Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2337-9622
Epidemiol. Serv. Saúde v.26 n.3 Brasília jul./sep. 2017
http://dx.doi.org/10.5123/s1679-49742017000300002
ORIGINAL ARTICLE
Dengue in pregnant women: characterization of cases in Brazil, 2007-2015*
1Universidade Federal de Goiás, Instituto de Patologia Tropical e Saúde Pública, Goiânia-GO, Brasil
2Ministério da Saúde, Secretaria de Vigilância em Saúde, Brasília-DF, Brasil
OBJECTIVE:
to characterize the probable cases of dengue in pregnant women reported in Brazil, from 2007 to 2015.
METHODS:
descriptive study of sociodemographic, epidemiological, clinical and laboratory characteristics, with data from the Information System for Notifiable Diseases (Sinan).
RESULTS:
the annual incidence of dengue in pregnant women ranged from 3.3 (2009) to 816.6 (2010) cases per 100 thousand live births; of the 43,772 probable cases of dengue in pregnant women, 81.6% were investigated, 34.1% were confirmed by laboratory tests, and 1.7% were severe cases; hospitalization and fatality rates were of 5.4% and 1.6‰, respectively; the risk of death due to dengue was higher in pregnant women than in the population of non-pregnant women at reproductive age (ratio=3.95; 95%CI=3.07;5.08), and higher in the third trimester of pregnancy (ratio=8.55; 95%CI=6.08;12.02).
CONCLUSION:
the results show the burden of dengue in pregnant women and their vulnerability to worsening of the disease and death.
Keywords: Pregnant Women; Dengue; Descriptive Epidemiology
Introduction
Dengue is an illness caused by a Flavivirus, considered the most relevant arbovirosis worldwide. The four serotypes of the virus (DENV1-4) are transmitted by the mosquito genus Aedes (Stegomya), causing acute fever that may vary from oligosymptomatic to severe forms, which can lead to death.1
Every year, there are approximately 390 million dengue cases worldwide; 96 million of them are symptomatic.2 The geographic expansion and the huge increase in the number of cases observed during the last decades have been attributed to factors such as population growth, urban agglomeration, use of fast means of transportation for people and goods, and, certainly, to the ecological conditions which favor the proliferation of the vector.3
In Brazil, since the reintroduction of the virus in 1986, dengue has become an endemic disease and one of the main public health problems in the country.4 It is a notifiable disease and has a well-established and representative surveillance system, capable of reflecting the real occurrence and magnitude of the event in the population and detecting trends of changes in the epidemiological profile.5 The suspected cases of dengue are reported through standardized forms, used throughout the country, which build the system databases.6
Dengue surveillance system has identified the continuous circulation of four viral serotypes, since the early 2010s, and an increase in the frequency and magnitude of epidemics, severe forms and deaths due to the disease. The largest transmission of the infection in the country occurred from 2010 to 2015, accounting to nearly 6 million probable cases of the disease. This hyperendemicity scenario also led to an increase in the number of cases in population segments which are under greater vulnerability of worsening of the disease, such as children, the elderly and pregnant women.7,8
Dengue infection during pregnancy has been associated with the development of preeclampsia, eclampsia, hemorrhage and maternal deaths, but not to the occurrence of congenital malformations.9-11 However, after the introduction of Zika virus in the country and the current evidence of causal relationship between prenatal infection by the virus and the development of microcephaly and other severe brain abnormalities, besides the circulation of new arboviruses,12 it became necessary to continuous monitor these cases in pregnant women, not only to characterize the cases of dengue in such group but also to establish baselines for comparison - and observation - of possible new trends of these diseases in Brazil.
This study aimed to characterize the probable cases of dengue in pregnant women reported in Brazil, from 2007 to 2015.
Methods
This is a descriptive study of the probable cases of dengue fever in pregnant women in Brazil, with initial symptoms from January 2007 to December 2015, conducted with data from the national database of the Information System on Notifiable Diseases (Sinan) of the Ministry of Health.
Brazil is the largest country in South America, with a total area of 8.5 million km2. It is politically and administratively organized into 26 states and one Federal District, distributed among five macroregions (North, Northeast, South, Southeast and Midwest). The climate is predominantly tropical. In 2015, the resident population in the country was estimated at 204,482,459 inhabitants, mostly women, and concentrated in urban areas (51%).13
In Brazil, dengue is a notifiable disease. According to the criterion of suspected case definition, used until 2013, every individual who presents fever lasting up to seven days, accompanied by at least two of the following symptoms - headache, retro-orbital pain, myalgia, arthralgia, prostration, skin rash - and with exposure to a dengue transmission area or area with presence of Aedes aegypti in the previous 15 days, must be notified to the epidemiological surveillance. Since 2014, there has been a change in this setting to 14 days of exposure to an area of transmission, the inclusion of nausea, vomiting, petechiae, positive tourniquet test and leukopenia among the symptomatic manifestations, and exclusion of prostration.6,14
Cases that meet the criteria for suspected cases of dengue are registered in the Individual Notification File (INF), covering variables related to the individual, time and place, and in the Individual Investigation File (IIF), which presents the characterization of the disease, including laboratory data and its final classification. The data collected by the disease surveillance are inserted in Sinan, which subsidizes data analysis of dengue epidemiological surveillance.14 During the period considered for this study, two versions of this system were in use: Sinan-Net, from 2007 to 2010; and Sinan Online, from December 2010 onwards.
