Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2337-9622
Epidemiol. Serv. Saúde vol.26 no.4 Brasília dic. 2017
http://dx.doi.org/10.5123/s1679-49742017000400009
ORIGINAL ARTICLE
Hospitalizations due to drug poisoning in under-five-year-old children in Brazil, 2003-2012*
1Ministério da Saúde, Departamento de Assistência Farmacêutica e Insumos Estratégicos, Brasília-DF, Brasil
2Fundação Oswaldo Cruz, Escola Nacional de Saúde Pública, Rio de Janeiro-RJ, Brasil
OBJECTIVE:
to describe hospitalizations due to drug poisonings in children under five years old, in Brazil, from 2003 to 2012.
METHODS:
descriptive study, with data from the National Hospital Information System (SIH/SUS); the drugs involved were divided into therapeutic classes, according to the Anatomical Therapeutic Chemical Classification (ATC).
RESULTS:
17,725 hospitalizations were identified, from which 22,395 poisonings were identified, and 75 deaths; the most common therapeutic classes were unspecified drugs (38.0%), antiepileptic/sedative-hypnotics/anti-parkinson drugs (19.8%), systemic antibiotics (13.4%) and non-opioid-analgesics/antipyretics (6.5%), varying among country regions and age groups; in 38.5% of the poisonings it was not possible to correlate therapeutic classes and ATC categories.
CONCLUSION:
the high frequency of unspecified drugs was a limitation; among the specified drugs, the most common were those that act in the central nervous system and those used in pediatric diseases (antibiotics and analgesics).
Keywords: Hospitalization; Poisoning; Pharmaceutical Preparations; Infant; Epidemiology, Descriptive
Introduction
Drug poisoning occurs when a medicine is used in doses higher than those prescribed for prophylaxis, diagnosis, treatment or change of physiological functions, either intentionally or not.1 In children, poisoning is related to (i) the curiosity inherent at this age, (ii) immature function of their organism,2 which enhances susceptibility to the toxic action of drugs or medicines and (iii) absence of child-resistant packaging, in addition to (iv) relatively little importance given to accident prevention,3 which favors, for example, incorrect storage, enabling children to reach the medicine.
Moreover, the use of medicines without clinical indications for children, which constitutes off-label use,4 difficulties to calculate the exact dose and measure of medicines,4 the practice of assisted self-medication (by parents or guardian)5 and the free advertising of medicines are also factors that favor poisonings.
In Brazil, the Toxicological Information and Care Centers (CIATox) are available to guide health professionals in public and private care, and the population in general; and, in some services, to assist exposed or poisoned individuals.6 The information received by CIATox is summarized by the National System of Toxic-Pharmacological Information (Sinitox), composing a national database of drug poisoning. In 2013, 34.3% of drug poisoning notifications involved children under 5 years old, making it the most common age group regarding drug poisoning.7 However, as it is not mandatory to send the information, it does not reflect the total number of country cases.
Due to the specificity of the Brazilian scenario, studies on drug poisoning usually use data provided by CIATox8 or hospitals.9 The National Hospital Information System (SIH/SUS) is still little explored in terms of hospitalization due to drug poisoning. However, the same SIH/SUS has been used in several local10 and national11 studies, enabling the knowledge on the profile of hospitalizations due to various health problems.
The objective of this study was to describe hospitalizations due to drug poisonings in children under five years, in Brazil, from 2003 to 2012.
Methods
A descriptive study was conducted with data available in SIH/SUS related to records of hospitalizations due to drug poisoning among under five-year-old children in Brazil, obtained from the Inpatient Hospital Authorization (IHA).
IHA is a tool used to record hospitalizations funded by SUS. The main diagnosis is related to the primary reason of hospitalization, whilst secondary diagnoses include all the coexisting conditions at the moment of hospitalization, those developed during hospitalization or those which affected the health care received and/or the length of time in hospital. The diagnoses are recorded by codes of the 10th International Statistical Classification of Diseases and Related Health Problems (ICD-10). The IHA gathers more than 50 variables which include information on the individual, characteristics of hospitalization and health care services performed. After its processing, the health care facilities receive the corresponding reimbursement for the conducted procedures.
