Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.26 n.2 Brasília abr./jun. 2017
http://dx.doi.org/10.5123/s1679-49742017000200002
Original article
Clinical-epidemiological description of live births with microcephaly in the state of Sergipe, Brazil, 2015
1Ministério da Saúde, Secretaria de Vigilância em Saúde, Brasília-DF, Brasil
2Secretaria de Estado da Saúde de Sergipe, Diretoria de Vigilância em Saúde, Aracaju-SE, Brasil
3Secretaria Municipal de Saúde de Aracaju, Diretoria de Vigilância em Saúde, Aracaju-SE, Brasil
4Ministério da Saúde, Departamento Nacional de Auditoria do Sistema Único de Saúde, Aracaju-SE, Brasil
OBJECTIVE:
to describe the clinical and epidemiological characteristics of microcephaly cases in live births in Sergipe, Brazil, and to calculate the prevalence in its municipalities.
METHODS:
this is a descriptive study on live births from September 1st to November 30th, 2015, with data from medical records and interviews with mothers.
RESULTS:
83 cases of microcephaly were confirmed, with three deaths; prevalence in the 26 municipalities with confirmed cases ranged from 18 to 185/10,000 live births; the median of head circumference was 31 cm (range: 22.5-33.0); agenesis of corpus callosum (26/43), lissencephaly (12/43), absence of midline (10/43) and ventriculomegaly (8/43) were observed in the transfontanellar ultrasound; 40 mothers reported rash in pregnancy, 23 in the first trimester, with pruritus, arthralgia and headache; seven were positive for infections potentially causing malformations.
CONCLUSION:
there was a high occurrence of cases of microcephaly, and reports of signs and symptoms compatible with Zika virus infection during pregnancy.
Keywords: Microcephaly; Zika Virus; Prevalence; Newborns Infant; Descriptive Epidemiology
Introduction
Microcephaly is a congenital malformation, in which the brain does not develop properly and the head circumference measures less than two standard deviations below the specific average for sex and gestational age. Microcephaly is considered severe when this measure is lower than three standard deviations.1 Microcephaly is characterized by structural or functional alterations at birth and originated during prenatal period.2
The most common causes of microcephaly are genetic and exposition to risk factors, such as: syphilis, toxoplasmosis, rubella, cytomegalovirus and simple herpes (STORCH), severe malnutrition (lack of nutrients or insufficient food) and exposition to harmful substances (alcohol, some medicines or toxic substances).1 Recently, infection by Zika virus was found to be related to microcephaly.3,4,5
Zika virus is a flavivirus transmitted by mosquitoes. The symptoms of the infection are similar to other infections by arbovirus, such as dengue fever, and include fever, skin rashes, conjunctivitis, muscle and joint pain, malaise or headache.6
The are two ways to diagnose microcephaly in the infant: during pregnancy, through an ultrasound examination performed during the second trimester of pregnancy onwards; and after the childbirth, through the measure of the head circumference and examinations, such as tomography and magnetic resonance.2
Microcephaly is a rare medical condition. Its occurrence varies from 2 to 12 per 10,000 live births in the United States.2 In Brazil, the historic average of microcephaly is of two cases per 10,000 live births.7 However, in 2015, the prevalence rate of microcephaly at birth was of 54.6 cases per 100 thousand live births.8
In October 2015, the Brazilian Ministry of Health was notified by Pernambuco State about the increase in the number of microcephaly cases, warning the country about this unusual event and stimulating notification by other states, especially those from the Northeast region, where Sergipe State is located.
After the change in the pattern of occurrence of microcephaly in Brazil, the Ministry of Health declared Public Health Emergency of National Concern.9 Based on preliminary results of clinical, epidemiological and laboratory investigations of microcephaly cases in the Northeast, in November 2015, the Ministry recognized the relation between the increase in microcephaly cases in Brazil and the infection by Zika virus during pregnancy.
On November 29th 2015, the World Health Organization (WHO) changed the classification of this event - under the International Health Regulations - to potential Public Health Emergency of International Concern.
There is no treatment or cure for microcephaly. However, in order to ensure a better quality of life to the child, it is important to follow up and give early stimulation.10
The objective of this study was to describe the clinical and epidemiological characteristics of microcephaly cases in live births in Sergipe, Brazil, and to calculate the prevalence in its municipalities.
Methods
A descriptive study was conducted with cases of microcephaly in live births of mothers resident in the Brazilian state of Sergipe, using births and reports from September 1st to November 30th 2015, that is, since the first cases were reported in the state until the beginning of field investigation. The cases investigation occurred from November 30th to December 21st 2015.
Sergipe State is located in the Brazilian Northeast region and is composed by 75 municipalities. It is the smallest Brazilian state, in territory (21,918.454km2).11 Sergipe has approximately 2,242,937 inhabitants, and in 2014, it registered 34,369 live births.12 The per capita monthly income in the state is BRL 758.00.13
For this study we included all the live births with microcephaly notified to Sergipe State Health Department (SES/SE), and also those which were not reported, but were captured in the health services, through active search for microcephaly cases.