The cases can be closed through laboratory criteria, after specific exams including serology (IgM), NS1 antigen detection, viral isolation, detection of viral genome (RT-PCR) or immunohistochemistry and histopathology, in cases of death. In epidemics situations, the cases can be confirmed by clinical and epidemiological criteria, after laboratory confirmation of the first cases in the area, although the laboratory confirmation is recommended in at least 10% of the cases in these periods.15 The cases that, after laboratory analysis show negative results for dengue or positive for another pathology or clinical and epidemiological criteria incompatible with dengue, are considered discarded.6,14,15
Up to 2013, the Brazilian Ministry of Health adopted the classification of dengue cases proposed by the World Health Organization (WHO) in 1997, ranking the cases as: classical dengue (CD), dengue hemorrhagic fever (DHF), dengue shock syndrome (DSS) and, additionally, an intermediate classification called ‘dengue fever with complications’ (DFC), which includes all cases that do not fit the diagnosis criteria of HDF and in which the CD rating is unsatisfactory given the severity of the clinical-laboratory conditions presented. From 2014 onwards, the national surveillance system adopted the WHO criteria set in 2009, ranking the cases of the disease as (i) dengue, (ii) dengue with warning signs, and (iii) severe dengue.6,14,16
During non-epidemic periods, every suspected case of dengue should be notified and investigated using the specific forms, INF and IIF, respectively; during epidemic periods, the suspected cases of severe dengue, HDF, DSS, DFC, deaths, pregnant women and children under 15 years old, and cases with unusual clinical manifestations must be investigated. Being pregnant and the trimester of pregnancy are variables used in both instruments, INF and IIF.15
Initially, the database of pregnant women reported with dengue in the country was subjected to exploratory analysis of the variables; nine inconsistent records for ‘sex’ were excluded. For the analysis, only pregnant women aged between 10 and 49 years were selected, as the Ministry of Health considers this age group as the reproductive age among women, and the ‘probable cases’; that is, the reports closed as discarded case were excluded.
The participants in the study were described according to their sociodemographic, epidemiological, and clinical and laboratory characteristics. Sociodemographic variables, were extracted from the database:
- date of early symptoms;
- federative unit and municipality of residence;
- age (in years: 10 to 14, 15 to 19, 20 to 29, 30 to 39, 40 to 49);
- ethnicity/skin color (white, black, Asian, brown, indigenous, unknown);
- education level (years of schooling: 0, 1 to 3, 4 to 7, 8 to 11, less than 12, unknown); and
- trimester of pregnancy (1st, 2nd, 3rd, unknown).
The epidemiological, clinical and laboratory data researched were:
- confirmation criteria (laboratory, clinical-epidemiological, under investigation, unknown);
- final classification used by the Ministry of Health up to 2013 (classical dengue, hemorrhagic dengue, dengue with complications, dengue shock syndrome, under investigation, unknown);
- final classification used by the Ministry of Health from 2014 onwards (dengue, dengue, dengue with warning signs, severe dengue, unknown);
- hospitalization (yes, no, unknown);
- outcome (cure, death by dengue, death due to other cause, unknown);
- serological test IgM (reagent, not reagent, inconclusive, not performed);
- NS1 antigen test (positive, negative, inconclusive, not performed);
- viral isolation (positive, negative, inconclusive, not performed);
- RT-PCR (positive, negative, inconclusive, not performed); and
- serotype (DENV 1, DENV 2, DENV 3, DENV 4).
The incidence of dengue in pregnant women was calculated by the ratio between the total number of probable cases of dengue in pregnant women and the population of live births registered in the Information System on Live Births (Sinasc). In the comparative analysis with the general population and non-pregnant women at reproductive age, the same definition criteria of probable cases for pregnant women was used.
The percentage of cases investigated was obtained from the proportion of cases with the variable ‘final classification’ filled among all probable cases, multiplied by 100.
The fatality due to dengue in pregnant women was calculated as the proportion of deaths by dengue among the probable cases, times 1,000 (‰).
The data analysis was conducted with the SPSS® (Statistical Package for the Social Sciences) version 23, Microsoft Office® Excel® 2010, TabWin® version 3.2, and Epi Info 7® version 7.1.5.2.
Although this is a descriptive study, having as its main objective the characterization of dengue in pregnant women according to the distribution of variables related to person, time and place, there has been a comparison between the studied group (pregnant women) with an external group (non-pregnant women at reproductive age). For that, we calculated the fatality ratio for dengue in pregnant women, according to the trimester of pregnancy, and fatality by dengue among non-pregnant women at reproductive age. The Chi-square test was used to compare those groups, with statistical significance level of 5%.
