive Care Units (ICU) beds. However, there are ethical and methodological concerns when attributing monetary value to the human life.
Converting clinical consequences into monetary units requires a balance of individual preferences and social values. The human capital and willingness to pay approaches are the most used.8 The procedures aim at estimating costs that could have been avoided in case the intervention and the strategy under investigation had been adopted. Taking as an example the comparison between the malaria rapid-test and the microscopy, the financial benefit to measure could be the costs avoided in consequence of medical-hospital expenses and the productivity loss avoided due to incapacity and early death.9
The human capital approach considers that the value of the benefit to health is equal to the profit caused by the intervention or strategy. The salary raise is usually based on the average salary, stratified by sex, age or occupation.
The willingness to pay approach is the most recommended method.8 Avoided expenses and how much people decide to spend to reduce the chance of an undesired health event are determined. This way, intangible benefits are also included in the analysis. To deduce the values, surveys with hypothetical scenarios are designed, in order to obtain the payment intention.
Information sources on the outcomes
Reliable clinical guidelines establish the diagnosis criteria, the treatment algorithm and the mechanisms for clinical monitoring. Thus, these documents are the first sources to be consulted when identifying clinically important outcomes.
Regardless of the type of outcome, the data reliability is influenced by the study design. Finding systematic reviews remain the first option to know the consequences of interventions. It is possible to use data from the local setting whenever this measurement can be conducted. The best measurements come from randomized clinical trials. They are particularly important when they add knowledge on the main intervention or analysis strategy.
In the absence of clinical trials on the topic, one can resort to observation designs. Most recently, researchers have decided to use big databases (big data) and electronic medical records as information sources. The cohort studies are hierarchically superior comparing to case-control, and this latter are preferred to the cross-sectional studies. It is also possible to use specialists' panels, which generate information located in a lower position on the evidence hierarchy.
Complementary analyses
Not only the costs, but also results in health need to be corrected if the time horizon was longer than a year. In order to analyze different time periods, a discount rate must be used, besides the inflation adjustment. In Brazil, there is a suggestion of applying a 5% discount rate.10