The following definitions were adopted for this study.10
a) Suspected case of microcephaly: Live births between September 1st and November 30th, 2015, in Sergipe, reported to SES/SE, or those not reported but captured through the active search for microcephaly cases.
b) Confirmed case of microcephaly: Suspected case, born at term (gestational age between 37 and 42 weeks), with head circumference smaller than or equal to 33cm, or suspected case, preterm, (gestational age 37 weeks) with circumference smaller than two standard deviations (or 3rd percentile) according to gestational age and sex (Fenton chart), or diagnostic of microcephaly by the physician.
c) Confirmed case of microcephaly suggestive of congenital infection: Case that presents alterations suggestive of congenital infection by any imaging examination (transfontanellar ultrasound, CT scan or magnetic resonance). In the present study, we considered only the cases confirmed through imaging exams, although there is another definition - through laboratory criteria - which was not used in this study because it was not available at the time.
d) Discarded case of microcephaly: Suspected cases with head circumference within the normal range (Fenton chart) according to gestational age and sex, or the absence of microcephaly diagnose registered on the medical records.
The aforementioned alterations suggestive of congenital infections were established in meetings with the medical society, institutions and specialists, after discussions based on previous experiences with microcephaly and the available scientific evidences, being defined as: ventricular disorders or calcification, or two minor signs of changes in the posterior fossa (cerebellar hypoplasia, hypoplasia of cerebellar vermis, large posterior fossa, agenesis, hypoplasia, or dysgenesis of the corpus callosum).10
From November 8th to December 12th, in the epidemiological weeks 45 to 49/2015, the Ministry of Health adopted a more sensitive criteria to detect microcephaly in live births at term, once it was a new event and of interest in terms of gathering most cases possible.10 Thus, in this study, we opted for describing all the cases that fit this definition, which did not differentiate sex.
From March 2016 onwards, the Ministry of Health10 began to use the Intergrowth chart as reference to measure the head circumference according to gestational age,14 for all preterm births (less than 37 gestational weeks), and the curves of WHO,1 for births at term (between 37 and 42 gestational weeks). A brief analysis of cases to be discarded was conducted, based on the new definition.
In the study for epidemiological description, the variables covered were: sociodemographic (sex, age, ethnicity/skin color, education level, among others), epidemiological background (municipality of residence, exposition to drugs, tobacco and others), exams conducted (STORCH), besides information on the obstetric and gynecological history, prenatal care and childbirth (ultrasounds, medical appointments), infectious manifestations during pregnancy (presence of rash, other infections), among others.
Data were obtained from medical records and records of medical assistance to pregnant women and newborns in the place the childbirth occurred. We also conducted interviews with the mothers, based on a semi-structured questionnaire and on the verification of the information in the pregnant woman health card and the infant health card. The questionnaire was divided into two blocks: one to register data of the live births and the other for maternal data. The interviews were conducted by professionals of the rapid response team of the Secretariat of Health Surveillance (SVS), of the Ministry of Health and by technicians of the municipal health departments.
The cases whose mother was not located by the interviewer, for any reason and after two attempts, were considered losses.
The cases were described using the absolute and relative measures of frequency, central tendency and dispersion. To calculate the prevalence of microcephaly in the municipalities, the following formula was applied: number of confirmed cases of microcephaly/number of live births in 2014, multiplied by 10,000. As denominator, we used the number of live births registered on the databases of the Information System on Live Births (Sinasc) in the last year available (2014). For data analysis, we used the softwares Epi Info™ 7, Microsoft Office Excel® 2010 and QGIS 2.6.1.
All the participants were advised and expressed their agreement and awareness by signing the Free Informed Term of Consent, regarding the information confidentiality and the anonymity of individual data, according to the Resolution of the National Health Council (CNS), No. 466, dated December 12, 2012. This study was conducted within the actions of epidemiological surveillance in the context of Public Health Emergency, being exempted of approval by an Ethics Research Committee.
Results
From September to November 2015, 95 live births suspected of microcephaly were notified: 83 fit the definition of confirmed case. Four were discarded and eight were losses. Of the confirmed cases there were three deaths. With regard to the occurrence of microcephaly cases, according to birth date of live births, there was a peak in the epidemiological week 46/2015 (n=16) (Figure 1).