The design of the study was approved by the Research Ethics Committee of Clinical Hospital of the Federal University of Goiás (CEP/HC/UFG) - Report No. 615,256/14 - on April 14th, 2014, and developed in accordance with the ethical principles established by the Resolution of the National Health Council (CNS) No. 466, dated December 12th, 2012.
Results
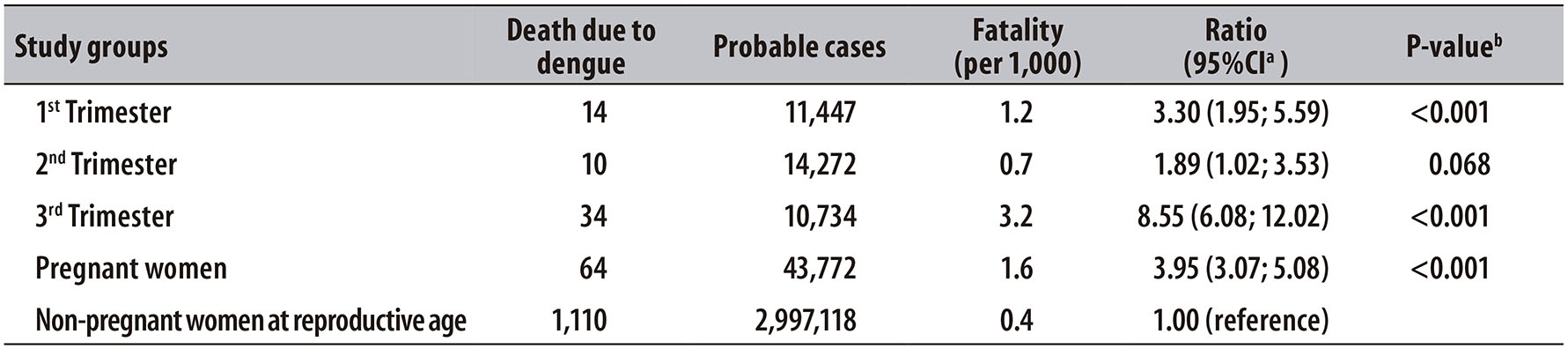
The 43,772 probable cases of dengue in pregnant women in Brazil, between 2007 and 2015, occurred in residents of 3,218 municipalities distributed in the five macroregions of the country, with higher concentration of cases in the Southeast region (44.3%), followed by the Northeast (24.8%), Midwest (17.1%), North (10.0%) and South (3.8%).
Overall, the incidence of dengue in pregnant women, in each region of the country, was similar to the occurrence of cases observed in the general population of these areas (Figure 1). The years with the highest number of dengue cases in the population showed a higher incidence of dengue in pregnant women (depending on the region): from 3.3 in 2009 to 816.6 cases per 100 thousand live births in 2010.
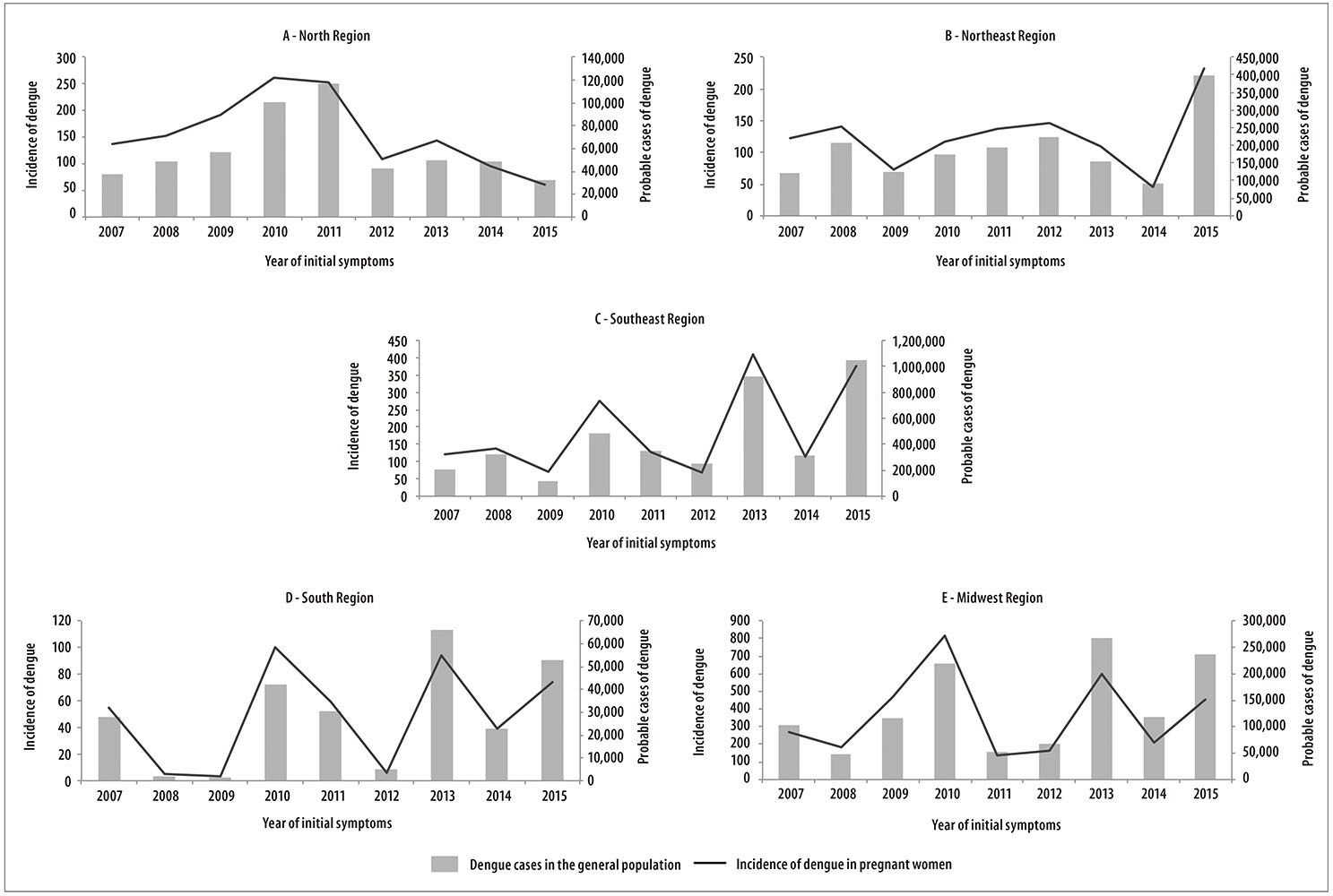
Figure 1 - Distribution of probable cases of dengue in the general population and incidence of dengue in pregnant women (per 100,000), in the country macroregions, Brazil, 2007-2015
In Table 1, the sociodemographic characteristics of pregnant women with dengue in Brazil are presented. There has been similar distribution over the years, with higher frequency of pregnant women between the ages of 20 and 29 (44.1%), ethnicity/skin color white and brown (74.2%), and with 8 to 11 years of schooling (34.1%). The proportion of cases by trimester of pregnancy showed similar distribution, with a slightly higher frequency in the second trimester of pregnancy (32.6%). The percentage of cases investigated was of 81.6% and 34.1% of the cases were confirmed by laboratory