Outcomes are measured in samples, in a way that there is a variety expected for the results, usually expressed through confidence intervals. Such results are used in sensitivity analyses, a subject that will be treated later in these series.
Most of times, it is possible to divide the target population into groups of better or worse prognosis. It can also be interesting to investigate the presence or absence of comorbidities in the analysis. These details increase the external validity of the economic model.
Conclusion
This paper presents the principles adopted in the selection of outcomes to be used in economic evaluation studies. Figure 1 presents a verification list to guide the work. The choice can be either for easily measured outcomes - for example, the occurrence of death -, or others which are more complex to obtain, for being multidimensional, such as life quality.
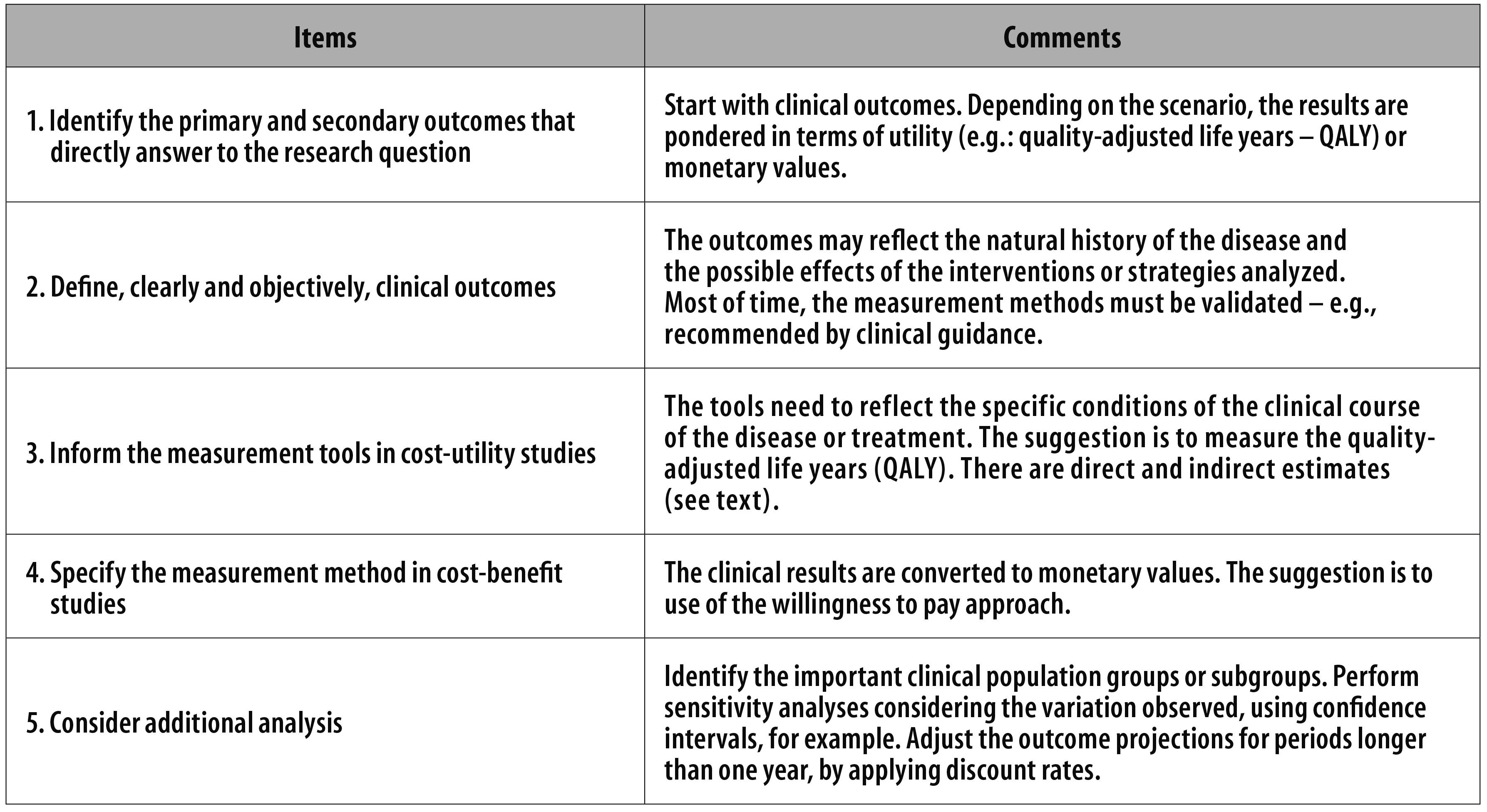
Figure 1 - Items to be verified when identifying and measuring outcomes on health economic evaluation studies
The choice for a certain outcome reflects the study perspective, the understanding of the clinical course of the disease and the analytical hypothesis. Multi-professional teams tend to make better choices. There is a considerable lack in outcomes research in Brazil. Tools that measure quality of life should be more frequently used, even in the health services routine.
Referências
1. Silva EN, Silva MT, Pereira MG. Estudos de avaliação econômica em saúde: definição e aplicabilidade aos sistemas e serviços de saúde. Epidemiol Serv Saude. 2016 jan-mar;25(1):205-7. [ Links ]
2. Willke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004 Dec;25(6):535-52. [ Links ]
3. Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta-analysis. Stat Methods Med Res. 2005 Oct;14(5):515-24. [ Links ]
4. la Cour JL, Brok J, Gotzsche PC. Inconsistent reporting of surrogate outcomes in randomised clinical trials: cohort study. BMJ. 2010 Aug;341: c3653 [ Links ]
5. Cordoba G, Schwartz L, Woloshin S, Bae H, Gotzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010 Aug;341:c3920 [ Links ]
6. Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009 Jul;339:b2688 [ Links ]
7. Rios-Diaz AJ, Lam J, Ramos MS, Moscoso AV, Vaughn P, Zogg CK, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016 Feb;11(2):e0148304. [ Links ]
8. Robinson R. Cost-benefit analysis. BMJ. 1993 Oct;307:924-6 [ Links ]
9. Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya S, Whitty CJ, et al. The impact of response to the results of diagnostic tests for malaria: cost-benefit analysis. BMJ. 2008 Jan;336(7637):202-5. [ Links ]
10. Vanni T, Luz PM, Ribeiro RA, Novaes HMD, Polanczyk CA. Avaliação econômica em saúde: aplicações em doenças infecciosas. Cad Saude Publica. 2009 dez;25(12):2543-52. [ Links ]











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI