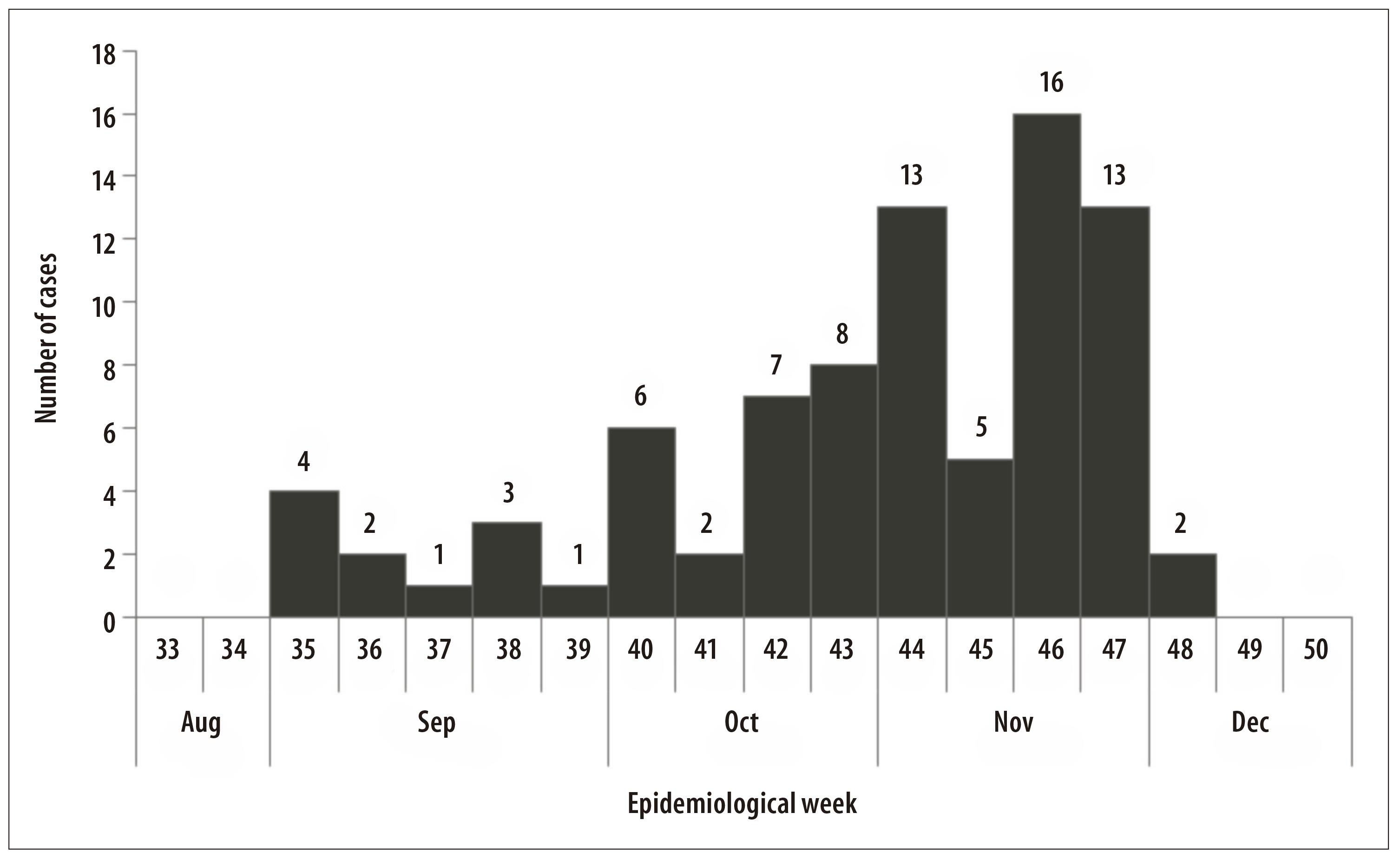
Figure 1 - Distribution of microcephaly cases (N=83) according to epidemiological week of childbirth occurrence, Sergipe, September-November 2015
The prevalence of microcephaly in Sergipe State was of 24.1 cases per 10,000 live births. The municipalities with the highest prevalences (cases per 10,000 live births) were São Miguel do Aleixo (185), Riachuelo (160), Tomar de Geru (110) and Pedrinhas (94) (Figure 2). There were confirmed cases of microcephaly in 26 of the 75 municipalities of the state.
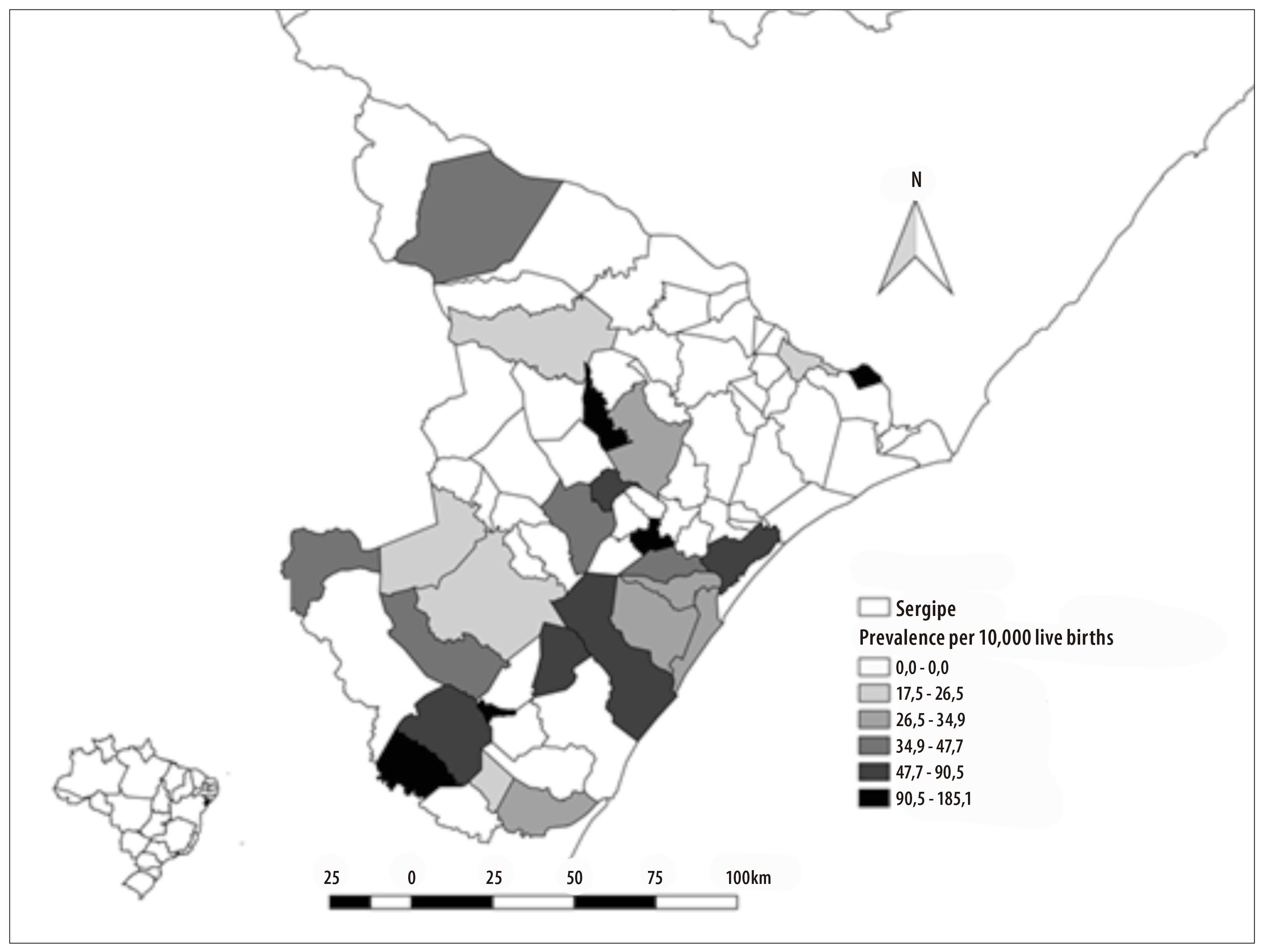
Figure 2 - Distribution of microcephaly prevalence per municipality Sergipe, September-November 2015
With regard to maternal sociodemographic data, the most common variables were: education level High School (n=39), marital status single (n=30) and occupation housewife (n=40). The median of age was 25 years old (range: 15 to 41 years), and of the per capita income, BRL 262.66 (Q1: BRL6.66; Q3: BRL2,666.00).
With regard to pregnancy, 47 mothers referred to be nulliparous and all the pregnancies were single. All the mothers attended prenatal care and the median of medical appointments was seven (Q1: 1; Q2: 16). Regarding the laboratory exams requested during prenatal care, of the 83 mothers in the study, (i) 64 were tested for toxoplasmosis and five presented IgM reagent, (ii) 39 were tested for rubella and none of them presented IgM reagent, (iii) 77 were tested for syphilis and two tested positive, totalizing seven women with laboratory evidence for infection potentially causative of congenital alterations in the infants. There was no positive result for the human Immunodeficiency Virus (HIV), hepatitis C, cytomegalovirus or herpes. From the total of mothers who underwent ultrasound exams during prenatal care, 70/83 were submitted to this procedures in the third trimester of pregnancy and in 30 of them the exams pointed to some alteration in the fetus development (e.g., asymmetric development incompatible with the gestational age, skull with altered morphology, ventriculomegaly, absence of cerebellar vermis and microcephaly, etc.).
In 53 (64%) of mothers, some kind of pregnancy complication could be observed, and 26 (49%) of them presented urinary tract infection, 12 (23%) had anemia and 10 (19%) had oligohydramnios. Only two mothers reported being treated for the infection.
