Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.26 n.1 Brasília jan./mar. 2017
http://dx.doi.org/10.5123/s1679-49742017000100007
Articles
Assessment of actions for breast cancer early detection in Brazil using process indicators: a descriptive study with Sismama data, 2010-2011
1Ministério da Saúde, Instituto Nacional de Câncer José Alencar Gomes da Silva, Rio de Janeiro-RJ, Brasil
2Secretaria de Estado de Saúde do Rio de Janeiro, Coordenação de Apoio à Gestão da Vigilância da Saúde, Rio de Janeiro-RJ, Brasil
OBJECTIVE:
to assess actions for breast cancer early detection in the Brazilian National Health System using process indicators.
METHODS:
this is a descriptive study with secondary data from the Breast Cancer Information System (Sismama), for the period from 2010 to 2011.
RESULTS:
5,759,503 mammograms and 44,892 histopathological tests were assessed; screening mammography was predominant (96.2%), with annual interval (44.6%) and 51.2% of the patients were in the recommended age group (50 to 69 years); mammogram report was emitted in 30 days in 61.7% of the cases; among the 17,343 malignant lesions confirmed in the histopathological tests, 66.4% were detected through clinical examination.
CONCLUSION:
screening actions and early diagnosis in disagreement with the Ministry of Health's recommendations may compromise its effectiveness and entail greater risk to women; it is necessary to improve professional’s adherence to screening guidelines, as well as enhance the control and assessment of the health services.
Key words: Breast Neoplasms; Early Detection of Cancer; Mass Screening; Mammography; Epidemiology, Descriptive
Introduction
Breast cancer is an important Public Health issue. In Brazil, 57,960 new cases were estimated in 2016, corresponding to approximately 30% of female cancers and representing the type of cancer with the highest incidence in women from almost every macroregions of the country: except for the North region, where cervical cancer occupies the first position.1 In Brazil and worldwide, breast cancer is also the most frequent cause of cancer death in women. In 2012, this disease’s mortality rate was 12.1 deaths per 100 thousand women.2
Breast cancer control action in Brazil have been progressively incorporated to public health policies since the end of 1980’s, as one of the guidelines of the plan of comprehensive care to women’s health. In 2005, the National Policy of Oncology Care3 - updated in 2013, as the National Policy of Cancer Prevention and Control4 - defined the control of cervical and breast cancers as one of the main components of Municipal and State Health Plans. Breast cancer control was then incorporated to the Strategic Action Plan for Tackling Chronic Non-communicable Diseases (NCDs) in Brazil - 2011 to 2022.5
According to World Health Organization (WHO),6 there are two strategies to early detect cancer: early diagnosis, or agile and timely approach of individuals with cancer signs and symptoms; and screening tests, with regular examination in apparently healthy individuals, belonging to a high risk age group for the disease, aiming to identify the disease in pre-clinical phase and to reduce mortality due this cause.
The recommendations to breast cancer early detection in Brazil, updated in 2015, propose early diagnosis and also screening tests of women in the age group from 50 to 69 years, through mammography every two years.7
The monitoring of actions to early detect breast cancer in the country has become possible with the Breast Cancer Information System (Sismama), implemented in 2009. At Sismama, all the information on mammography, cytopathologic and histopathological tests performed by the Brazilian National Health System (SUS) are registered.8
The offer of screening mammograms has increased in the past years. According to data of the National Ambulatorial Information System (SIA/SUS), there was a progressive increase in mammograms financed by SUS, from 1,869,285 tests, in 2012, to 4,713,530, in 2014. However, the increased use does not necessarily guarantee the achievement of results expected by screening actions, once it depends on the adequacy of these tests in terms of quality, target population and frequency of examination.9
Indicators that are usually used to evaluate screening actions results, as survival time and distribution per cancer staging, are subject to biases, such as anticipation time and overdiagnosis at screening.10 Because of this, mortality rate is the most adequate measure to evaluate screening actions, although the impact can only be measured years after its implementation. Besides that, other factors, such as improvement of access to early diagnosis of symptomatic cases and treatment improvement, can influence mortality rates and hamper interferences over the causality relation between the studied interventions and possible modifications in these rates.11
Given the importance of the process assessment in planning and management, this present study aimed to assess actions for breast cancer early detection in the Brazilian National Health System - SUS - using process indicators.
Methods
An evaluation study of process indicators was carried out, with secondary data related to the production of mammograms and breast histopathology tests, by biopsy or surgical specimen, financed by SUS in 2010 and 2011.
