Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.27 no.1 Brasília mar. 2018 Epub 11-Ene-2018
http://dx.doi.org/10.5123/s1679-49742018000100004
ORIGINAL ARTICLE
Assessment of the production of cervical cancer care procedures in the Brazilian National Health System in 2015*
1Instituto Nacional de Câncer José Alencar Gomes da Silva, Divisão de Detecção Precoce e Apoio à Organização de Rede, Rio de Janeiro, RJ, Brasil
2Universidade do Estado do Rio de Janeiro, Instituto de Medicina Social, Rio de Janeiro, RJ, Brasil
Objective:
to assess the procedures production of screening, diagnostic investigation and precursor lesions treatment for cervical cancer in the Brazilian National Health System (SUS).
Methods:
normative evaluation with calculation of estimates of need based on national screening guidelines in two scenarios: organized (1) and opportunistic (2) screening; data were obtained from the SUS Ambulatory Care Information System and the Cervical Cancer Information System.
Results:
considering the scenario 1, the production of cytopathologic exams (-46,9%) and biopsies (-44.9%) was below the necessary in Brazil, whereas colposcopy (61.3%) and precursor lesions treatments (37.4%) were above; in scenario 2, biopsies were below the necessary (-48.5%) whilst colposcopy (193.9%) and precursor lesions treatments (28.4%) were above.
Conclusion:
there were deficits in cytopathologic exams and biopsies and excess of colposcopies and treatment of precursor lesions in Brazil.
Keywords: Mass Screening; Uterine Cervical Neoplasms; Health Planning; Health Information Systems; Evaluation Studies
Introduction
Although the incidence of cervical cancer has been decreasing worldwide, there are still significant differences between countries; approximately 85% of the cases are believed to occur in developing countries.1,2 The main reasons for these differences are the implementation of screening programs, the access to early diagnosis, and the timely treatment.2,3
In Brazil, despite its ongoing status as a major disease - with an estimate of 16,000 new cases in 2017 -,4 cervical cancer mortality has notably decreased, except for some municipalities in the North and Northeast regions.5,6 There are significant differences in the incidence rate of this type of cancer among national macroregions, ranging from 23.9 per 100,000 women in the North to 11.3 per 100,000 women in the Southeast.4 The identification of regional differences in the organization and in the supply of health care services compared to the demand is essential to understand the inequality in the access to these measures and the quality of the assistance provided to cancer patients.
The National Policy of Cancer Prevention and Control emphasizes the importance of comprehensive health care.7 Its guidelines highlight the need for planning, monitoring, and evaluating cancer control procedures and services using avaiable health care and epidemiological data.7
The higher coverage for preventive testing and the treatment of 100% of women with precursor lesions until 2022 are among the goals signed by Brazil in the Strategic Action Plan for Tackling Chronic Non-communicable Diseases.8 So as to accomplish this strategy of early detection, it is essential to ensure coverage and quality of cytopathologic testing, as well as provide every woman with altered results the access to diagnostic investigation and treatment procedures, when recommended. Thus, it is vital that the production of procedures related to these actions are monitored and assessed.7,9,10
The purpose of the present study was to assess the production of cervical cancer screening procedures, diagnostic investigation, and precursor lesions treatment in the Brazilian National Health System (SUS).
Methods
This is a descriptive study based on secondary data recorded in SUS health information systems.
The Brazilian Ministry of Health recommends the conduction of cytopathologic tests as a cervical cancer screening method for women aged 25 to 64.10 The choice of the age group used in the present study followed such recommendation. The year 2015 was chosen for its recent and complete databases, available at the moment of data collection.
We used databases - with no individual identification - from the SUS Ambulatory Care Information System (SIA/SUS) and from the Cervical Cancer Information System (SISCOLO), besides the data tabulator from the National Regulatory Agency for Private Health Insurance and Plans (ANS), all of which are available at the website of SUS IT Department (Datasus).11 Data from the Cancer Information System (SISCAN) were extracted from reports by the Brazilian National Cancer Institute (INCA). The data were accessed in February 2017. Data referring to procedures were taken from SIA/SUS,11 a system that does not record cytopathologic tests results, which is why such information was obtained from SISCOLO and from reports that contained data from SISCAN.
