Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.25 n.4 Brasília oct./dic. 2016
http://dx.doi.org/10.5123/S1679-49742016000400020
Economic evaluation
Analytical models in economic evaluation studies
1Universidade de Brasília, Faculdade de Ceilândia, Brasília-DF, Brasil
2Universidade de Sorocaba, Programa de Pós-Graduação em Ciências Farmacêuticas, Sorocaba-SP, Brasil
3Universidade de Brasília, Faculdade de Medicina, Brasília-DF, Brasil
Introduction
In health economics, analytical model is the most common term used to designate the mathematical framework that represents reality with enough details in order to inform a clinical or political decision.1 The use of economic evaluation modelling can be justified by the fact that information comes from various sources and results are extrapolated into the future.2 For instance, randomized clinical trials usually analyze a restrict number of interventions, have a short follow-up period and rarely bring information on the costs and other relevant elements for decision making.3
The research field on analytical modelling has expanded in the past decades, as well as the offer of softwares to run these models. Consequently, complex mathematical and statistical procedures have become more accessible. In this paper, the main characteristics, advantages and limitations of four analytical models are presented. Figure 1 summarizes all the information.
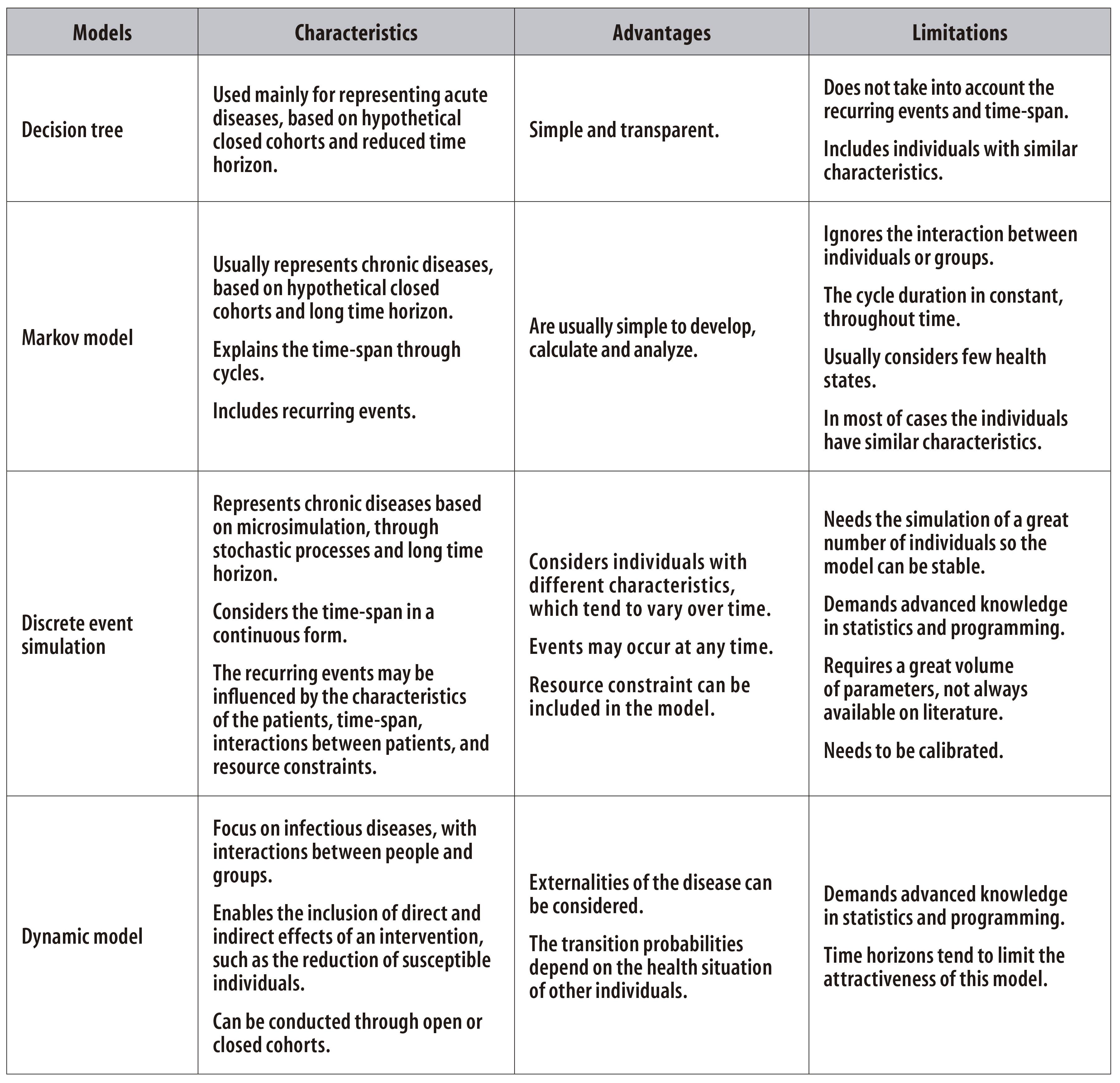
Figure 1 - Main characteristics, advantages and limitations of analytical modelling in economic evaluation studies
Decision tree
Decision tree is the simplest way of representing decision modelling.4 Graphic resources are used to draw possible pathways that patients would follow in case they were under the strategies or interventions investigated. In these pathways, the events and their respective probabilities of occurrence are included. In the end, costs and health outcomes are assigned to each itinerary taken by the individuals.