Data were extracted from Sismama, using TabNet available at SUS IT Department website (Datasus). Mammograms and breast histopathology tests performed in men and those classified by Sismama as inconsistent (records with different information from the rules settled by the system) were excluded from the analysis. The following variables were considered:
Mammograms
a) Clinical indication (screening, when performed in asymptomatic women; and diagnostic, when performed in patients with breast cancer signs and/or symptoms).
b) Age group (in years: under 40; from 40 to 49; from 50 to 69; and 70 or older).
c) Results (presented in the seven categories of Breast Imaging Reporting and Data System [BI-RADS®], which classify radiologic findings, according to the suspicion level): 0 (inconclusive); 1 (without findings); 2 (benign finding); 3 (finding possibly benign); 4 (suspicious finding); 5 (highly suspicious finding); and 6 (finding with cancer diagnosis, not treated).
d) Examination time (interval between the requesting date and report emission date by the service provider, categorized in days: under 30; from 31 to 60; and over 60).
e) Time interval between the current test and the last screening mammogram.
f) Presence of palpable nodule (bigger than 20 millimeters) in screening mammograms (the cut point of 20mm was defined for being an easier size to be palpable,12 and due to this analysis objective, which is to identify cases that present problems in the diagnostic investigation of women with signs and symptoms).
Histopathological tests
a) Diagnosis type (benign or malignant).
b) Form of detection of suspicious lesions and lesions confirmed as malignant (palpable lesion or detection by image).
With regard to process indicators (some of them used in screening programs in other countries13), the following are considered:
1) Recall proportion due to abnormal results in the age group from 50 to 69 years: proportion of mammograms classified as BI-RADS® 0, 4 or 5, among all screening mammograms. ‘Initial screening mammograms’ were considered as the tests which informed that the woman had no previous mammograms, and ‘subsequent mammogram’ when there was information upon previous tests.
2) Estimate of predictive positive value (PPV) of screening. This indicator was estimated in two ways. In the first one (PPV1), the ratio between malignant lesions in cases coming from mammographic screening and the total of altered screening mammograms (BI-RADS® 0, 4 or 5) was used. In the second way (PPV2), only the cases with biopsy recommendation (BI-RADS® 4 or 5) were included in the denominator. These ratios must be closer to the real PPV, because it is expected to come from women with alterations in the screening that have performed diagnostic confirmation in the period of two years.
3) Ratio between biopsies with diagnosis of benign and malignant lesions. This indicator was stratified according to the material origin (surgical biopsy or core needle biopsy).
4) Proportion of screening mammograms performed in the target age group: percentage of screening mammograms performed in women inside the age group recommended by the Ministry of Health (from 50 to 69).
5) Proportion of screening mammograms performed every two years: percentage of screening mammograms in women inside the target-age group whose interval between the tests was of two years.
6) Proportion of breast histopathological tests, from biopsies and surgical specimen, performed within 30 days: percentage of histopathological tests, regardless of detection through imaging or not, whose time interval between the material removal for analysis and the test result did not exceed 30 days.
7) Proportion of unsatisfactory histopathological tests: percentage of unsatisfactory histopathological tests, among the total of histopathological tests.
8) Proportion of nodules bigger than 20mm found in screening mammograms: percentage of mammograms classified as screening in which nodules bigger than 20 millimeters were registered in the results.
The analyses were performed with the program Microsoft® Office Excel® version 5.0.
This study has been exempted from ethic-scientific analysis by the Ethics Research Committee from the National Cancer Institute José Alencar Gomes da Silva in January 21st, 2014, in accordance with the Resolution of the National Health Council (CNS) No. 466, dated December 12th, 2012.
Results
Data referring to 5,759,503 mammograms and to 44,892 histopathological tests performed in 2010 and 2011, in Brazil, were assessed (Table 1). According to exclusion criteria, 153,078 (2.6%) mammograms and 3,167 (6.6%) histopathological tests were not considered.