All screening tests, diagnostic investigation, and precursor lesions treatment conducted in women aged 25 to 64 that were on the records of SUS information systems for 2015 were included in this study.
We obtained the following indicators: (i) supplemental health coverage (proportion of the population covered per region of residence); and (ii) number of procedures performed by SUS (cervical cytopathologic test, anatomopathological test of cervical biopsy material, large loop excision of the transformation zone (LEETZ), and conization).
The procedures used are described in the Table of SUS Procedures, Drugs, Orthotic Devices, Prostheses, and Special Materials,10 with the following codes:
a) Screening exams - cervicovaginal/vaginal flora cytopathologic exam (020301001-9); cervicovaginal/vaginal flora screening exam (020301008-6)-;
b) Diagnostic investigation exams - colposcopy (021104002-9) and anatomopathological cervical exam, biopsy (0203020081) -; and
c) Precursor lesion treatment - large loop excision of the transformation zone (LEETZ) (0409060089) and conization (0409060038).
The Brazilian female population aged 25 to 64, divided by macroregion of residence, estimated for 2015 by the Brazilian Institute of Geography and Statistics (IBGE), was used as reference.11 The supplemental health coverage used to calculate the target population covered by health insurance plans was provided by the National Regulatory Agency for Private Health Insurance and Plans (ANS) regarding the female population aged 20 to 69, according to region of residence, in 2015.11 This age group was chosen for being the closest to the target age group for screening tests.
To estimate female users of SUS, we subtracted the percentage of women covered by health insurance plans from the total population in that age group (Table 1).
Table 1 - Female population used as reference in the study, female population covered by supplemental health, and users of the Brazilian National Health System, according to region of residence, Brazil, 2015
| Region | Female population aged 25-64 years (N) a | Supplemental health coverage (%)b | Female population user of SUS (N) |
|---|---|---|---|
| North | 3,984,897 | 14.2 | 3,419,042 |
| Northeast | 14,448,717 | 15.1 | 12,266,961 |
| Midwest | 4,133,744 | 25.9 | 3,063,104 |
| Southeast | 23,681,008 | 41.8 | 13,782,347 |
| South | 7,981,068 | 29.3 | 5,642,615 |
| Brazil | 54,229,434 | 29.7 | 38,123,292 |
a) Female population estimated for 2015 by the Brazilian Institute of Geography and Statistics.
b) Supplemental health coverage for women aged 20 to 69 years in 2015.
Figure 1 presents the source of all data collected, as well as the codes applied to the selected items.
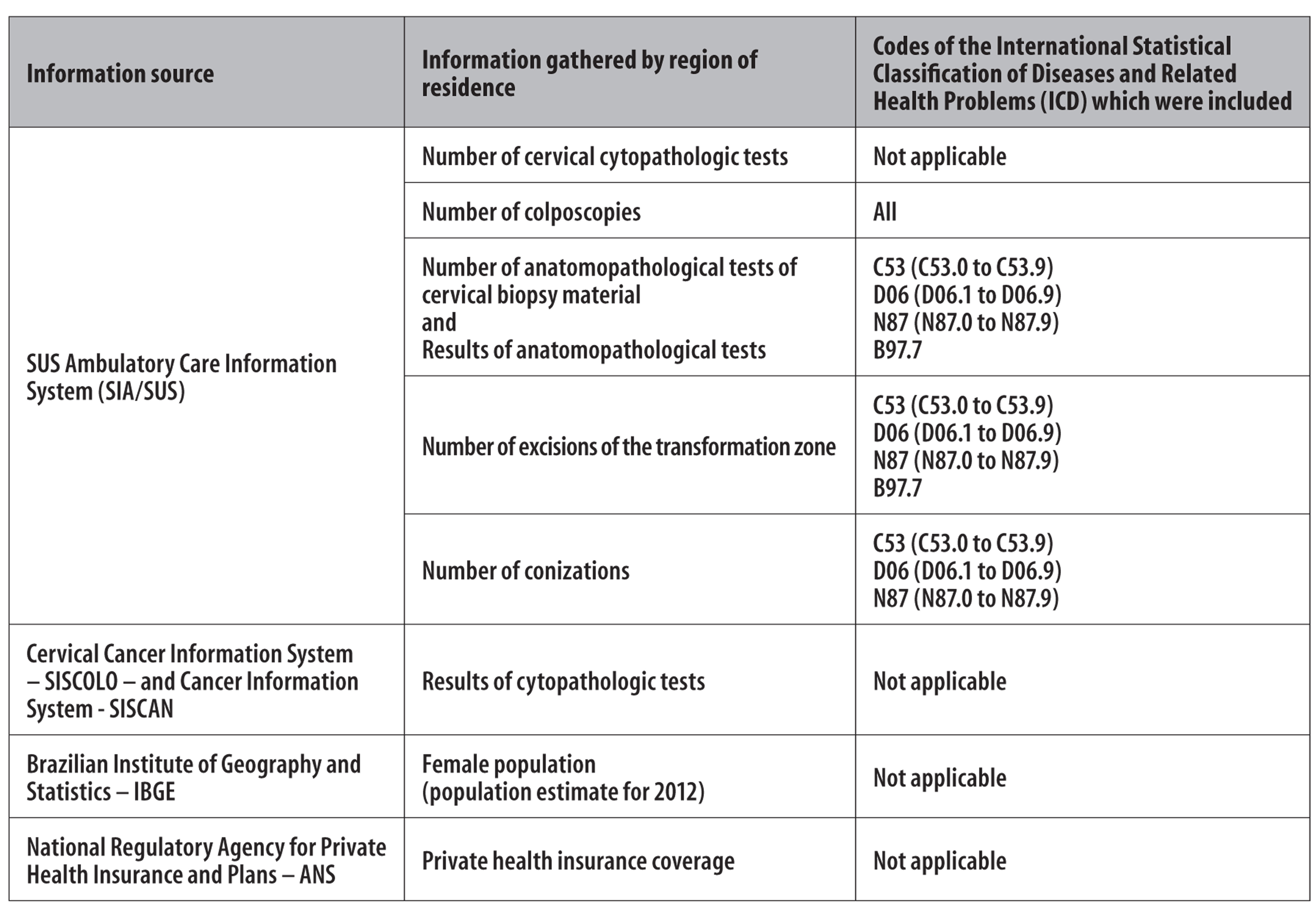
Figure 1 - Sources, information, and the respective codes used in the research, referring to women aged 25 to 64 years
We calculated the annual needs indicators considering two different scenarios: one, hypothetical, in which the screening would be organized, with 100% coverage for the SUS user target population; and the second, a realistic scenario for the country, in which screening is opportunistic and demands actions for continuity of care. Both scenarios can be described as follows:
Scenario 1 - Considers the screening test needs so as to (i) achieve 100% screening coverage for the target population that uses SUS health care services (population not covered by supplemental health), and (ii) meet the demand for diagnosis confirmation and treatment of precursor lesions.
Scenario 2 - Considers the needs of diagnostic investigation exams and treatment of precursor lesions from the screening exams effectively conducted by SUS in a scenario of opportunistic screening.
The criteria adopted for the calculation of procedure needs considered the (i) conducts proposed by the Ministry of Health - defined in the national guidelines for cervical cancer screening in Brazil -, recommended for diagnostic investigation that starts from alterations in the screening test,10 the (ii) results of screening tests and diagnostic investigation exams recorded in SUS information systems; (iii) data from the national literature; and (iv) data from the international literature, when absent from the information systems or national literature.