criteria. According to the final classification, 1.7% of the records were characterized as severe forms of the disease (HDF, DSS, DFC and severe dengue), and the rates of hospitalization and fatality rate due to dengue were 5.4% and 1.6‰, respectively. The year of 2015 concentrated the largest number of recorded cases of the disease (18.4%), followed by 2013 (18.2%) and 2010 (16.4%); the year of 2014 (6.1%) presented the lowest occurrence.
Table 1 - Sociodemographic and epidemiological characteristics of the probable cases of dengue in pregnant women (n = 43,772), per year, Brazil, 2007-2015
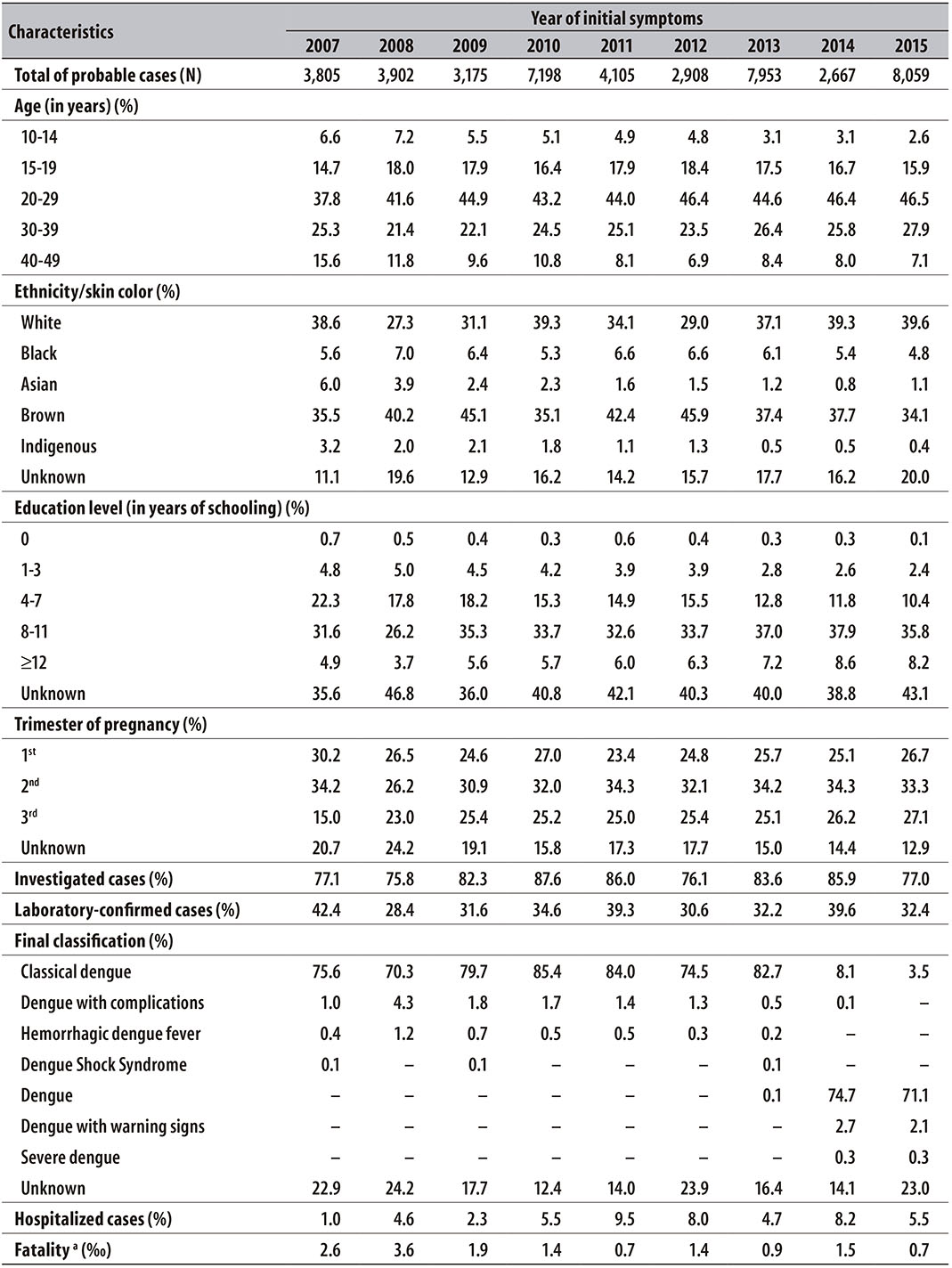
a) The fatality rate was estimated by dividing the number of deaths due to dengue by the number of probable cases of the disease, times 1,000.
Of all the pregnant women with laboratory confirmation, 84.2% underwent serology tests and 10.7% underwent tests for the NS1 antigen. Among the 150 pregnant women who underwent viral serotype identification, DENV1 was found in 62.7%, DENV4 in 20.0%, DENV2 in 11.3% and DENV3 in 6.0%.
In Figure 2, the fatality rate distribution for dengue among non-pregnant women at reproductive age is presented, with variation from 0.2 to 0.6 per 1,000 cases, being lower than those observed in the pregnant women in the study. Pregnant women with infection during the third trimester, who reached the fatality rate of 11.7 per 1,000 cases in 2008, stood out.
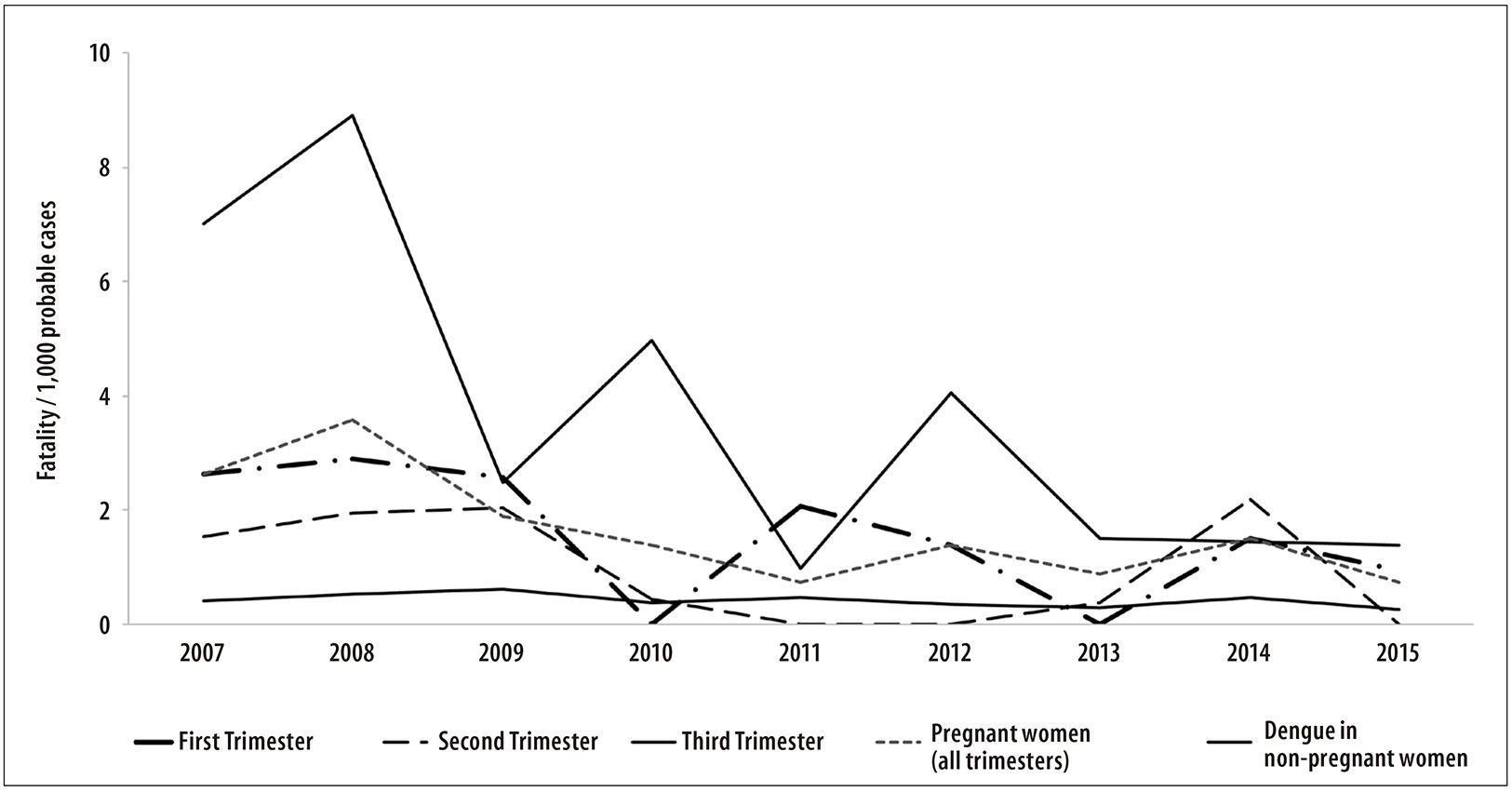
Figure 2 - Fatality rate of dengue in pregnant women (per 1,000 probable cases), according to the trimester of pregnancy, and fatality by dengue among non-pregnant women at reproductive age, Brazil, 2007-2015