Table 1 - Mammograms and breast histopathological tests distribution according to tests characteristics, by age group. Brazil, 2010-2011
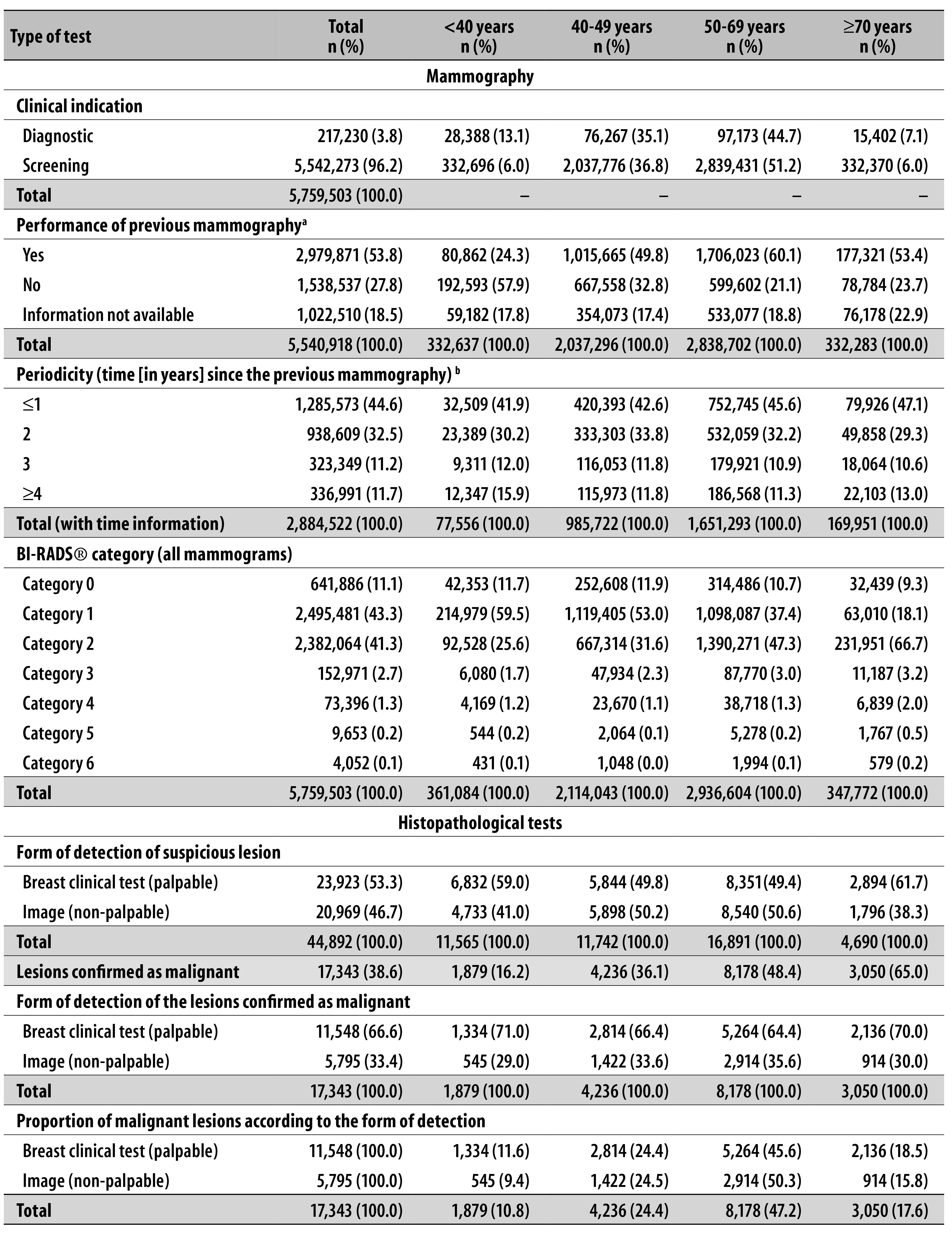
a) 1,355 mammograms were excluded due to inconsistence in the tab tool (TabNet).
b) 95,349 tests in which mammography time was in blank or ignored were excluded.
The distribution of mammograms and breast histopathological tests results per age group is presented in Table 1. In the analyzed period, 96.2% of the mammograms had the screening as clinical indication and 3.8% were diagnostic. Among the screening mammograms, 51.2% of them were performed in women in the recommended age group by Ministry of Health: from 50 to 69 years. In 53.8% of the cases, women answered to have already undergone previous mammograms, from which 44.6% had undergone it one year or less before (Table 1).
The proportion of results in the category BI-RADS® 1 has decreased as women aged, whilst the frequency of categories BI-RADS® 2 and 3 has increased with aging. Categories 4 and 5 have presented similar distribution among the age groups, with increase only among women older than 70 years (Table 1).
Although 61.7% of the screening mammograms in Brazil have the result issued within 30 days, in the national distribution there was no difference in the time of report emission between screening and diagnostic mammograms.
Among histopathological tests, 53.3% came from palpable lesions and 46.7% from findings in mammographic image. Malignant neoplasm diagnosis has occurred in 38.6% of the tests and the malignancy percentage has increased with aging. This percentage was also higher amongst tests whose lesion was identified in the clinical test, with most expressive difference being among women under 40 years old and over 70 years. In the reports analysis, 20.2% have presented as result ‘other malignant neoplasms’ (without specification of the tumor’s histologic type). Among the tests with malignant results, the proportion of tests coming from non-palpable lesions in the age group from 50 to 69 years (50.3%) have presented more than the double that was found in the age group from 40 to 49 years (24.5%) (Table 1).