Calculation of indicators in scenario 1
The need for the cytopathologic tests was measured by adding:
a) 1/3 of female SUS users, aged 25 to 64, given the triennial periodicity of the screening tests recommended by the Ministry of Health;10
b) the expected number of repetition exams, considering the proportion of results that urged for immediate repetition (unsatisfactory and reject specimen), or for a 6-month repetition (atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesion);
c) the expected number of follow-up exams for cases with abnormal test results (atypical squamous cells can not exclude high-grade lesion and atypical glandular cells) but normal colposcopy; since there is no information system that records results of colposcopy, we used the proportions of normal colposcopies for each cytological diagnosis found in a study conducted in a reference center for diagnostic investigation in a Brazilian municipality over the course of a year (21.3% for atypical squamous cells can not exclude high-grade lesion, and 58.3% for atypical glandular cells);13 and
d) the expected number of conizations and LEETZ, considering the recommended cytological follow-up of women submitted to these procedures.
The need for colposcopies was measured by adding:
a) the number of altered cytopathologic exams that require an immediate colposcopy (atypical squamous cells can not exclude high-grade lesion; atypical glandular cells, high grade squamous intraepithelial lesion, squamous cell carcinoma, and adenocarcinoma); and
b) the number of colposcopies for the evaluation of women who showed the same cytological abnormalities in the repeated test after the first cytological diagnosis of atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesion. This information is not available in information systems and we could not find national studies that offered that criterion, so we chose to use the value found in a study conducted in 2013, in Norway, where 27.9% of women with this diagnosis had an altered result after a repeated cytological test.14
To calculate the need for biopsies, we considered the number of altered colposcopies, which require a follow-up in diagnostic investigation. Since there is no record of colposcopy results in the information systems, we applied the ratio of altered colposcopies according to first diagnosis, found in the aforementioned study, conducted in a reference center for diagnostic investigation over the course of a year (43% for low grade squamous intraepithelial lesion and atypical squamous cells of undetermined significance; 59% for high grade squamous intraepithelial lesion and atypical squamous cells can not exclude high-grade lesion; and 16.7% for alterations in glandular cells or adenocarcinoma).13
The need for precursor lesion treatment (LEETZ and conization) was measured considering the recommendations of an LEETZ or conizations for biopsies with results that showed moderate or severe cervical intraepithelial neoplasia (CIN2/3), or carcinoma in situ. Therefore, we applied the ratio of these results to the estimated number of biopsies.
Calculation of indicators in scenario 2
In scenario 2, we estimated the need for diagnostic investigation and precursor lesions treatment procedures in women that were realistically screened by SUS. Thus, the need for cytopathologic tests was not calculated.
The criteria used were the same as in scenario 1. Nonetheless, the basis for the calculation was not formed by women in the target age group for screening, but by the number of cytopathologic tests recorded in SIA/SUS in 2015.
In order to estimate the percentage of deficit or excess of cervical cancer screening, diagnostic investigation, and precursor lesions treatment procedures, we calculated the difference between the number of procedures conducted and the demand for those, divided by the number of necessary procedures, all of that multiplied by 100. The results were expressed in absolute and relative frequency.
The study project was exempted from ethical-scientific analysis by the Ethics Research Committee of the Brazilian National Cancer Institute - José Alencar Gomes da Silva (INCA) on May 11th, 2017, because it exclusively used secondary data, without any individual identification, in accordance with the recommendations by the Resolution No.466 of the National Health Council (CNS), dated December 12th, 2012.
Results
In 2015, it was estimated that over 54 million women were in the target age group for cervical cancer screening (25 to 64 years old), and over 38 million of them would undergo the testing at SUS (Table 1).
Considering scenario 1, the production of screening exams (-46.9%) and biopsies (-44.9%) was below the estimated minimum required to meet the demand of the population in all Brazilian macroregions. The productions of colposcopies, excisions of the transformation zone, and conizations were above the required amount, except in the North and Midwest regions (Table 2).