The result of the association between fatality rate by dengue and pregnancy and the trimester of occurrence of the disease is presented in Table 2. The risk of death by dengue was higher in the population of pregnant women in the study (ratio=3,95; 95%CI=3.07;5.08>;p<0.001), if compared to the same risk in the population of non-pregnant women at reproductive age. When stratified by trimester of pregnancy, the risk was higher during the third trimester (ratio=8.5595%CI = 6.08;12.02; p<0.001); and statistically significant in the first trimester, but not in the second.
Discussion
This study presents a detailed characterization of the probable cases of dengue in pregnant women, from 2007 to 2015. The occurrence of more than 40 thousand cases of pregnant women with dengue in Brazil at that period highlights the burden of this disease in one of the groups that require special attention from health services. As expected, the incidence of dengue in pregnant women accompanied the occurrence of cases in the general population, in all regions of the country, with high proportion of cases investigated by the surveillance system. Fatality by dengue among pregnant women was higher than in the population of women at reproductive age, with greater risk of death in the third trimester of pregnancy.
The dengue surveillance system in Brazil was established since the introduction of the virus in the country, and assessed as consistent, timely and representative for the notification of cases, with appropriate positive predictive value.5 In comparison with data for the general population available in Sinan (unpublished data), the percentage of cases investigated during the same period was of 62.8% (95%CI=62.8;62.9) and laboratory confirmation of 30.4% (95%CI=30.4;30.5); lower values than the percentages observed for pregnant women in this study. Although the attributes of the dengue surveillance system for special groups of the population have not been previously assessed, the results of this study reinforce the surveillance quality for the group of pregnant women.