Selected records covered children aged 0 days to 4 years and 11 months, whose primary and/or secondary diagnosis was related to the following codes of ICD-10: F11.0, F.13.0, F.15.0, F.19.0, F55, P93, T36, T37, T38, T39, T40.2, T40.3, T40.4, T41, T42, T43, T44, T45, T46, T47, T48, T49, T50, T96, X40, X41, X43, X44, X60, X63, X64, X85, Y10, Y11, Y13 or Y14, according to a previously standardized methodology.12-13
The IHA variables employed were: year of hospitalization; sex (male, female); age (in years); outcome (death or absence of death); primary diagnosis; secondary diagnosis; type of health care facility; and Federative Unit (FU) of hospitalization. From IHA variables, the variables ‘age’ and ‘type of health care facility’ were categorized. The age categories were: 0 days; 1 to 28 days; 29 to 364 days; 1 year; 2 years; 3 years; and 4 years. For type of health care facility, the categories were: private; public; and SUS-affiliated private health service facility. The therapeutic classes of medicines involved in poisoning were characterized according to the the Anatomical Therapeutic Chemical Classification (ATC), of the World Health Organization (WHO), from the diagnoses reported by the ICD-10 codes.
We used summarized IHA files from SIH/SUS databases, available at the website of the IT Department of the Brazilian National Health System (Datasus). The files corresponding to data from all FU, including the Federal District, were included. Those files did not identify the individuals, nor the type of health care procedure performed. Data access occurred from April to June 2014.
Hospitalizations with more than 30 days generated a new IHA: the long-term IHA. Thus, in order to avoid a possible bias and duplication of hospitalizations, we searched for long-term IHA, and their respective short-term IHAs were excluded.
This study comprised all the records from SIH/SUS for hospitalizations due to drug poisoning in children under five years old, from 2003 to 2012. The database of the study was formed by the records with at least one of the described diagnoses, according to Figure 1.

Figure 1 - Method employed to build the databases on hospitalizations due to drug poisoning in children under five years of age recorded in the National Hospital Information System (SIH/SUS), Brazil, 2003-2012
The data were analyzed using descriptive statistics, through absolute and relative frequencies. The softwares TabWin32 Analysis System (SAS 9.3) and Statistical Package for the Social Sciences (SPSS 20) were used for data extractions and analysis.
Calculation of proportion of deaths considered deaths and hospitalizations due to drug poisoning in children under five. For comparison purposes, we calculated, separately, the proportion of deaths for hospitalizations involving poisoning by a toxic agent and by two toxic agents, using only deaths and hospitalizations observed in each of these groups.
This study used free-access public databases and data, with no identification of individuals. It was approved by the Ethics in Research Committee of the National School of Public Health Sérgio Arouca (CEP/ENSP): Report No. 01/2014, dated May 16th 2014.
Results
A total of 17,725 hospitalizations due to drug poisonings in children under five years old were identified for the period from 2003 to 2012 (Table 1). Of those, 13,055 hospitalizations involved only one poisoning diagnosis (primary or secondary diagnosis) and 4,670 not only had a primary diagnosis for poisoning but also a secondary diagnosis, considering poisonings from different toxic agents in the same hospitalization, totaling 9,340 cases. In sum, 22,395 toxic agents were involved in the 17,725 hospitalizations (Figure 2).
Table 1 - Frequency of hospitalizations of children under five years of age due to drug poisoning, recorded in the National Hospital Information System (SIH/SUS), Brazil, 2003-2012
| Characteristics | (N) | % |
|---|---|---|
| Age | ||
| 0 days | 313 | 1.77 |
| 1-28 days | 395 | 2.23 |
| 29-364 days | 2,270 | 12.81 |
| 1 year | 3,665 | 20.68 |
| 2 years | 4,302 | 24.27 |
| 3 years | 3,891 | 21.95 |
| 4 years | 2,889 | 16.30 |
| Sex | ||
| Male | 9,384 | 52.94 |
| Female | 8,341 | 47.06 |
| Year | ||
| 2003 | 2,134 | 12.04 |
| 2004 | 1,944 | 10.97 |
| 2005 | 1,833 | 10.34 |
| 2006 | 1,713 | 9.66 |
| 2007 | 1,721 | 9.71 |
| 2008 | 1,912 | 10.79 |
| 2009 | 1,810 | 10.21 |
| 2010 | 1,608 | 9.07 |
| 2011 | 1,482 | 8.36 |
| 2012 | 1,568 | 8.85 |
| Country macroregion | ||
| North | 835 | 4.71 |
| Northeast | 3,726 | 21.02 |
| Midwest | 8,283 | 46.73 |
| Southeast | 3,086 | 17.41 |
| South | 1,795 | 10.13 |
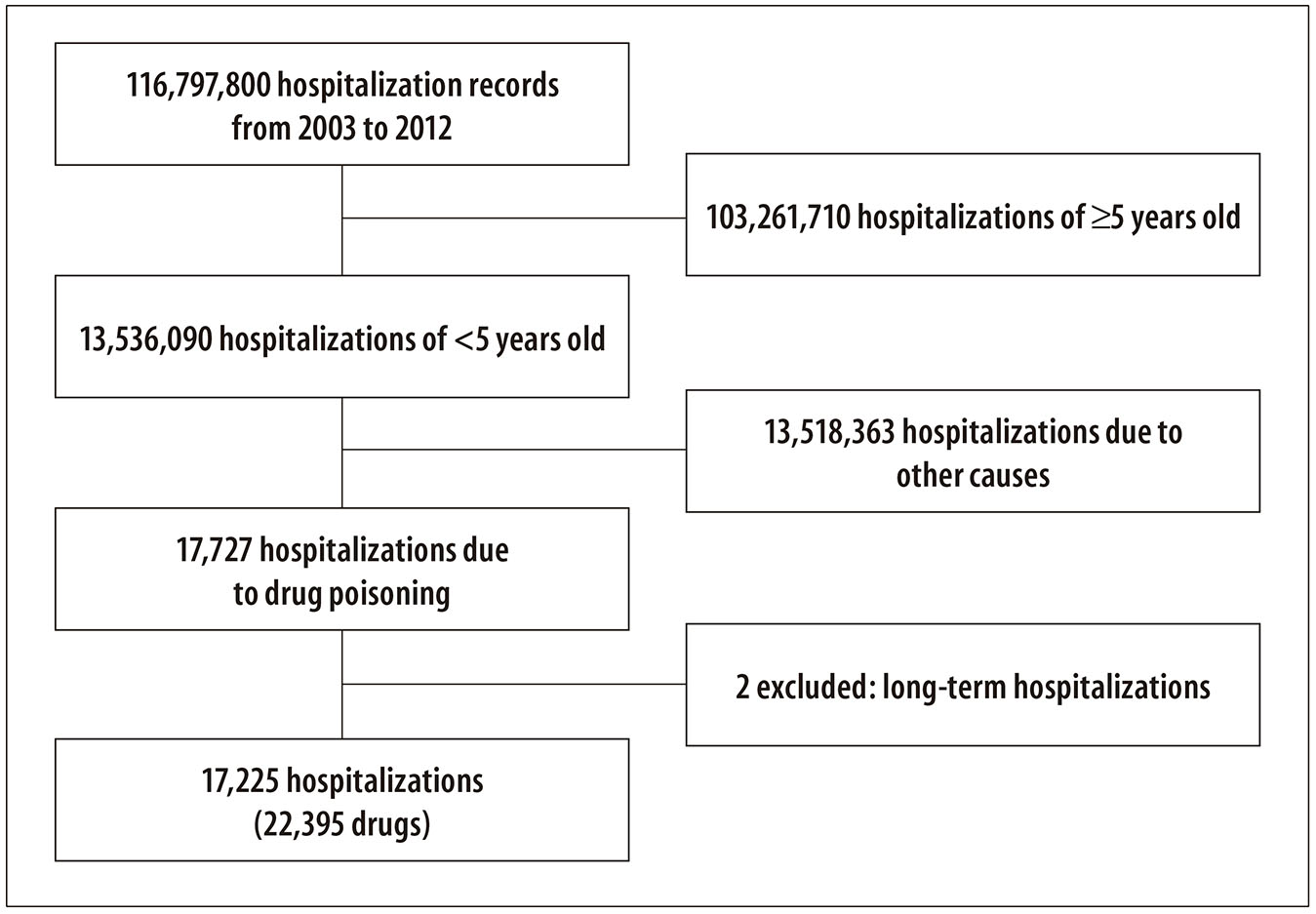
Figure 2 - Hospitalizations due to drug poisoning in children under five years of age recorded in the National Hospital Information System (SIH/SUS), Brazil, 2003-2012
The most common therapeutic classes which led to hospitalization were: unspecified drugs (38.0%); antiepileptic/sedative-hypnotics/anti-parkinson drugs (19.8%); systemic antibiotics (13.4%); and non-opioid-analgesics/antipyretics (6.5%) (Table 2).