Given the limited circumstances of this type of modelling, regarding its use, only acute short-term diseases are considered here. For situations where it is necessary to represent recurring events and long time horizons - chronic diseases, for example -, there are other options of decision models, as follows.
Markov model
The Markov model has three essential characteristics. The first one is about the restricted number of clinically and economically relevant events that a cohort of individuals may experience during the evolution of the disease. These events are called health states. The second element refers to the period of time in which individuals remain in each health state, called Markov cycle. The third element relies on the transition probabilities from one health state to others. For instance, when considering breast cancer, possible health state would be: (i) local cancer; (ii) metastasis; (iii) cure; and (iv) death. Each cycle would last six months, i.e., every six months there would be a change in the health state (from local cancer to metastasis) or remaining in the same stage (local cancer to local cancer). If the time horizon were ten years, 20 cycles would be repeated, and, at the end of each cycle costs and health outcomes would be determined - these topics were discussed in the two last articles of this series.5,6
These characteristics of Markov model are appealing for chronic diseases analyses,7 since the recurring events and the explanation of time through cycles can be easily included. Usually, Markov models are calculated through hypothetical closed cohorts of individuals,8 cases in which its use presents some limitations. The first one is about the strictness of cycles' definition by Markov, which are always constant. In real life, the length of each cycle tends to vary with time, due to the natural history of the disease. The second limitation is about the memoryless between two cycles, that is, the past is not taken into consideration. For instance, there would be the same possibility of transition from metastasis to death, regardless of the individual has been with metastasis for one or four cycles, which is not supported by clinical data. An individual with metastasis for twenty-four months (a total of four cycles) has higher probability of evolving to death than an individual that has been with metastasis for only six months (only one cycle). The third limitation of Markov model is related to its interaction between individuals and groups, a characteristic that appears in the next models presented.
Discrete event simulation
The model of discrete event simulation is adequate for cases in which interactions between individuals or groups should be considered, when time-to-event is best described stochastically, when individual pathways through the model are influenced by multiple characteristics of the individuals, and when there are resource constraints.9 Different characteristics mean the possibility of including patients with different traits, such as several age groups, various family histories concerning diseases, comorbidities. The resource constraint represents a situation in which there is no intervention enough for all the individuals who need it, in a certain moment. This occurs, for example, when the health system is not able to offer a satisfactory amount of elective surgeries to the population, resulting in waiting lists. Time, in turn, may influence both the characteristics of the individuals and the events. Keeping the example of elective surgery, the longer the individual waits, the worse his/her health situation will become, that is, the individual's characteristics will get worse. A long period waiting for care also increases the probability of occurrence of events, such as reaching a more advanced stage of the disease.
Dynamic model
The dynamic model is especially appropriate to the analysis of strategies or interventions that aim to control infectious diseases.10 In this models, the direct and indirect effects of transmissions between groups are introduced, also known as externalities of the disease. The transition probabilities depend on the health situation of other individuals. For instance, if a vaccination campaign reduces the number of cases in the population, then the risk of transmission to non-ill people will be lower. In the case of chronic diseases, such as those represented by Markov models, this characteristic does not exist, because the reduction in the prevalence of cardiac diseases, for example, does not affect the individual risk of having heart problems.
For dynamic models, an important measure is the basic reproduction number, an indicator of the disease spread among the population. Values higher than one indicate an exponential growth in the number of infected cases among the susceptible population, that is, in a higher proportion. For instance, when the basic number reproduction is 3, it means that, on average, a new case generates new three cases, and each of them will generate three new cases, resulting in nine new cases, and those, in 27 new cases, and so on.
Concluding remarks
This article discussed the main analytical models recommended for guiding the decisions on the inclusion or exclusion of technology in health systems or services. As these models have different degrees of complexity, the option for one or another should happen observing the characteristics of the disease under investigation and its essential elements, such as recurring events, time horizon, interaction between individuals or groups, resource constraints and direct and indirect effects of the disease.
Referências
1. Caro JJ, Briggs AH, Siebert U, Kuntz KM; ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012 Sep-Oct;15(6):796-803. [ Links ]
2. Silva EN, Silva MT, Pereira MG. Estudos de avaliação econômica em saúde: definição e aplicabilidade aos sistemas e serviços de saúde. Epidemiol Serv Saude. 2016 jan-mar;25(1):205-7 [ Links ]
3. Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011 Feb;342:d1766 [ Links ]
4. Soárez PC, Soares MO, Novaes HMD. Modelos de decisão para avaliações econômicas de tecnologias em saúde. Cienc Saude Coletiva. 2014 out;19(10):4209-22 [ Links ]
5. Silva EN, Silva MT, Pereira MG. Identificação, mensuração e valoração de custos em saúde. Epidemiol Serv Saude.2016 abr-jun;25(2):437-39 [ Links ]
6. Silva MT, Silva EN, Pereira MG. Desfechos em estudos de avaliação econômica em saúde. Epidemiol Serv Saude. 2016 jul-set; 25(3):663-6 [ Links ]
7. Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998 Apr;13(4):397-409 [ Links ]
8. Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al; ISPOR-SMDM Modeling Good Research Practices Task Force. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health. 2012 Sep-Oct;15(6):812-20. [ Links ]
9. Karnon J1, Stahl J, Brennan A, Caro JJ, Mar J, Möller J; ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--4. Value Health. 2012 Sep-Oct;15(6):821-7. [ Links ]
10. Pitman R, Fisman D, Zaric GS, Pstma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value Health. 2012 Sep-Oct;15(6):828-34. [ Links ]











 texto en
texto en 
 Curriculum ScienTI
Curriculum ScienTI