With regard to maternal background, none of them mentioned any contact with pesticides, 10 (12%) declared to take continuous use medication, use tobacco and/or drugs. None of the mothers referred any microcephaly case in the family, although we observed one congenital malformation (myelomeningocele) and consanguinity.
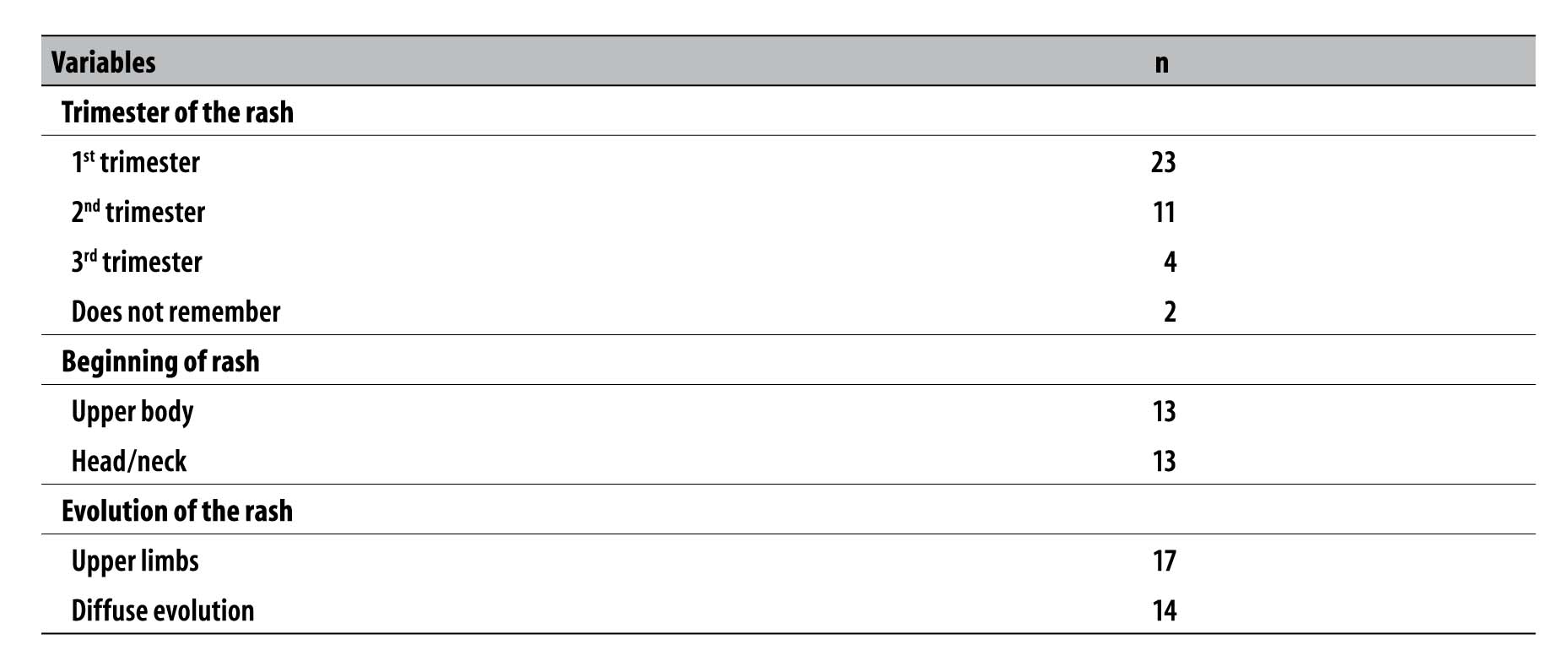
During the pregnancy, 40 women presented rashes, of whom 23 had it in the first trimester, with median of time length of five days (range from 1 to 15 days). Regarding the area where the rash began, 13 women referred the upper body and 13 referred the head/neck, spreading to the upper limbs in 17 cases and with diffuse evolution in 14 cases (Table 1). Simultaneously with the clinical picture of rash, 21 women reported pruritus, 18 arthralgia, 16 headache, 16 fever, 12 myalgia, nine conjunctival hyperemia, three edema and one running nose. Four mothers with laboratory results positive for STORCH also presented rash.
Table 1 - Characteristics of the mothers of live births who developed rash (N=40), Sergipe, September-November 2015
Fifty-two cases were born through vaginal delivery, 58 infants were female and 71 were born at term. Seventeen cases presented some type of malformation, being 11 musculoskeletal malformations (Table 2). Three infants had Dandy-Walker syndrome.
The median of gestational age at the moment of childbirth was of 39 weeks and of the head circumference measured at birth, of 31cm (range: 22.5 to 33cm). The median of the 1st Apgar score was nine (range: 1 to 10) and of the 2nd Apgar was 10 (range: 2 to 10).
Table 2 - Characteristics of the newborns according to the type of malformation (n=17), Sergipe, September-November 2015

With regard to possible infections in the confirmed cases, 12 cases were tested for syphilis and 12 for cytomegalovirus: three of them tested positive for syphilis, two with mother IgM reagent and one with a non-tested mother, and one had IgM reagent for cytomegalovirus. For the identification of Zika virus, in six confirmed cases of microcephaly, the mothers reported to have undergone exams; however there are only three records of tests, which were not detected. For chikungunya fever virus, five cases underwent exams, with negative results.