Table 2 - Therapeutic classes involved in hospitalizations due to drug poisoning in children under five years of age recorded in the National Hospital Information System (SIH-SUS), Brazil, 2003-2012
| Therapeutic class | Poisoning | Deaths | |
|---|---|---|---|
| N | % | N | |
| Diagnostic agentsa | 82 | 0.36 | - |
| Analeptics and opioid receptor antagonists | 9 | 0.04 | 1 |
| Non-opioid analgesics/antipyretics | 1,455 | 6.50 | 9 |
| Anaesthetics and therapeutic gases | 84 | 0.38 | 1 |
| Systemic antibiotics | 3,010 | 13.44 | 17 |
| Antidotes and chelating agents, not elsewhere classified | 5 | 0.02 | - |
| Antiepileptic, sedative-hypnotics and anti-parkinson drugs | 4,424 | 19.75 | 4 |
| Diuretics | 41 | 0.18 | - |
| Enzymes, not elsewhere classified | 16 | 0.07 | - |
| Antiallergic and antiemetic drugs | 146 | 0.65 | - |
| Antineoplastic and immunosuppressive drugs | 8 | 0.04 | - |
| Stimulants | 33 | 0.15 | - |
| Psychotropic drugs | 852 | 3.80 | 1 |
| Drugs affecting the autonomic nervous system | 660 | 2.95 | 4 |
| Drugs affecting uric acid metabolism | 14 | 0.06 | - |
| Drugs acting on the circulatory system | 447 | 2.00 | 4 |
| Drugs acting on smooth and skeletal muscles and on the respiratory system | 413 | 1.84 | 2 |
| Hormones and their synthetic substitutes and antagonists | 501 | 2.24 | 3 |
| Appetite inhibitors | 19 | 0.08 | - |
| Multiple drugs and psychoactive agents | 93 | 0.42 | - |
| Narcotics | 181 | 0.81 | 1 |
| Drugs acting on electrolyte, calorie and water balance | 8 | 0.04 | - |
| Anti-infective and antiparasitic agents | 397 | 1.77 | - |
| Agents affecting the gastrointestinal system | 525 | 2.34 | 4 |
| Topical agents | 389 | 1.74 | 4 |
| Vitamins | 58 | 0.26 | - |
| Sequelaeb | 6 | 0.03 | - |
| Non-specified drugs | 8,519 | 38.04 | 32 |
| Total | 22,395 | 100.00 | 87 |
a) Radiopharmaceuticals, contrasts and any other medicine used for diagnosis.
b) Non-therapeutic class presented in the table for calculation.
Note: There was no intoxication by drugs acting in blood and coagulation.
In all Brazilian macroregions, unspecified drugs were the most common causes; 4.3% of those poisonings occurred in the North region, whereas 47.5% occurred in the Southeast. Poisoning by systemic antibiotics presented the following distribution among macroregions: North (5.0%); Midwest (8.5%; South (17.1%); Northeast (30.5%); and Southeast (38.9%). The distribution of poisonings by antiepileptic/sedative-hypnotics/anti-parkinson drugs within regions was presented as follows: 60.3% in the Southeast; 17.5% in the Northeast; 11.9% in the South; 8.3% in the Midwest; and 2.1% in the North. The region with the highest number of deaths was the Southeast (29 deaths), followed by the Northeast (23 deaths), North (9 deaths) and Midwest and South (7 deaths each; data not presented in table or figure).
There were 75 hospitalizations with death as an outcome, of which 63 had diagnosis of drug poisoning (primary or secondary). In 12 deaths, the primary and secondary diagnoses were drug poisoning. Thus, 87 toxic agents were involved in the hospitalizations which ended in death. Considering the 13,055 hospitalizations with only primary and/or secondary diagnosis of drug poisoning, the proportion of deaths was of 0.48%. With regard to the 4,670 hospitalizations with primary and secondary diagnoses, the proportion of deaths was of 0.26%. However, proportion of deaths for the 17,725 hospitalizations was of 0.42% (data not presented in table or figure). The most common therapeutic classes involved in poisoning were unspecified drugs, systemic antibiotics, and non-opioid-analgesics/antipyretics (Table 2).
Hospitalizations of infants under 28 days of life involved a smaller number of therapeutic classes than hospitalizations among older children (data not presented).
It was possible to correlate therapeutic classes and ATC categories in 13,777 poisonings, which represented a loss of 8,618 poisonings (38.5%). For poisonings in which this linkage was possible, the most common toxic agent belonged to category N - medicines which act in the central nervous system (Table 3).