Table 2 - Comparison between the estimated annual needs and the actual supply of procedures in a scenario of organized screening covering 100% of women aged 25 to 64, users of the Brazilian National Health System, according to region of residence, Brazil, 2015
| Region | Cytopathologic tests | Colposcopies | Biopsies | ETZ a and conizations | ||||
|---|---|---|---|---|---|---|---|---|
| Needed | Conducted (difference in %) | Needed | Conducted (difference in %) | Needed | Conducted (difference in %) | Needed | Conducted (difference in %) | |
| North | 1,193,741 | 403,059 (-66.2) | 18,398 | 9,436 (-48.7) | 8,654 | 1,631 (-81.2) | 2,602 | 1,835 (-29.5) |
| Northeast | 4,240,969 | 1,717,084 (-59.5) | 40,758 | 96,807 (137.5) | 19,078 | 7,415 (-61.1) | 3,372 | 4,009 (18.9) |
| Midwest | 1,059,187 | 462,209 (-56.4) | 15,692 | 8,814 (-43.8) | 7,520 | 2,777 (-63.1) | 2,049 | 997 (-51.3) |
| Southeast | 4,736,365 | 3,096,968 (-34.6) | 61,679 | 112,641 (82.6) | 27,986 | 22,893 (-18.2) | 4,319 | 8,011 (85.5) |
| South | 1,928,176 | 1,295,567 (-32.8) | 20,439 | 24,864 (21.7) | 9,572 | 5,317 (-44.5) | 2,672 | 4,262 (59.5) |
| Brazil | 13,125,078 | 6,974,887 (-46.9) | 156,568 | 252,562 (61.3) | 72,670 | 40,033 (-44.9) | 13,912 | 19,114 (37.4) |
a) ETZ: excision of the transformation zone.
In a scenario closer to reality, in which screening is opportunistic and there is not enough coverage for the entire target population, we observed an excess of colposcopies conducted in all Brazilian macroregions, with a production that was three-fold above the necessary. In contrast, biopsy was insufficient (-48.5%), with an alarming deficit of 70% in the North. With regard to treatment procedures of precursor lesions, only the Midwest presented deficit (-40.2%) (Table 3).
Table 3 - Comparison between the estimated annual needs and the actual supply of follow-up procedures for women aged 25 to 64 screened for cervical cancer and users of the Brazilian National Health System, according to region of residence, in a scenario of opportunistic screening, Brazil, 2015
| Region | Cytopathologic tests | Colposcopies | Biopsies | ETZ a and conization | |||
|---|---|---|---|---|---|---|---|
| Conducted | Needed | Conducted (difference in %) | Needed | Conducted | Needed | Conducted (difference in %) | |
| North | 403,059 | 6,507 | 9,436 (45.0) | 5,429 | 1,631 (-70.0) | 1,633 | 1,835 (12.4) |
| Northeast | 1,717,084 | 17,115 | 96,807 (465.6) | 15,179 | 7,415 (-51.1) | 2,683 | 4,009 (49.4) |
| Midwest | 462,209 | 7,104 | 8,814 (24.1) | 6,121 | 2,777 (-54.6) | 1,668 | 997 (-40.2) |
| Southeast | 3,096,968 | 41,579 | 112,641 (170.9) | 39,480 | 22,893 (-42.0) | 6,092 | 8,011 (31.5) |
| South | 1,295,567 | 14,079 | 24,864 (76.6) | 12,557 | 5,317 (-57.7) | 3,505 | 4,262 (21.6) |
| Brazil | 6,974,887 | 85,935 | 252,562 (193.9) | 77,786 | 40,033 (-48.5) | 14,892 | 19,114 (28.4) |
a) ETZ: excision of the transformation zone.
Discussion
The production of cytopathologic tests was only half of the estimated necessary to screen 100% of SUS target population in 2015. The number of biopsies was below the minimum, whilst colposcopies and precursor lesion treatments were above, both in the organized screening scenario and in the scenario that would meet the demand for continuity of care for women who were actually screened.