Concerning the imaging exams, 45 live births underwent one of these exams: three underwent CT scan, two, magnetic resonance and 43, transfontanellar ultrasound. Among those who underwent ultrasound exams, the main findings were agenesis of corpus callosum (26/43), lissencephaly (12/43), absence of midline (10/43) and ventriculomegaly (8/43) (Table 3). Of the 45 live births who underwent imaging exams, 32 presented some kind of alteration, and there were positive results for infection in two newborns: one for cytomegalovirus, and another one for syphilis (mother and infant), in which the mother did not receive treatment. Still with regard to the newborns who presented alteration in the exams, 17 presented results suggestive of congenital infection.
Table 3 - Main findings of imaging exams in microcephaly cases, Sergipe, September-November 2015
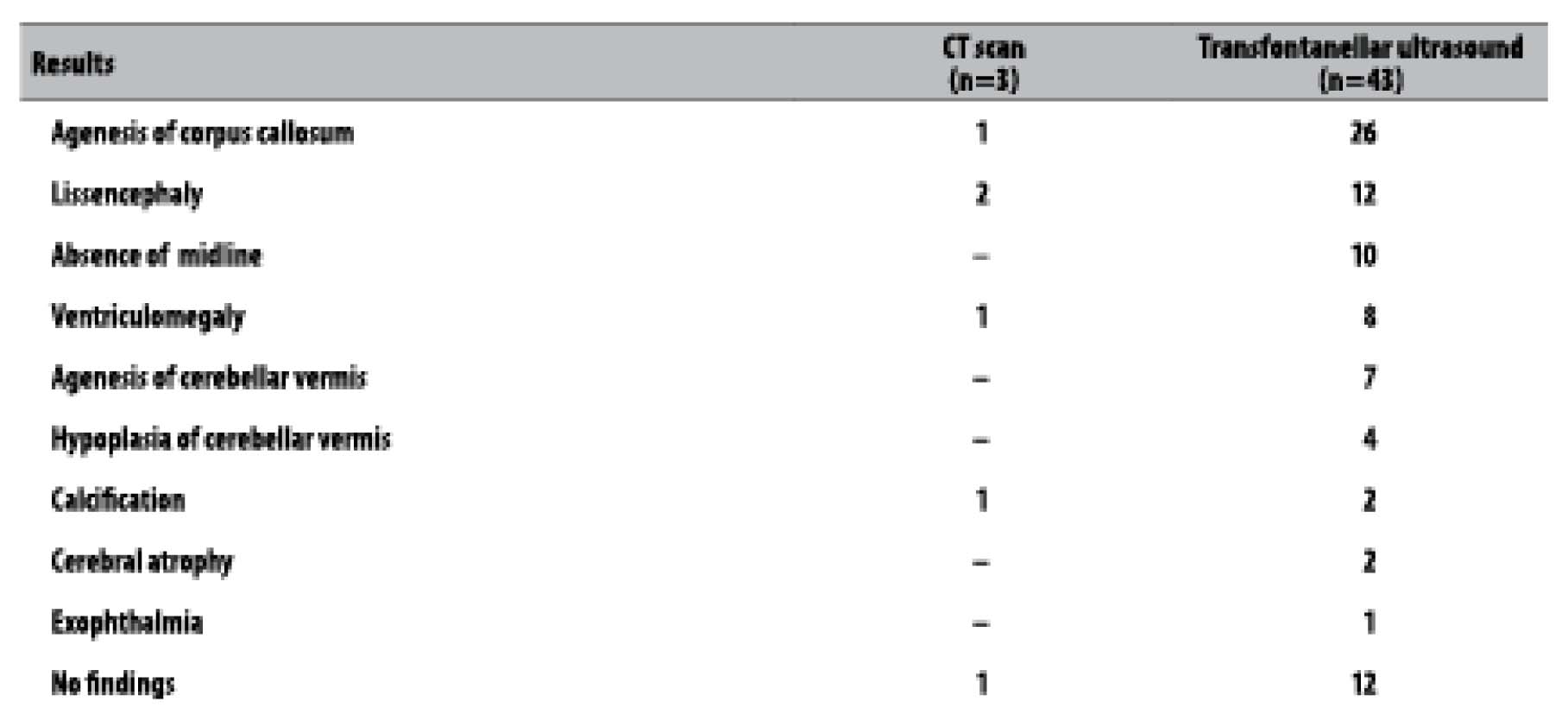
Note: There may have more than one result per exam.
Ophthalmic exams were performed in 43 live births and speech-hearing exams, in 44 live births. Eye alterations were found in nine cases (inclination of the palpebral fissure, ecchymosis, chorioretinitis, macular pucker), and six cases presented speech-hearing problems (absent or below standard otoacoustic emissions).
Among the three deaths, the average of live days was of 26 days; two females were born at term, with average head circumference of 26.2 cm. In one death, musculoskeletal malformation was observed; in two deaths, there were ophthalmic problems. In those three cases microcephaly had been detected previously through ultrasound exams (intrauterine detection); two of them underwent transfontanellar ultrasound, though which lissencephaly, agenesis of corpus callosum, absence of midline and agenesis of cerebellar vermis were observed. Two of the mothers reported rash (one in the first trimester and one in the second trimester of pregnancy); one of them had a congenital anomaly (myelomeningocele) and consanguinity with the infant’s father (1st degree cousins), in which, according to the doctors, chromosomopathy could not be excluded.
With regard to the analysis of the notified cases - under the new case definition adopted by the Brazilian Ministry of Health -, of the 83 confirmed cases by the present study, 23 would be discarded for having head circumference over the cut point. Regarding the cases that would be discarded, two mothers reported rash during pregnancy, and three tested positive for infectious diseases (one for syphilis and two for toxoplasmosis). Nine newborns who would be excluded from the study underwent imaging exams and two of them presented alterations: one case was suggestive of congenital infection (the mother presented IgM reagent for toxoplasmosis during pregnancy).
Discussion