Table 3 - Distribution of drug poisonings which led to hospitalizations, according to the therapeutic class described in the 10th International Statistical Classification of Diseases and Related Health Problems (ICD-10) and the Anatomical Therapeutic Chemical Classification (ATC), among under five-year-old children recorded in the National Hospital Information System (SIH/SUS), Brazil, 2003-2012
| Therapeutic class according to ICD-10 | ATC Classification | Total | |||
|---|---|---|---|---|---|
| Code ICD - 10 | Therapeutic Class | Code | Category | N | % |
| F13.0, T42, X41, X61, Y11 | Antiepileptic, sedative-hypnotics and anti-parkinson drugs | N03A, N05C, N04 | 4,424 | 19.74 | |
| T43 | Psychotropic drugs | N | 852 | 3.80 | |
| F11.0, T40.2, T40.3, T40.4, X42 | Narcotics | N | Central nervous system | 181 | 0.81 |
| F15.0 | Stimulants | N06B | 33 | 0.15 | |
| T50.7 | Analeptics and opioid receptor antagonists | N06 | 9 | 0.04 | |
| T36 | Systemic antibiotics | J | General anti-infectives for systemic use (J) and antiparasitic products, insecticides and repellents | 3,010 | 13.44 |
| T37 | Anti-infectives and antiparasitic agents | J, P | 397 | 1.77 | |
| T39, X40, X60, Y10 | Non-opioid analgesics/antipyretics | N02A, M01A | 1,455 | 6.50 | |
| T44, X43, X63, Y13 | Drugs affecting the autonomic nervous system | N04A, N07A, A03A, A03B, A03C, A03D, M03AB, R03AL, R03BB, S01FA, R03DA02, R03DB02 | 660 | 2.95 | |
| T38 | Hormones and their synthetic substitutes and antagonists | H, G03 | 501 | 2.24 | |
| T49 | Topical agents | D, S, M02A, C05A, R01A | Multiple correlations | 389 | 1.74 |
| T45.0 | Antiallergic and antiemetic drugs | R06A, D04A, A04A | 146 | 0.65 | |
| T41.0 | Anaesthetics and therapeutic gases | N01, V03AN | 84 | 0.38 | |
| T50.8 | Diagnostic agents | V04, V09, S01J | 82 | 0.37 | |
| T45.3 | Enzymes, not elsewhere classified | A09A, A16AB, B01AD, B06AA, D03BA, M09AB | 16 | 0.07 | |
| T46 | Drugs affecting the circulatory system | C | Cardiovascular system (C) | 447 | 2.00 |
| T50.0 a T50.2 | Diuretics | C03 | 41 | 0.18 | |
| T48 | Drugs acting on smooth and skeletal muscles and on the respiratory system | M, R | Musculoskeletal system (M) and respiratory system (R) | 413 | 1.84 |
| T50.4 | Drugs affecting uric acid metabolism | M04AA, M04AB | 0.06 | ||
| T47 | Agents affecting the gastrointestinal system | A | 525 | 2.34 | |
| T50.5 | Appetite inhibitors | A08A | Digestive system and metabolism (A) and blood and hematopoietic organs | 19 | 0.08 |
| T45.2 | Vitamins | A11 | 58 | 0.26 | |
| T50.3 | Drugs acting on electrolyte, calorie and water balance | A, B | 8 | 0.04 | |
| T45.4 a T45.8 | Drugs acting in blood and coagulation | B | - | - | |
| T45.1 | Antineoplastic and immunosuppressive drugs | L01, L04 | Antineoplastic and immunotherapeutic drugs (L) | 8 | 0.04 |
| T50.6 | Antidotes and chelating agents, not elsewhere classified | V03AB | Several (V) | 5 | 0.02 |
| F19.0 | Multiple drugs and psychoactive agents | - | No correspondence | 93 | 0.42 |
| T96 | Sequelae | - | 6 | 0.03 | |
| T45.9, T50.9, X44, X64, Y14, X85, P93, F55 | Non-specified drugs | - | 38.04 | ||
| Total | 22,395 | 100.00 | |||
Discussion
The most common therapeutic classes which led to hospitalization of children under five due to drug poisoning, funded by SUS in Brazil were: unspecified drugs, antiepileptic/sedative-hypnotics/anti-parkinson drugs, systemic antibiotics, and non-opioid analgesics/antipyretics. This classification varied depending on the country region and the children’s age. The identification of therapeutic classes, according to the ICD-10 and the ATC classification was possible in more than half of the poisonings.
The occurrence of hospitalizations with more than one poisoning diagnosis reflects the children’s exposure to more than one therapeutic class. As most child poisonings occur accidentally,14 poisonings show children have access to different medicines, probably due to inadequate storage or caregivers’ negligence.15
In this study, we observed the participation of up to two toxic agents per hospitalization. However, it is important to highlight that this limit was imposed due to how the IHAs are recorded, since only two diagnoses are allowed to be inserted. Therefore, these poisonings may have been caused by more than two toxic agents; however, it was not possible to record it. The proportion of deaths for one toxic agent (0.48%) was higher than what was observed for two toxic agents (0.26%), showing that the toxic agent´s therapeutic class is likely to have more influence on the evolution of hospitalization than the number of toxic agents involved.