Among screening mammograms with nodule information, the proportion of nodules bigger than 20mm was 11.4% in Brazil, with variations among the states, with the lowest percentage found in Rio Grande do Norte (8.1%) and the biggest in Tocantins (20.4%), Acre (20.9%) and Maranhão (21.1%). The South and Southeast regions have presented the lowest proportions of nodules bigger than 20mm in screening mammograms (Figure 1).
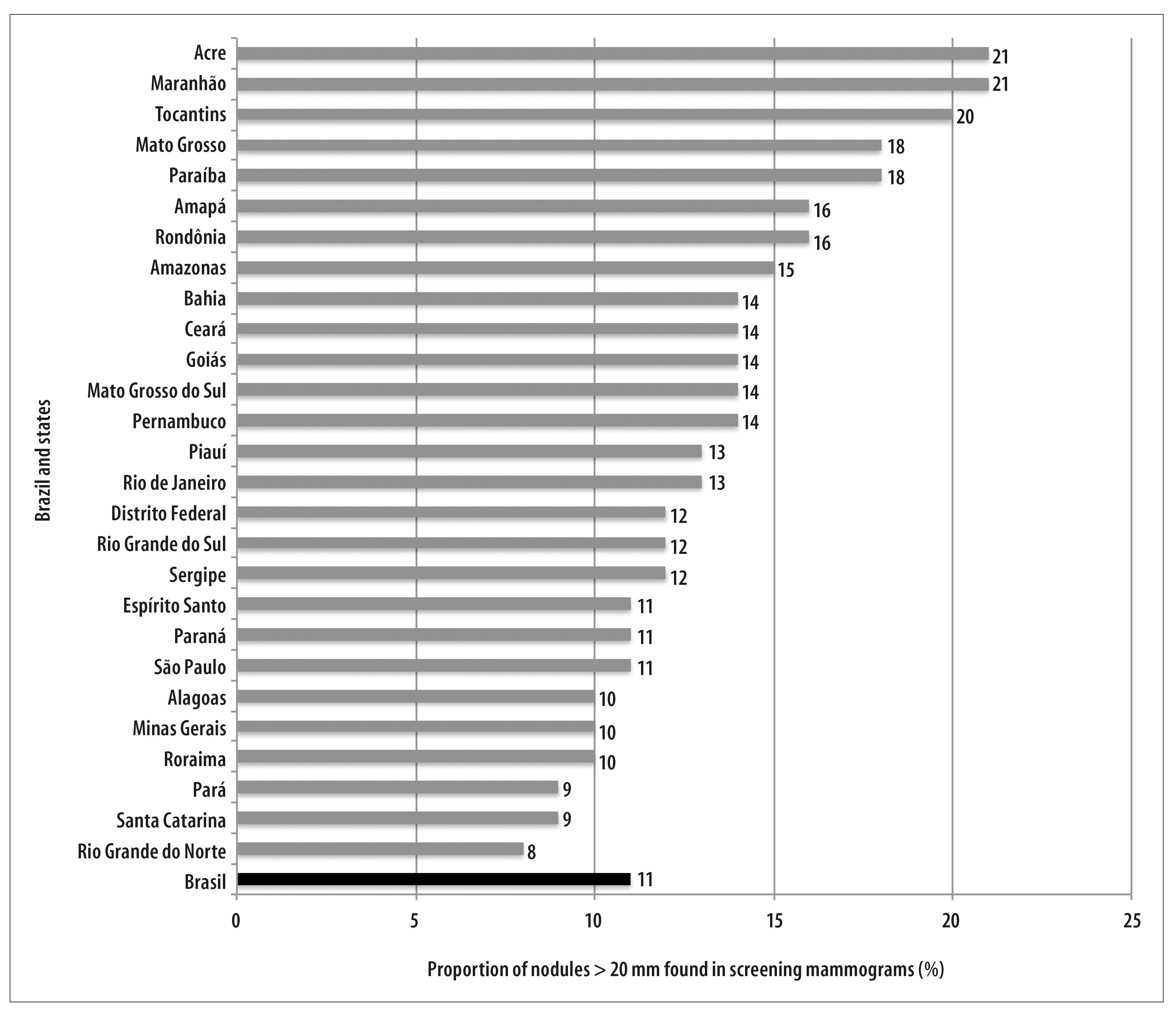
Figure 1 - Proportion distribution of nodules bigger than 20mm found in screening mammograms, per state. Brazil, 2010-2011
In Table 2 some process indicators referring to the production of mammograms and breast histopathological tests are presented. Recall proportion due to abnormal results was of 12.1%, being 11.2% to initial screening and 12.5% to subsequent screening steps. The time to receive the result of breast histopathological tests was up to 30 days in 78.2% of the tests registered in the country, and the proportion of screening mammograms performed in the recommended periodicity was of 32.2%. The percentage of unsatisfactory tests to histopathological analysis was of 0.9% (Table 2).