There was an increase in the number of cases of microcephaly notified in Sergipe State in 2015, when compared to the previous year (2014), when two cases had been registered. In November 2015 (epidemiological week 46), a higher number of microcephaly cases was observed, although in the beginning of September 2015 (epidemiological week 35), the four first cases of that year had already been registered.
The first months of pregnancy of the mothers of infants with microcephaly - between January and March 2015 - corresponded to the months of Zika virus circulation, which was later identified in laboratory procedures, in Brazil.15 Given the epidemiological finding that the first months of pregnancy of women with infants with microcephaly corresponded to the period of higher circulation of the virus in the Northeast region,10 we can observe a time relation between the circulation of the etiologic agent and the occurrence of microcephaly in those live births.
The prevalence of microcephaly (number of cases per 10,000 live births) in Sergipe State was higher when compared to the prevalence in the French Polynesia (2.0)16 between October 2013 and April 2014, in Europe17 (1.9) between 1996 and 2001, and in other states of the Brazilian Northeast region, such as Pernambuco (16.6) and Paraíba (10.8), in 2015.18 The variation of prevalence among the municipalities may be explained by the numerator (number of live births in each municipality), which varied a lot, as well as by the virus circulation, possibly different among these places.
Concerning the mothers’ sociodemographic characteristics, it is important to highlight the fact that most of them were single, unemployed and had per capita income almost three times lower than the population of their own state.19 This female condition requires efforts to reduce the negative social effects that the individuals and their families may live, being necessary to fully ensure all the health assistance, through public policies, such as the receptivity, insertion, follow-up, referral and, mainly, financial support, once these children need special care for their development.
Environmental factors may influence the etiopathogenesis of microcephaly, such as medication, toxic substances (alcohol, tobacco and drugs) and congenital infections (STORCH).20 In the present study there were five cases of toxoplasmosis and two of syphilis in pregnant women, besides one case of syphilis in a live birth (mother reagent) and one of cytomegalovirus, whose mother had not been tested.