The main limitation of the present study was the use of secondary data from information systems that record procedures for billing purpose. Due to the lack of some specific information in the systems we had to use data obtained in the literature for the estimates adopted, which constituted another limitation for this study.
The use of private health insurance coverage for a broader age group than the screening target to measure SUS user population was also another limitation, although it may not have altered the expected results since the age groups are not too far apart. Data from the 2013 National Health Survey (PNS), estimated that supplemental health coverage was quite close between both age groups: 30.9% (95%CI 30.1%;31.9%) for women between 20 and 69; and 31.9% (95%CI 30.9%;32.8%) for those aged 25 to 64.11
The deficit in the production of cytopathologic tests in Brazil has been discussed in other local and national studies.15-17 According to the 2013 PNS, self-reported coverage of Pap smear for women aged 25-69 reached 78.8%.18 However, the odds of women undergoing a Pap smear was three-fold higher among those covered by private health insurance in comparison to those who depended exclusively on SUS.18
According to reports by the Brazilian National Institute of Cancer, in 2013 only 11% of exams were conducted within the recommended periodicity.19 It is possible that the deficit found in the present study is an underestimate since many of the exams conducted could be repeated tests of the same women.
The colposcopy was the procedure with the highest excess in production for both analyzed scenarios, which could be a result of courses of action that are not in line with what is recommended by the Ministry of Health. Studies that assessed the follow-up of women with altered screening results found that there was an excess of referrals to different levels of attention for women whose cytological diagnosis would only warrant a recommendation for a 6-month repetition of the test.20,21
This study found biopsy to be the procedure with the highest deficit, in all macroregions. In a cross-sectional study conducted in Mato Grosso do Sul State, the access to high-complexity exams was reported as a major shortcoming in SUS.20 The need for biopsies could be overestimated in that study, considering that in some cases, when the colposcopy alteration is consistent with the cytological results of high grade squamous intraepitheial lesion, it is possible to conduct excisional treatment through the ‘see-and-treat’ method, rendering biopsy unnecessary.22,23 Nevertheless, even if all cases of high-grade lesion were to be treated with such method, the production of biopsies would still be below the bare minimum.
The production of procedures for precursor lesion treatment (LEETZ and conization) was above the estimated needs for Brazil. Here are some possible reasons for this finding: (i) use of the ‘see-and-treat’ method (not considered in this study); (ii) such procedures conducted out of line with the Ministry recommendations; (iii) flawed recording of these procedures; and (iv) use of these services by women who have private health insurance plans that cover only outpatient procedures, and who seek more complex procedures in the services offered by SUS.24,25 Another point to consider is the fact that conization might be recommended in the treatment of early stage invasive cancer for women at reproductive age, so as to preserve their fertility.26 However, this hypothesis, in all likelihood, does not explain the difference we found, considering that this is not common.
The organized screening of cervical cancer is known to possibly reduce the incidence and mortality from this disease.27 The opportunistic nature of screening in Brazil, added to flawed information systems records and to low adherence to national protocols all make it difficult to monitor, assess, and organize actions of early detection of the disease.15,28
The highest deficit in procedures was found in the North, Midwest, and Northeast regions, where the highest incidence and mortality rates of cervical cancer are found,4 suggesting that difficulties in the organization of the health care network have major repercussions in the effective control of the disease.