Table 2 - Process indicators related to mammograms and breast histopathological tests financed by the Brazilian National Health System (SUS). Brazil, 2010-2011
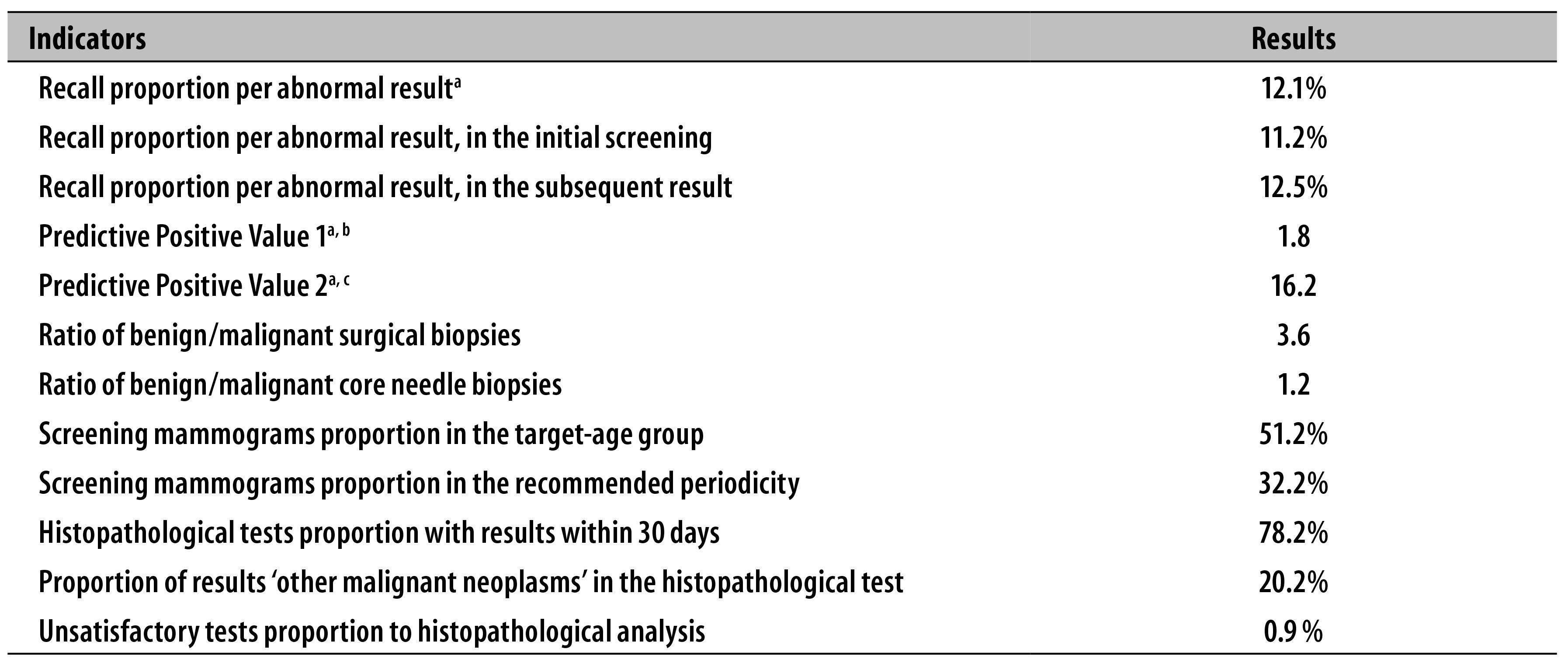
a) Indicators calculated to screening in the target-age group (from 50 to 69 years).
b) Predictive Positive Value 1 - ratio between malignant lesions detected in mammographic screening and the total of altered screening mammograms (BI-RADS® 0, 4 or 5).
c) Predictive Positive Value 2 - ratio between malignant lesions detected in mammographic screening and the total of screening mammograms with biopsy indication (BI-RADS® 4 or 5).