Unspecified drugs were responsible for most poisonings that led to hospitalization, which implies a considerable loss of information. The lack of specification of the involved agents in the records had already been observed in hospitalizations with adverse effects,16 identified from 2008 to 2012 at SIH/SUS, and may be caused by the similarity of signs and symptoms of poisonings by different therapeutic classes, and intensified due to the fact that children do not express themselves clearly. Still, the great number of unspecified records involving SIH/SUS may be inherent to this information system, a consequence of the poor quality of IHA records16 and the lack of familiarity of the health care team with ICD-10.
Antiepileptic, sedative and hypnotics was the second most common therapeutic class, which is similar to a study on a CIATox from Paraná State, conducted in 2010.17 However, hospitalizations associated to this therapeutic class were reduced after 2008. Coincidentally, at that same period, the National Controlled Product Management System (SNGPC) was implemented. Those drugs had already been submitted to specific regulation,18 but the SNGPC, created by a Resolution of the National Health Oversight Agency - RDC No. 27, dated March 30th 2007 -,19 increased surveillance, hampering their commercialization without a medical prescription. This extra control effort is likely to have led to an outcome related to rational use, with a reduction in hospitalizations.
Similarly, there has been a reduction in the number of hospitalizations due to poisoning by systemic antibiotics after 2010. Since that year, with the release of RDC No. 44/201020 and No. 20/2011,21 which obliged pharmacies to withhold the medical prescription in the sale of antibiotics, there was reduction in the number of hospitalizations. The literature relays evidence on the use of regulatory measures to improve the rational use of medicines.22
For all Brazilian regions, unspecified drugs was the main therapeutic class found. There was a difference among regions regarding the second most common cause:systemic antibiotics in the North and the Northeast and antiepileptic, sedative-hypnotics and anti-parkinson drugs in the other regions. We can observe regional differences in the use of medicines, either due to different cultures or prescribing habits, or due to epidemiological differences or health-system voids, which can lead to worsening of communicable diseases. For example, in the North region, there was higher proportion of poisoning by anti-infectives and antiparasitic agents, including antimalarials and drugs for endemic diseases, pointing to prevalent illnesses and outbreaks observed in latter years in the Brazilian Amazon.23
Infants under 28 days of age were poisoned by a smaller variety of therapeutic classes. The literature corroborates this pattern in poisoning profile.24 Younger children are dependent of their caregivers, and drug poisonings occur because of medication errors involving medicines for this age group and not because of accidental ingestion, which is the case for older children. Some preventive actions targeted at caregivers are necessary, such as attention in handling the dose,25-26 and more effective answers from sanitary authorities, such as promotion of pediatric clinical research,27 besides more appropriate and safer formulas, in concentration and dosage form.8
In admissions that evolved to death, the most common therapeutic class was that of unspecified drugs. The correct identification of the therapeutic class involved in poisoning allows the administration of antidotes, support measures or decontamination.28 Difficulties in diagnosing and, consequently, providing the adequate treatment may be associated to greater mortality with use of these drugs.
It was not possible to link some therapeutic classes to a specific ATC category, so they could not be classified. As ICD-10 is less specific than ATC, because it does not focus on medicines but on diagnoses, some important information on drugs poisoning is lost. Poisoning by medicines that act in the central nervous system (category N) and antibiotics and antiparasitic agents (respectively, categories J and P) stood out, which corroborates with the literature.29
The main limitation of this study is the definition of the therapeutic classes (ATC) from the diagnosis recorded by ICD-10 codes at IHA, which focuses on the disease and not on the drug. Some therapeutic classes grouped different medicines, hampering in-depth data analysis. The use of ICD-10 can lead to dangerous generalization and not clearly show the sanitary risk of certain medicines for more vulnerable age groups. Thus, the analysis based on ATC using the ICD-10 classification was an effort to bring drug therapy issues forth, allowing a better identification of medicines involved in poisoning.
There could be a possible assessment bias, since the diagnosis recorded at IHA may not reflect the clinical picture of the inpatients. However, this limitation is inherent to studies involving health information systems. It is possible that the number of hospitalizations due to drug poisoning in Brazil between 2003 and 2012 is higher, considering that these hospitalizations may have occurred in health facilities not linked to SUS. Despite this fact, we did not find other recorded researches in Brazil with the same approach, for comparison purposes.
The high number of diagnoses involving undetermined therapeutic classes stood out. This may be related to hospitalizations in which drug poisoning was not confirmed, involving a clinical hypothesis, uncertainty about the therapeutic class involved or difficulties in classifying the clinical picture with any of the ICD-10 codes.
To overcome this scenario, in cases of unspecific diagnosis on poisoning at IHA, we would recommend the creation of a field in the system to allow the insertion of information on signs and symptoms and clinical hypothesis regarding the medicine involved. This information would complement and facilitate the monitoring of the most common therapeutic classes involved in poisoning.