In conclusion, the deficit in screening exams and biopsies could represent a significant obstacle in the cervical cancer system of care. Such deficit should be assessed and addressed, with the priority being to determine the reason behind the excess of some procedures - especially colposcopy - so that better screening results of this kind of cancer are possible.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar;136(5):E359-86. [ Links ]
2. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012 Aug;13(8):790-801. [ Links ]
3. Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013 Oct;49(15):3262-73. [ Links ]
4. Ministério da Saúde (BR). Instituto Nacional de Câncer José de Alencar Gomes da Silva. Estimativa 2016: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA; 2015 [citado 2017 14 set]. 122 p. Disponível em: Disponível em: http://www.inca.gov.br/estimativa/2016/estimativa-2016-v11.pdf [ Links ]
5. Girianelli VR, Gamarra CJ, Azevedo e Silva G. Disparities in cervical and breast cancer mortality in Brazil. Rev Saúde Pública. 2014 Jun;48(3):459-67. [ Links ]
6. Gonzaga CMR, Freitas-Junior R, Barbaresco AA, Martins E, Bernardes BT, Resende APM. Cervical cancer mortality trends in Brazil: 1980-2009. Cad Saúde Pública. 2013 Mar;29(3):599-608. [ Links ]
7. Brasil. Ministério da Saúde. Portaria GM no 874, de 16 de maio de 2013. Institui a Política Nacional para a Prevenção e Controle do Câncer na Rede de Atenção à Saúde das Pessoas com Doenças Crônicas no âmbito do Sistema Único de Saúde (SUS). Diário Oficial da República Federativa do Brasil, Brasília (DF), 2013 maio 29; Seção 1:29. [ Links ]
8. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Análise de Situação de Saúde. Plano de ações estratégicas para o enfrentamento das doenças crônicas não transmissíveis (DCNT) no Brasil, 2011-2022 [Internet]. Brasília: Ministério da Saúde; 2011 [citado 2017 set 14]. 160 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/plano_acoes_enfrent_dcnt_2011.pdf [ Links ]
9. Malta DC, Silva Júnior JB. Strategic Action Plan to Combat Non-Communicable Diseases in Brazil after three years of implementation, 2011-2013. Epidemiol Serv Saúde. 2014 Jul-Sep;23(3):389-95. [ Links ]
10. Ministério da Saúde (BR). Instituto Nacional de Câncer José Alencar Gomes da Silva. Diretrizes brasileiras para o rastreamento do câncer do colo do útero [Internet]. 2. ed. rev. atual. Rio de Janeiro: INCA; 2016 [citado 2017 set 14]. 114 p. Disponível em: Disponível em: http://colposcopia.org.br/files/consensos/diretrizesparaorastreamentodocancerdocolodoutero2016corrigido-1448538996.pdf [ Links ]
11. Ministério da Saúde (BR). Departamento de Informática do SUS. Informações de saúde (TABNET) [Internet]. 2017 [citado 2017 mar 23]. Disponível em: Disponível em: http://datasus.saude.gov.br/informacoes-de-saude/tabnet [ Links ]
12. Brasil. Ministério da Saúde. Portaria GM no 2.848, de 06 de novembro de 2007. Aprova a tabela de procedimentos, medicamentos, órteses, próteses e materiais especiais - OPM do Sistema Único de Saúde. Diário Oficial da República Federativa do Brasil, Brasília (DF), 2007 nov 7; Seção 1:54. [ Links ]
13. Albuquerque ZBP, Manrique EJC, Tavares SBN, Souza ACS, Guimarães JV, Amaral RG. Women with atypical, precursor lesions and invasive cervical cancer: behaviors according to the recommendations of the Ministry of Health. Rev Bras Ginecol Obstet. 2012 Jun;34(6):248-53. [ Links ]
14. Budal EB, Haugland HK, Skar R, Maehle BO, Bjorge T, Vintermyr OK. HPV DNA testing improves CIN2+ risk stratification and detection of CIN2+ in delayed triage of ASCUS and LSIL. A population-based follow-up study from Western Norway. Cancer Med. 2014 Feb;3(1):182-9. [ Links ]