The ratio between biopsies with benign lesions and malignant neoplasms was 3.6 in surgical biopsies (2.7 to palpable lesions and 5.6 to non-palpable lesions) and 1.2 in core needle biopsy (0.6 to palpable lesions and 2.3 to non-palpable lesions) (Table 2).
The estimates of predictive value referring to tests performed in women between 50 and 69 years was of 1.8 (PPV1) and 16.2% (PPV2) (Table 2). In women under 50 years, the values found to PPV1 and PPV2 were 0.64 and 7.47 respectively.
Discussion
The present assessment of actions to early detect breast cancer pointed that the recommendations of the Ministry of Health have not been followed with regard to age groups and screening periodicity, and there are possible errors on information records, which hampers the monitoring of actions and the achievement of the impact objective on mortality due to this disease.
Among the screening mammograms, over 10% of nodules bigger than 20mm were detected, which can indicate: (i) difficulties in the access to health services; (ii) low awareness and/or of prepare by the physician to detect clinical suspicious alterations; and (iii) lack of information by women upon alert signs of breast cancer. It is important to highlight that the cut point used to classify tumors as palpable was conservative, considering that Skinner et al. have identified an average size of palpable T1 tumors of 14mm.12
The majority of mammograms being conducted within one year or less of interval goes against the best available scientific evidences, which say that biennial periodicity preserves almost all benefits of annual screening, reducing the risks nearly by half.14 The elevated proportion of annual interval found in this study cannot be justified by the occurrence of category BI-RADS® 3, in which repetition is recommended in less than one year to radiologic control,15 once the results proportion in this category was only of 2.7%.
The expressive offer of screening mammograms to the female population under 50 years old (42.8%) shows a high frequency of non-adherence to the recommendation of the proposed age group.
The trend observed in BI-RADS® results was similar to the one found by Azevedo e Silva et al,16 in which the frequency in category 1 is higher in younger women, whilst categories 2 and 3 increase in older women. Still according to that study, categories 4 and 5 were also more frequent in women aged 70 years old or over.16
In histopathological tests resulting from non-palpable lesions, it was verified that the proportion of cancer detected in the age group from 50 to 69 years was, approximately, 40% higher than in the age group from 40 to 49, which must be explained by the lowest prevalence of this cancer and worst mammography acuity in this latter age group.
According to the United States Preventive Services Task Force (USPSTF), an independent panel of specialists in primary health care and prevention that systematically revises the efficiency evidence and develops recommendations to preventive clinical services, there are still important lacks in the scientific evidences about mammographic screening benefits in the age group from 40 to 49 years. However, there are evidences that the balance between possible benefits and damages are more unfavorable than the observed in the age group from 50 to 69 years.17
Evidences are also insufficient to women older than 70 years, among which there are higher risks of overdiagnosis and overtreatment due to competitive causes of mortality and to existence of cancers of slow evolution that would not arise in their lifetime.17 These risks are potentially worsened in the Brazilian reality, where life expectation is lower than in countries with organized screening programs,18 so the risk of death due to other causes is high before manifestation and evolution of breast cancer.
The existing epidemiologic differences between Brazil and countries where clinical trials about mammographic screening were performed, probably, also enhance the risks and harms associated to the occurrence of false-positives, considering that the predictive value of screening tests is influenced by the prevalence of the disease in a given population.19
Until the present moment, no studies were found about the efficiency of breast cancer mammographic screening in low incidence regions. In these regions, the balance between risks and benefits is more unfavorable than in regions with elevated incidence. In these cases, early diagnosis actions can be a more effective strategy and less harmful than the development of screening actions.20 The elevated proportion of lesions bigger than 20mm in states from the Brazilian North region possibly justifies the intensification of early diagnosis strategies. Most of confirmed cases for breast cancer were diagnosed from palpable or symptomatic lesions. The same was observed in countries with organized screening programs and great coverage of mammographic screening, as the United Kingdom, where, after almost 20 years of implementation of the national program of population screening, this proportion was high (46%), going against common sense.21
A systematic review on the findings of all studies published on MEDLINE and Embase databases throughout the twentieth century demonstrated that delays of over three months between the beginning of the symptoms and the beginning of the treatment significantly decrease the survival rate in five years.22 In the assistance network organization, diagnostic mammography must be prioritized because they are associated to clinical signs of the disease, which demand differential diagnosis and timely treatment. We could not observe a pattern of prioritization on the emission of screening mammography reports in the country, and, in many states the percentage of screening mammography with results within 30 days or less is bigger among screening mammograms, which can delay diagnostic investigation on symptomatic women.