The surveillance routine recommends the investigation of all cases in this group, with priority for laboratory confirmation.15 The characterization of pregnant women in this study found a high percentage of investigated cases, reflecting the surveillance system's attention to this group. However, less than one third of the cases in pregnant women were confirmed by laboratory criteria, hence there is need for greater priority for the closure of cases by specific laboratory criteria, once pregnant women are considered a special group in health care.
The incidence of dengue in pregnant women has followed the trend of the occurrence of the disease in the general population, that is, higher number of probable cases in the years with epidemics. The incidence of dengue in pregnant women is higher than the incidence in the general population, in all country regions and years. This higher incidence may be associated with the increasing demand for health services by pregnant women, when they feel the symptoms of the disease, and the better attention of such services to pregnant women, since they are considered a special group in health care.7,17 In this scenario, the underreporting of cases may be lower, and the data on the occurrence of the disease more reliable, when compared to data of dengue among the general population.
In the Northeast region, there has been an increase in the number of cases of dengue in pregnant women, especially in the first months of 2015. Such growth was not observed significantly in other years, or in the other regions of the country. This period coincides with the occurrence of cases with dengue-like symptoms, yet without laboratory confirmation of Zika virus circulation, held only in April of that year.18 Considering the similarity of clinical manifestations between dengue and Zika virus, the results suggest that the peak of notifications of dengue in pregnant women observed is the reflection of premature identification - by the dengue surveillance system - of the Zika virus epidemic in that region.