As observed in another study on microcephaly conducted in Brazil,21 most of the mothers reported having rash in the first trimester of pregnancy, a rate similar to what was found in this study. The embryonic period is considered the period with the highest risk of multiple complications due to infections.10 The signs and symptoms observed corroborate with those presented in a cohort study conducted from September 2015 to February 2016, with pregnant women who presented severe fever and rash, in Rio de Janeiro,22 and the findings of an analysis of the Zika virus outbreak, occurred in 2007.23 Other diseases may also present rash, such as rubella, measles and scarlet fever, for example. However, Brazil has been declared free from measles and rubella,24 and the laboratory evidence that the Zika virus is autochthonous in the Northeast, and is transmitted by a vector which is very common in that region,25 suggests possible infection by this agent among the pregnant women.
Regarding the complications of pregnancy observed, the oligohydramnios (few amniotic fluid) may be caused by many factors, among them, the congenital anomalies,26 such as microcephaly.
Some infants presented ophthalmic alterations, which was also found in other studies, such as macular and optic disc alterations.27,28 Other congenital malformations were also identified, mainly musculoskeletal malformations,29 corroborating with literature findings.10 Dandy-Walker syndrome, also observed in this study, is an anomaly that strikes the central nervous system.30
Half of the microcephaly cases described in this study were submitted to transfontanellar ultrasound, which is more accessible to the local population. The imaging findings are suggestive of congenital infections, corroborating with other studies,22,29 and there was no report of microcephaly for other causes.
Concerning the three deaths, although it was not possible to collect samples for the identification of Zika virus, there are evidences of this infection as being responsible for the deaths occurred in other states from the Northeast, with the identification of the viral RNA of Zika, and negative results for known viruses, such as dengue, chikungunya and others.10,23,29
This study presented some limitations, such as: (i) divergences/lack of information regarding the records of the health professionals - such as medical records and pregnant women’s health card -, which may have reduced the accuracy of the epidemiological description; (ii) difficulty of the mothers to report signs and symptoms, which may have underestimate or overestimate the data; (iii) no collection of clinical samples for the identification of Zika virus in mothers and infants; (iv) no measurement of the head circumference in babies born through vaginal delivery after 24 hours, which may have overestimated the number of cases of microcephaly; and (v) lack of data to evaluate whether the infant was small for the gestational age, situation in which the reduced head circumference could not represent microcephaly.
In face of the situation of Public Health Emergency, characterized by the increase in the number of microcephaly cases in Sergipe, it is necessary to organize the assistance and surveillance to conduct imaging diagnoses and laboratory techniques, aiming to follow up these newborns. Moreover, we recommend the conduction of analytic and laboratory studies that can evidence the relation between the behavior of Zika virus and the congenital malformations, and, with the awareness of the pregnancy and the possibility of infection for Zika virus, provide health education directed to pregnant women, promoting the use of repellents for Aedes aegypti mosquito and other forms of prevention.
REFERENCES
1. World Health Organization. Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero [Internet]. Genebra: World Health Organization; 2016 [Cited 2016 Mar 4]. Available from: Available from: http://apps.who.int/iris/bitstream/10665/204475/1/WHO_ZIKV_MOC_16.3_eng.pdf?ua=1 [ Links ]
2. National Birth Defects Prevention Network. Major birth defects data from population-based birth defects surveillance programs in the United States, 2006-2010. Birth Defects Res Part A Clin Mol Teratol. 2013;97:S1-S172. [ Links ]
3. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016 May;374(20):1981-7. [ Links ]
4. Ellington SR, Devine O, Bertolli J, Martinez Quiñones A, Shapiro-Mendonza CK, Perez-Padilla J, et al. Estimating the number of pregnant women infected with Zika virus and expected infants with microcephaly following the Zika virus outbreak in Puerto Rico. JAMA Pediatr. 2016 Oct;170(10):940-5. [ Links ]
5. Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med . 2016 Mar;374:951-8. [ Links ]
6. Organização Mundial da Saúde. Doença do virus Zika [Internet]. Geneva: World Health Organization; 2016 [citado 2016 jul 8]. Disponível em: Disponível em: http://www.who.int/mediacentre/factsheets/zika/pt/ [ Links ]
7. Butler D. Microcephaly surge in doubt. Nature. 2016 Feb;530(7588):12-3. [ Links ]
8. Marinho F, Araújo VEM, Porto DL, Ferreira HL, Coelho MRS, Lecca RCR, et al. Microcefalia no Brasil: prevalência e caracterização dos casos a partir do Sistema de Informações sobre Nascidos Vivos (Sinasc), 2000-2015. Epidemiol Serv Saude. 2016 out-dez;25(4):701-12. [ Links ]
9. Brasil. Ministério da Saúde. Portaria GM nº 1.813, de 11 de novembro de 2015. Declara Emergência em Saúde Pública de importância Nacional (ESPIN) por alteração do padrão de ocorrência de microcefalias no Brasil. Diário Oficial da República Federativa do Brasil, Brasília (DF) , 2015 nov 12;Seção 1:51. [ Links ]
10. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Protocolo de vigilância e resposta à ocorrência de microcefalia relacionada e/ou alterações do Sistema Nervoso Central (SNC): Emergência de Saúde Pública de Importância Internacional - ESII [Internet]. Brasília: Ministério da Saúde; 2015 [citado 2016 abr 1]. Disponível em: Disponível em: http://portalsaude.saude.gov.br/images/pdf/2016/marco/24/Microcefalia-Protocolo-vigil--ncia-resposta-versao2.1.pdf [ Links ]
11. Instituto Brasileiro de Geografia e Estatística. Estados [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2016 [citado 2016 nov 9]. Disponível em: Disponível em: http://www.ibge.gov.br/estadosat/perfil.php?sigla=se [ Links ]
12. Ministério da Saúde. Secretaria de Vigilância em Saúde. Datasus [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2016 abr 5]. Disponível em: Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinasc/cnv/nvuf.def . [ Links ]
13. Instituto Brasileiro de Geografia e Estatística. Sala de imprensa [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2015 [citado 2016 abr 5]. Disponível em: Disponível em: http://saladeimprensa.ibge.gov.br/noticias?view=noticia&id=1&busca=1&idnoticia=2833 [ Links ]
14. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the Intergrowth-21st Project. Lancet; 2014 Sep;384(9946):857-68. [ Links ]
15. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Confirmação do Zika vírus no Brasil [Internet]. Brasília: Ministério da Saúde, 2015 [citado 2016 jul 07]. Disponível em: Disponível em: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/noticias-svs/17702-confirmacao-do-zika-virus-no-brasil [ Links ]
16. Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016 May;387(10033):2125-32. [ Links ]
17. European Surveillance of Congenital Anomalies. Project 2001/RD/10014 of the DG Sanco Rare Diseases Programme. Final Activity Report (Eurocat), final activity report 2002-2003. Reino Unido: European Surveillance of Congenital Anomalies; 2005 [cited 2016 Jul 7]. Available from: Available from: http://ec.europa.eu/health/ph_threats/non_com/docs/eurocat_en.pdf . [ Links ]
18. Oliveira WK, Cortez-Escalante J, Oliveira WT, Carmo GM, Henriques CM, Coelho GE, et tal. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016 Mar;65(9):242-7. [ Links ]
19. Instituto Brasileiro de Geografia e Estatística. Sala de imprensa: notícias: IBGE divulga rendimento domiciliar per capita segundo a PNAD contínua para o FPE [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2014 [citado 2016 abr 5]. Disponível em: Disponível em: http://saladeimprensa.ibge.gov.br/noticias?view=noticia&id=1&busca=1&idnoticia=2833 [ Links ]
20. García Peñas JJ, Andújar FR. Alteraciones del perímetro craneal: microcefalia y macrocefalia. Pediatr Integr. 2003;7:587-600. [ Links ]
21. Schuler-Faccini L, Ribeiro EM, Feitosa IML, Horovitz DDG, Cavalcanti DP, Pessoa A, et al. Possível associação entre a infecção pelo vírus zika e a microcefalia: Brasil, 2015. MMWR. 2016 jan;65(3):59-62. [ Links ]
22. Brasil P, Pereira Júnior JP, Moreira ME, Nogueira RMR, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016 Dec;375(24):2321-34. [ Links ]
23. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009 Jun;360:2536-43. [ Links ]
24. Organização Pan-Americana da Saúde. Temas da saúde. Sarampo. Região das Américas é declarada livre de sarampo [Internet]. Brasília: Organização Pan-Americana da Saúde; 2016 [citado 2016 dez 28]. Disponível em: Disponível em: http://www.paho.org/bra/index.php?option=com_joomlabook&task=display&id=255&Itemid=232 [ Links ]
25. Campos, GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015 Oct;21(10):1885-6. [ Links ]
26. Ribeiro AM, Guimarães MJ, Lima MC, Sarinho SW, Coutinho SB, Coutinho SB. Fatores de risco para mortalidade neonatal em crianças com baixo peso ao nascer. Rev Saude Publica. 2009;43(2):246-55. [ Links ]
27. Ventura CV, Maia M, Ventura BV, Linden VV, Araújo EB, Ramos RC, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uter us Zika virus infection. Arq Bras Oftalmol. 2016 Feb;79(1):1-3. [ Links ]
28. Ashwal S, Michelson D, Plawner L, Dobyns WB. Practice parameter: evaluation of the child with microcephaly (an evidence-based review). Neurology. 2009 Sep;73(11):887-97. [ Links ]
29. Carvalho FH, Cordeiro KM, Peixoto AB, Tonni G, Moron AF, Feitosa FEL, et al. Associated ultrasonographic findings in fetuses with microcephaly because of suspected Zika virus (ZIKV) infection during pregnancy. Prenat Diagn. 2016 Sep;36(9):882-7. [ Links ]
30. Ewald O, Scremim F, Busch F, Von Hertwig R. Alterações oculares em paciente pediátrico portador de malformações de Dandy-Walker: relato de caso. Arq Bras Oftalmol. 2006 jan-fev;69(1):97-9. [ Links ]
2Cabral CM, Nóbrega MEB, Saad E, Souza PB, Souza MSF, Teixeira DCP, Carvalho RAS, Cavalcante TF, Lima RGS and Tavares LMSA contributed to the conception, design of the study, critical review of its intellectual content, analysis and interpretation of the results. Santelli ACFS and Leite PL contributed to the analysis and interpretation of results and critical review of the intellectual content of the manuscript. All the authors participated in the manuscript’s writing, approved its final version and declared to be responsible for all aspects of the study, ensuring its accuracy and integrity.
Received: September 30, 2016; Accepted: November 29, 2016











 texto em
texto em 