The proportion of tests classified as BI-RADS® 0 was a little above the corresponding proportion to the Canadian screening program, of 10%.23 An analysis in the states, municipalities and mainly in the radiology services is necessary, to identify the needs of qualifying the staff responsible for the mammography reports and responsible for the requests, according to the pointed inadequacies.
Recall proportion due to abnormal results to the subsequent screening in the population from 50 to 69 years was significantly higher than the established parameter to abnormal call rate in screening programs (<5%).24 Even though this direct comparison is still not possible, due to different characteristics of mammographic screening in Brazil and in developed countries, these data call attention because, maybe, they indicate possible quality problems in the screening mammograms, associated to the quality of images and/or reports.
The ratio between malignant lesions detected in mammographic screening and the total of altered screening mammograms (PPV1) was used as proxy of the positive predictive value of screening mammograms and has presented considerable inferior value to the patterns found in literature (5 to 10 for every 100),25 and the ratio between malignant lesions and the total of screening mammograms with biopsy indication (PPV2) (25 to 40%) was also inferior.25 The age group under 50 years presented values even lower to both indicators. These findings are also not directly comparable, but can point to the existence of problems in the quality of screening or diagnostic confirmation tests. In Brazil, the great proportion of screening in women under 50 years, age group in which breast cancer prevalence is lower, and the disease low incidence in the country macroregions may negatively influence the results.
The ratio between benign lesions and malignant neoplasms in surgical biopsies of palpable lesions was more than three times superior to the pattern internationally adopted (≤2).23 This indicator, together with the positive predictive value, seems to suggest the existence of an excessively elevated number of false-positive results in the screening. This implies the performance of unnecessary surgical procedures and possible resulting complications, besides psychological impacts given the possibility of cancer diagnosis. This is an important finding, considering that the predicted number of false-positive results in breast cancer screening is already elevated.26 In addition, there is a great number of breast cancer histopathological tests classified as ‘others’, which demonstrates problems in the quality of histopathological tests or on the record of information in the system.
This study presents some limitations, which are intrinsic to the use of secondary data, such as: (i) out of date database in some states and consequent differences between Sismama national database and the billing database of these tests; (ii) lack of information on the clinical indication of the previous mammography in the space ‘mammography periodicity’; and the fact that (iii) the register unit is tests and not women, hampering the joined analysis of mammograms and histopathological tests in the individual level without using methods of probabilistic relation.16
Furthermore, there is the possibility of failures when filling the clinical indication, given the form simplicity to screening tests and the differential financing for these tests.
The comparison of indicators here presented with the screening programs organized in other countries should be seen with cautious, due to the opportunist characteristic of breast cancer screening in Brazil.8
The assessment of indicators showed that great part of screening actions have been offered in disagreement with the Ministry of Health recommendations, which can compromise its results and the expected impact, besides increasing the risks to which women are subjected. Moreover, this study results suggest that there is still a significant group of women that present palpable lesions, so early diagnosis strategies can be improved in order to have timely diagnoses, optimizing treatment chances and reducing mortality caused by breast cancer.
We expect the present study to contribute to stimulate the culture of assessment and planning of health policies, and to reinforce the importance of progressive qualification of the Breast Cancer Information System as a resource to improve breast cancer early detection in Brazil.
REFERENCES
1. Ministério da Saúde (BR). Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2016: incidência de câncer no Brasil. Rio de Janeiro: Instituto Nacional de Câncer José Alencar Gomes da Silva; 2015. [ Links ]
2. Ministério da Saúde (BR). Instituto Nacional de Câncer. Atlas on-line de mortalidade [Internet]. Rio de Janeiro: Instituto Nacional de Câncer; 1996 [citado 2016 ago 26]. Disponível em: Disponível em: https://mortalidade.inca.gov.br/MortalidadeWeb/ [ Links ]