The occurrence of severe forms of dengue is associated with viral risk factors, such as the continuous circulation of four serotypes, host organism factors such as immune status, besides age, the presence of comorbidities, and also genetic predisposition.2,3,19 The proportion of severe cases observed in pregnant women was higher than the 0.1 to 0.5% variation identified in the systematic review with data from the general population of Brazil (2000 to 2010).7 Dengue infection during pregnancy has been associated with adverse maternal outcomes, such as hemorrhage and maternal death.9,20-24 Studies with pregnant women in the municipality of Rio de Janeiro, in 2007-2008, and in the African and Asian continents, in 2008-2009, verified fatality rates ranging from 30 to 220‰,10,21,25 superior to those found in this present study. However, those studies were conducted among populations of pregnant women from different scenarios, such as pregnant women hospitalized or in countries located in Asia, where there are higher rates of severe cases and mortality from dengue, or in places with a predominance of the viral serotype DENV2, which may be associated with increased virulence, with efficient replication and high viral burden, causing inflammatory and toxic effects.26
The occurrence of maternal death in pregnant women with dengue infection has been linked mainly to hemorrhage related to thrombocytopenia and endothelial dysfunction during acute infection, and the complications arising from direct damage to the placenta in the first trimester of pregnancy, leading to anatomical and functional anomalies.9,22 The fatality found in pregnant women investigated was similar to that found for the general population in Brazil, whose rates are below 3‰.7,27 However, a more appropriate comparison would be with the group of women at reproductive age, which usually does not present other factors associated to death by dengue, as being over 45 years of age or present comorbidity.27-29
When comparing the fatality rates in pregnant women with the fatality of the population of women at reproductive age, during the period of the study, we observed a risk of death by dengue about four times higher, especially in the third trimester of pregnancy. Similarly, another study, conducted in Rio de Janeiro State in 2007-2008, identified a higher risk for pregnant women of developing severe forms and of death due to the disease, especially during the third trimester of pregnancy.10
This study finds some limitations inherent to the use of secondary data from the passive surveillance system. The underreporting of cases and the existence of incomplete records may compromise the analysis of some variables. Notwithstanding, the occurrence of underreporting, a prior assessment of the dengue surveillance system characterized the activities as consistent, which makes this system able to identify trends.5
This characterization of dengue among pregnant women was carried out in the period of greatest transmission of the disease in Brazil, with over 40 thousand cases coming from the five macroregions of the country. Given the importance of Brazil in the world scenario of dengue transmission, the findings reinforce the recognition of pregnant women as a vulnerable group, due to the worsening of the disease, or to the occurrence of maternal death by dengue. Moreover, the large number of pregnant women with dengue expresses the magnitude of the potential risk of pregnant women to be infected by other arboviruses, which can lead to serious infection and neonatal death. It is the case of infected mothers at delivery, of chikungunya fever infection, and of microcephaly and other congenital anomalies, in live births from pregnant women infected with the Zika virus.12,30 Specific measures, such as treatment priority, investigation of cases and the collection of specific exams, already adopted by the Ministry of Health, should be maintained and strengthened. The assessment of new prevention technologies targeted at pregnant women, and its implementation should also be expanded, with the aim of reducing the risk of infection by dengue and other arboviruses in this population group.
REFERENCES
1. World Health Organization. Global strategy for dengue prevention and control 2012-2020. Geneva: World Health Organization; 2012 [Cited 2016 set 10]. Available from: http://www.who.int/denguecontrol/9789241504034/en/ [ Links ]
2. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013 Apr;496(7446):504-7. [ Links ]
3. Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014 Mar;22(3):138-46. [ Links ]
4. Rodriguez-Barraquer I, Cordeiro MT, Braga C, Souza WV, Marques ET, Cummings DA. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis. 2011 Jan;5(1):e935. [ Links ]
5. Barbosa JR, Barrado JCS, Zara ALSA, Siqueira Júnior JB. Avaliação da qualidade dos dados, valor preditivo positivo, oportunidade e representatividade do sistema de vigilância epidemiológica da dengue no Brasil, 2005 a 2009. Epidemiol Serv Saude. 2015 jan-mar;24(1):49-58. [ Links ]
6. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Guia de vigilância epidemiológica. 7 ed. Brasília: Ministério da Saúde; 2009. (Série A. Normas e Manuais Técnicos). [ Links ]
7. Teixeira MG, Siqueira Júnior JB, Ferreira GLC, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000-2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013 Dec;7(12):e2520. [ Links ]
8. Martelli CM, Siqueira Júnior JB, Parente MP, Zara AL, Oliveira CS, Braga C, et al. Economic impact of dengue: multicenter study across four brazilian regions. PLoS Negl Trop Dis. 2015 Sep;9(9):e0004042. [ Links ]
9. Basurko C, Carles G, Youssef M, Guindi WE. Maternal and fetal consequences of dengue fever during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009 Nov;147(1):29-32. [ Links ]
10. Machado CR, Machado ES, Rohloff RD, Azevedo M, Campos DP, Oliveira RB, et al. Is pregnancy associated with severe dengue? A review of data from the Rio de Janeiro surveillance information system. PLoS Negl Trop Dis. 2013 May;7(5):e2217. [ Links ]
11. Restrepo BN, Isaza DM, Salazar CL, Ramírez JL, Upegui GE, Ospina M, et al. Prenatal and postnatal effects of dengue infection during pregnancy. Biomédica. 2003 Dec;23(4):416-23. [ Links ]
12. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016 May;374(20):1981-. [ Links ]
13. Ministério da Saúde (BR). Datasus. Estimativas de 1992 a 2015 utilizadas pelo TCU para determinação das cotas do FPM. [Internet]. Brasília: Ministério da Saúde ; 2015 [citado 2016 out 9 ]. Disponível em: Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?ibge/cnv/poptuf.def [ Links ]
14. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de Vigilância em Saúde. Brasília: Ministério da Saúde ; 2014. [ Links ]
15. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Diretrizes nacionais para a prevenção e controle de epidemias de dengue. Brasília: Ministério da Saúde ; 2009. (Série A. Normas e Manuais Técnicos). [ Links ]
16. World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention, and control. Geneva: World Health Organization ; 2009. [ Links ]
17. Fares RC, Souza KP, Añez G, Rios M. Epidemiological scenario of dengue in Brazil. Biomed Res Int. 2015;2015:321873. [ Links ]
18. Heukelbach J, Alencar CH, Kelvin AA, Oliveira WK, Cavalcanti PGL. Zika virus outbreak in Brazil. J Infect Dev Ctries. 2016 Feb;10(2):116-20. [ Links ]
19. Yacoub S, Mongkolsapaya J, Screaton G. Recent advances in understanding dengue. F1000Res. 2016 Jan;5:1-10. [ Links ]
20. Hung LP, Nghi TD, Anh NH, Van Hieu M, Thien LN, Phuoc Long N, et al. Case report: postpartum hemorrhage associated with dengue with warning signs in a term pregnancy and delivery. F1000Res. 2015 Dec;4:1483. [ Links ]
21. Pouliot SH, Xiong X, Harville E, Paz-Soldan V, Tomashek KM, Breart G, et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv. 2010 Feb;65(2):107-18. [ Links ]
22. Hanf M, Friedman E, Basurko C, Amaury R, Bruncher P, Dussart P, et al. Dengue epidemics and adverse obstetrical outcomes in French Guiana: a semi-ecological study. Trop Med Int Health. 2014 Feb;19(2):153-8. [ Links ]
23. Ismail NAM, Rahim WERWA, Salleh SA, et al. Seropositivity of dengue antibodies during pregnancy. Sci World J. 2014;2014:436975. [ Links ]
24. Sharma S, Jain S, Rajaram S. Spectrum of maternofetal outcomes during dengue infection in pregnancy: an insight. Infect Dis Obstet Gynecol. 2016;2016:45046091. [ Links ]
25. Kariyawasam S, Senanayake H. Dengue infections during pregnancy: case series from a tertiary care hospital in Sri Lanka. J Infect Dev Ctries. 2010 Nov;4(11):767-75. [ Links ]
26. Halstead SB. Pathogenesis of dengue: dawn of a new era. F1000Res. 2015 Nov;4:1-8. [ Links ]
27. Cardoso IM, Cabidelle ASA, Borges PCL, Lang CF, Calenti FG, Nogueira LO, et al. Dengue: formas clínicas e grupos de risco em município de alta incidência do sudeste do Brasil. Rev Soc Bras Med Trop. 2011 jul-ago;44(4):430-5. [ Links ]
28. Díaz-Quijano FA, Waldman EA. Factors associated with dengue mortality in Latin America and the Caribbean, 1995-2009: an ecological study. Am J Trop Med Hyg. 2012 Feb;86(2):328-34. [ Links ]
29. Moraes GH, Duarte FE, Duarte EC. Determinants of mortality from severe dengue in Brazil: A population-based case-control study. Am J Trop Med Hyg. 2013 Apr;88(4):670-6. [ Links ]
30. Calvet GA, Santos FB, Sequeira PC. Zika virus infection: epidemiology, clinical manifestations and diagnosis. Curr Opin Infect Dis. 2016 Oct;29(5):459-66. [ Links ]
* Article based on the PhD thesis by Laura Branquinho do Nascimento, entitled 'Dengue em Gestantes e a Associação entre a Infecção Sintomática e Desfechos Desfavoráveis em Nascidos Vivos: um Relacionamento entre os Dados dos Sistemas de Informação em Saúde no Brasil de 2007 a 2013', defended to the Post-graduation Program in Public Health and Tropical Medicine of the Federal University of Goiás, in 2016. Study funded with resources from the Fundação de Amparo à Pesquisa do Estado de Goiás (Fapeg): Public Call No. 04/2015 - Scholarships for Master’s and Doctoral Degree programs.
Received: November 15, 2016; Accepted: March 21, 2017











 texto en
texto en