15. Dias MBK, Tomazelli JG, Assis M. Rastreamento do câncer de colo do útero no Brasil: análise de dados do Siscolo no período de 2002 a 2006. Epidemiol Serv Saúde. 2010 jul-set;19(3):293-306. [ Links ]
16. Costa RF, Longatto-Filho A, Pinheiro C, Zeferino LC, Fregnani JH. Historical analysis of the Brazilian cervical cancer screening program from 2006 to 2013: a time for reflection. PLoS One. 2015 Sep;10(9):e0138945. [ Links ]
17. Nascimento GWC, Pereira CCA, Nascimento DIC, Lourenço GC, Machado CJ. Cervical cancer screening coverage in the state of Minas Gerais, Brazil between 2000-2010: a study using data from the Cervical Cancer Information System (SISCOLO). Cad Saúde Colet. 2015 Jul-Sep;23(3):253-60. [ Links ]
18. Theme Filha MM, Leal MD, Oliveira EF, Esteves-Pereira AP, Gama SG. Regional and social inequalities in the performance of Pap test and screening mammography and their correlation with lifestyle: Brazilian national health survey, 2013. Int J Equity Health [Internet]. 2016 Nov [cited 2017 Jun 26];15(1):136. Available in: Available in: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5112710/ [ Links ]
19. Ministério da Saúde (BR). Instituto Nacional de Câncer José de Alencar Gomes da Silva. Informativo detecção precoce. Informativo detecção precoce. Monitoramento das ações de controle dos cânceres do colo do útero e da mama. Boletim [Internet]. 2015 jan-abr [citado 2017 mar 24];6(1);1-8. Disponível em: Disponível em: http://www1.inca.gov.br/inca/Arquivos/comunicacao/deteccao_precoce_12015.pdf [ Links ]
20. Farias ACB, Barbieri AR. Seguimento do câncer de colo de útero: estudo da continuidade da assistência à paciente em uma região de saúde. Esc Anna Nery [Internet]. 2016 out-dez [citado 2017 mar 23];20(4):e20160096. Disponível em: Disponível em: http://www.scielo.br/scielo.php?script=sci_abstract&pid=S1414-81452016000400213&lng=en&nrm=iso&tlng=pt [ Links ]
21. Araújo ES, Barbosa FM, Ázara CZS, Ferreira TXAM, Tavares SBN, Amaral RG. Avaliação do seguimento de mulheres com exames citopatológicos alterados de acordo com as condutas preconizadas pelo Ministério da Saúde do Brasil em Goiânia, Goiás. Rev Bras Cancerol. 2014;60(1):7-13. [ Links ]
22. Monteiro AC, Russomano F, Reis A, Camargo MJ, Fialho SA, Tristão MA, et al. Effectiveness of see-and-treat for approaching pre-invasive lesions of uterine cervix. Rev Saúde Pública. 2009 Oct;43(5):846-50. [ Links ]
23. World Health Organization. WHO guidelines for treatment of cervical intraepithelial neoplasia 2-3 and adenocarcinoma in situ: cryotherapy, large loop excision of the transformation zone, and cold knife conization. [Internet]. Geneva: World Health Organization; 2014 [cited 2017 Jul 7]. 52 p. Available in: Available in: https://www.ncbi.nlm.nih.gov/books/NBK206769 / [ Links ]
24. Santos IS, Ugá MAD, Porto SM. O mix público-privado no Sistema de Saúde Brasileiro: financiamento, oferta e utilização de serviços de saúde. Ciênc Saúde Colet. 2008 out;13(5):1431-40. [ Links ]
25. Ocké-Reis CO. O público e o privado na saúde. Cad Saúde Pública. 2006 dez;22(12):2722-4. [ Links ]
26. He Y, Wu YM, Zhao Q, Wang T, Wang Y, Kong WM, et al. Clinical value of cold knife conization as conservative management in patients with microinvasive cervical squamous cell cancer (stage IA1). Int J Gynecol Cancer. 2014 Sep;24(7):1306-11. [ Links ]
27. Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013 May;2:35. [ Links ]
28. Jorge M, Gotlieb S, Laurenti R. Avaliação dos sistemas de informação em saúde no Brasil. Cad Saúde Colet [Internet]. 2010 jan-mar[citado 2017 jul 31];18(1);7-18. Disponível em: Disponível em: http://www.iesc.ufrj.br/cadernos/images/csc/2010_1/artigos/Modelo%20Livro%20UFRJ%201-a.pdf [ Links ]
Received: July 11, 2017; Accepted: August 21, 2017










 texto en
texto en