3. Brasil. Ministério da Saúde. Portaria nº 2.439/GM, de 8 de dezembro de 2005. Institui a Política Nacional de Atenção Oncológica: promoção, prevenção, diagnóstico, reabilitação e cuidados paliativos, a ser implantada em todas as Unidades Federadas, respeitadas as competências das três esferas de gestão. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2005 dez 09; Seção 1:80. [ Links ]
4. Brasil. Ministério da Saúde. Portaria no 874, de 16 de maio de 2013. Institui a Política Nacional para a Prevenção e Controle do Câncer na Rede de Atenção à Saúde das Pessoas com Doenças Crônicas no âmbito do Sistema Único de Saúde (SUS). Diário Oficial da República Federativa do Brasil, Brasília (DF), 2013 maio 17; Seção 1:29. [ Links ]
5. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Análise de Situação de Saúde. Plano de ações estratégicas para o enfrentamento das doenças crônicas não transmissíveis (DCNT) no Brasil, 2011-2022. Brasília: Ministério da Saúde; 2011. [ Links ]
6. World Health Organization. Cancer control: knowledge into action: WHO guide for effective programmes; module 3 [Internet]. Geneva: World Health Organization; 2007 [cited 2016 Aug 26]. Avaliable from: http://www.who.int/cancer/modules/Early%20Detection%20Module%203.pdf [ Links ]
7. Ministério da Saúde (BR). Instituto Nacional de Câncer José Alencar Gomes da Silva. Diretrizes para detecção precoce do câncer de mama no Brasil [Internet]. Rio de Janeiro: Instituto Nacional de Câncer José Alencar Gomes da Silva ; 2015 [citado 2016 ago 26]. Disponível em: http://www1.inca.gov.br/inca/Arquivos/livro_deteccao_precoce_final.pdf [ Links ]
8. Passman LJ, Farias AM, Tomazelli JG, Abreu DM, Dias MB, Assis M, et al. SISMAMA: implementation of an information system for breast cancer early detection programs in Brazil. Breast. 2011 Apr; 20 Suppl 2:S35-9. [ Links ]
9. Migowski A. Direito à saúde e incorporação de tecnologias: o caso do rastreamento mamográfico no Brasil. Rev APS. 2012 abr-jun;15(2):235-6. [ Links ]
10. Bleyer A, Welch G. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012 Nov;367(21):1998-2005. [ Links ]
11. Jatoi I. The impact of advances in treatment on the efficacy of mammography screening. Prev Med. 2011 Sep;53(3):103-4. [ Links ]
12. Skinner KA, Silberman H, Sposto R, Silverstein MJ. Palpable breast cancers are inherently different from nonpalpable breast cancers. Ann Surg Oncol. 2001 Oct;8(9):705-10. [ Links ]
13. Perry N, Broeders M, Wolf C, Törnberg S, Holland R, Von Karsa L, editors. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities;2006. [ Links ]
14. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009 Nov;151(10):727-37 [ Links ]
15. American College of Radiology (US). Breast imaging reporting and data system (BI-RADS™). 5th ed. Reston: American College of Radiology; 2013. [ Links ]
16. Azevedo e Silva G, Bustamante-Teixeira MT, Aquino EML, Tomazelli JG, Santos-Silva I. Acesso à detecção precoce do câncer de mama no Sistema Único de Saúde: uma análise a partir dos dados do Sistema de Informações em Saúde. Cad Saude Publica. 2014 jul;30(7):1537-50. [ Links ]
17. Siu AL, U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016 Feb;164(4):279-96. [ Links ]
18. Word Health Organization. Life expectancy at birth (years), 2000-2015 [Internet]. Geneve: World Health Organization; 2016 [cited 2016 Aug 26]. Avaliable from: Avaliable from: http://gamapserver.who.int/gho/interactive_charts/mbd/life_expectancy/atlas.html [ Links ]
19. Gordis L. Epidemiology. 5th. Philadelphia: Elsevier; 2013. [ Links ]
20. Leung GM, Lam TH, Thach TQ, Hedley AJ. Will screening mammography in the East do more harm than good? Am J Public Health. 2002 Nov; 92(11):1841-6. [ Links ]
21. NHS Cancer Screening Programmes. All Breast Cancers Report. London: NHS Breast Cancer Screening Programme; 2009. [ Links ]
22. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999 Apr;353(9159):1119-26. [ Links ]
23. Public Health Agency of Canada (CA). Organized breast cancer screening programs in Canada: report on program performance in 2001 and 2006. Ottawa: Public Health Agency of Canada; 2011. [ Links ]
24. Canadian Partnership Against Cancer (CA). Report from the evaluation indicators working group: guidelines for monitoring breast cancer screening program performance. 3th ed. Toronto: Canadian Partnership Against Cancer; 2013. [ Links ]
25. Colégio Brasileiro de Radiologia e Diagnóstico por Imagem. Sistema de laudos e registro de dados de imagem da mama. Rio de Janeiro: Colégio Brasileiro de Radiologia e Diagnóstico por Imagem; 2005. [ Links ]
26. Canadian Task Force on Preventive Health Care, Tonelli M, Connor Gorber S, Joffres M, Dickinson J, Singh H, et al. Recommendations on screening for breast cancer in average-risk women aged 40-74 years. CMAJ. 2011 Nov;183(17):1991-2001. [ Links ]
Received: February 01, 2016; Accepted: August 08, 2016











 texto em
texto em 