Besides these measures, it is highly important to restrict children’s access to medicines, by promoting a domestic safety culture among parents and guardians. In this sense, it is also necessary to discuss the adoption of special packaging in medicines to protect children, in order to avoid or reduce the occurrence of poisoning.30
If education measures are important to increase domestic safety, regulation on rational use is essential do reduce drug poisoning. Furthermore, the use of the ICD-10 classification as a proxy to determine the ATC classification may be a path for other studies on poisoning. By using a classification related to drug therapy, these studies may offer managers of the Brazilian National Health System new evidence and more reliable data on therapeutic classes involved in poisoning.
In conclusion, we verified that the higher proportion of drug poisoning, as well as deaths caused by it, was attributed to unspecified therapeutic classes, which limits the data analysis. Notwithstanding, among drug poisoning with defined therapeutic classes, medicines acting in the central nervous system were the most common, followed by non-opioid analgesics/antipyretics, which are frequently used in the under-five-year age group.
REFERENCES
1. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Gerência de Farmacovigilância. Guias de farmacovigilância para detentores de registro [Internet]. Brasília: Ministério da Saúde, 2010 [citado 2017 ago 14]. 156 p. Disponível em: Disponível em: http://portal.anvisa.gov.br/documents/33868/2894051/Guias+de+Farmacovigil%C3%A2ncia+para+Detentores+de+Registro+de+Medicamentos+-+documento+completo/f3fc06a5-97e6-4bbc-848d-750bcefb99e0?version=1.2 [ Links ]
2. Batchelor HK, Marriott JF. Formulations for children: problems and solutions. Br J Clin Pharmacol. 2015 Mar;79(3):405-18. [ Links ]
3. Bond GR; Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012 Feb;160(2):265-70. [ Links ]
4. Moraes CG, Mengue SS, Tavares NUL, Pizzol TSD. Utilização de medicamentos entre crianças de zero a seis anos: um estudo de base populacional. Ciênc Saúde Coletiva. 2013 jan-dez;18(12):3585-93. [ Links ]
5. Telles Filho PCP, Pereira Junior AC. Automedicação em crianças de zero a cinco anos: fármacos administrados, conhecimentos, motivos e justificativas. Esc Anna Nery. 2013 abr-jun;17(2):291-7. [ Links ]
6. Brasil. Ministério da Saúde. Portaria nº 1.678, de 02 de outubro de 2015. Institui os Centros de Informação e Assistência Toxicológica (CIATox) como estabelecimentos de saúde integrantes da linha de cuidado ao trauma, da Rede de Atenção às Urgências e Emergências no âmbito do Sistema Único de Saúde - SUS. Diário Oficial da União da República Federativa do Brasil, Brasília (DF), 2015 out 06; Seção 1:55. [ Links ]
7. Ministério da Saúde (BR). Fundação Oswaldo Cruz. Sistema Nacional de Informações Toxico-Farmacológicas. Dados de intoxicação [Internet]. Rio de Janeiro; 2016 [citado 2016 dez 28]. Disponível em: Disponível em: http://sinitox.icict.fiocruz.br/dados-nacionais [ Links ]
8. Takahama CH, Turini CA, Girotto E. Perfil das exposições a medicamentos por mulheres em idade reprodutiva atendidas por um Centro de Informações Toxicológicas. Ciênc Saúde Coletiva. 2014 abr;19(4):1191-99. [ Links ]
9. Domingos SM, Borghesan NBA, Merino MFGL, Higarashi IH. Internações por intoxicação de crianças de zero a 14 anos em hospital de ensino no Sul do Brasil, 2006-2011. Epidemiol Serv Saúde. 2016 jun;25(2):343-50. [ Links ]
10. Junqueira RMP, Duarte EC. Internações hospitalares por causas sensíveis à atenção primária no Distrito Federal, 2008. Rev Saúde Pública. 2012;46(5):761-8. [ Links ]
11. Andrade SSCA, Jorge MHPD. Internações hospitalares por lesões decorrentes de acidentes de transporte terrestre no Brasil, 2013: permanência e gastos. Epidemiol Serv Saúde. 2017 jan;26(1):31-8. [ Links ]
12. Lessa MA, Bochner R. Análise das internações hospitalares de crianças menores de um ano relacionados a intoxicações e eventos adversos de medicamentos no Brasil. Rev Bras Epidemiol. 2008 dez;11(4):660-74. [ Links ]
13. Rozenfeld S. Agravos provocados por medicamentos em hospitais do Estado do Rio de Janeiro, Brasil. Rev Saúde Pública . 2007 fev;41(1):108-15 [ Links ]
14. Oliveira FFS, Suchara EA. Perfil epidemiológico das intoxicações exógenas em crianças e adolescentes em município do Mato Grosso. Rev Paul Pediatria. 2014 dez;32(4):299-305. [ Links ]
15. Tavares EO, Buriola AA, Santos JAT, Ballani TSL, Oliveira MLF. Fatores associados à intoxicação infantil. Esc Anna Nery. 2013 jan-mar;17(1):31-7. [ Links ]
16. Martins ACM. Eventos adversos a medicamentos: bancos de dados administrativos de pacientes hospitalizados e registro de óbito como fonte de informação [tese]. Rio de Janeiro (RJ): Escola Nacional de Saúde Pública; 2015. [ Links ]
17. Hahn RC, Labegalini MPC, Oliveira MLF. Características de intoxicações agudas em crianças: estudo em um Centro de Assistência Toxicológica. Braz J Surg Clin Res. 2013 set-nov;4(1):18-22. [ Links ]
18. Brasil. Ministério da Saúde. Portaria nº 344, de 12 de maio de 1998. Aprova o regulamento técnico sobre substâncias e medicamentos sujeitos a controle especial. Diário Oficial da União da República Federativa do Brasil, Brasília (DF), 1998 maio 15; Seção 1:3. [ Links ]
19. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC nº 27, de 30 de março de 2007. Dispõe sobre o Sistema Nacional de Gerenciamento de Produtos Controlados - SNGPC, estabelece a implantação do módulo para drogarias e farmácias e dá outras providências. Diário Oficial da União da República Federativa do Brasil, Brasília (DF), 2007 abr 02; Seção 1:62. [ Links ]
20. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC nº 44, de 26 de outubro de 2010. Dispõe sobre o controle de medicamentos à base de substâncias classificadas como antimicrobianos, de uso sob prescrição médica, isoladas ou em associação e dá outras providências. Diário Oficial da União da República Federativa do Brasil, Brasília (DF), 2010 out 28; Seção 1:76. [ Links ]
21. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC nº 20, de 5 de maio de 2011. Dispõe sobre o controle de medicamentos à base de substâncias classificadas como antimicrobianos, de uso sob prescrição médica, isoladas ou em associação e dá outras providências. Diário Oficial da União da República Federativa do Brasil, Brasília (DF), 2011 maio 09; Seção 1:39. [ Links ]
22. Mueller T, Östergren PO. The correlation between regulatory conditions and antibiotic consumption within the WHO European Region. Health Policy. 2016 Aug;120(8):882-9. [ Links ]
23. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Saúde Brasil 2014: uma análise da situação de saúde e causas externas [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2017 ago 14]. 464 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/saude_brasil_2014_analise_situacao.pdf [ Links ]
24. Mendonça DR, Menezes MS, Matos MAA, Rebouças DS, Conceição Filho JN, Assis RS, et al. Acute poisoning in children in Bahia, Brazil. Glob Pediatr Health. 2016 Feb;3:1-7. [ Links ]
25. Richey RH, Shah UU, Peak M, Craig JV, Ford JL, Barker CE, et al. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013 May;13:81-8. [ Links ]
26. Parand A, Garfield S, Vincent C, Franklin BD. Carers’ medication administration errors in the domiciliary setting: a systematic review. PLoS One. 2016 Dec; 11(12):e0167204. [ Links ]
27. Turner MA, Catapano M, Hirschfeld S, Giaquinto C. Paediatric drug development: the impact of the evolving regulations. Adv Drug Deliv Rev. 2014 Jun;73:2-13. [ Links ]
28. Waring WS. The acute management of poisoning. Medicine. 2017 Feb;45(2):104-109. [ Links ]
29. Oliveira DH, Suchara EA. Intoxicações medicamentosas em hospital público de Barra do Garças-MT, no período de 2006 a 2009. Rev Ciênc Méd Biol. 2014 jan-abr;13(1):55-9. [ Links ]
30. Malhotra S, Arora RK, Singh B, Gakhar U, Tonk R. Child resistant packaging: a prime concern for packaging of medicinal products. Int J Pharm Sci Ver Res. 2013 Sep-Oct;22(2):79-88. [ Links ]
*Article based on the Master’s thesis, entitled “Hospitalizations due to drug poisoning in children under five years old in Brazil”, presented by the author, Marta da Cunha Lobo Souto Maior, to the Post-graduation Program in Public Health of the National School of Public Health Sérgio Arouca, in 2015.
Received: May 15, 2017; Accepted: July 13, 2017











 texto en
texto en 


