Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.27 no.2 Brasília jun. 2018 Epub 07-Jun-2018
http://dx.doi.org/10.5123/s1679-49742018000200011
ORIGINAL ARTICLE
Magnitude and distribution of deaths due to hantavirus in Brazil, 2007-2015*
1Universidade de Brasília, Faculdade de Ciências da Saúde, Programa de Pós-Graduação em Saúde Coletiva, Brasília, DF, Brasil
2 Ministério da Saúde, Secretaria de Vigilância em Saúde, Brasília, DF, Brasil
3Universidade de Brasília, Faculdade de Medicina, Brasília, DF, Brasil
Objective:
to describe the magnitude and temporal and spatial distribution of hantavirus cases and deaths in Brazil between 2007 and 2015.
Results:
1,060 cases and 410 deaths were reported in the period; hantavirus lethality was 39.0%, varying according to month (from 28.6% in November to 50.7% in December), sex (37.4% in males and 42.6% in females), age (higher lethality in the elderly and children) and Brazilian regions (46.2% in the North, 32.9% in the South); most of the individuals who died lived in urban areas (58.3%) and were infected in rural area (70.2%).
Conclusion:
high lethality in certain population groups, months of the year and regions of Brazil point to low clinical suspicion of the disease in groups with low exposure, which may compromise proper case management.
Keywords: Hantavirus Infections; Hantavirus; Lethality; Zoonoses; Descriptive, Epidemiology
Introduction
Hantavirus in Brazil is considered a public health problem1 of great importance, due to its high lethality and high social and economic cost.2
The etiologic agent of the disease is a virus of the Bunyaviridae family. In Brazil, nine variants of hantavirus have been identified: seven of them in the municipalities of Araraquara, Juquitiba, Castelo dos Sonhos, Anajatuba, Laguna Negra, Paranoá and Rio Mamoré associated with hantavirus cardiopulmonary syndrome ; and two variants in the municipalities of Rio Mearim and Jaborá which - at the time this report was concluded - were only detected in rodents.3,4 Transmission among rodents is horizontal, while in humans it occurs through exposure to tiny droplets of infected rodent secretion/excretion present in the air.5,6
The disease occurs in all regions of Brazil; however, the South and Southeast regions have the largest number of registered cases.3 Hantavirus lethality in Brazil is higher in comparison to other South American countries , such as Chile (32%),7 Argentina (12 to 40%)8 and Paraguay (11.3%).9
Clinical manifestations in the initial appearance of infection include fever, asthenia and headache.5 The worst prognoses are presented by individuals with septicemia, dyspnea, need for mechanical ventilation and hemoconcentration.10,11
Hantaviruses lethality is not, however, homogeneous among population groups and few studies describe the magnitude and distribution of mortality in Brazil in recent historical series.10,12
The objective of this study is to describe the magnitude, the temporal and spatial distribution of hantavirus cases and deaths in Brazil between 2007 and 2015.
Methods
This is a descriptive study of hantavirus cases and deaths notified on the Notifiable Diseases Information System (SINAN) between 2007 and 2015 in Brazil.
The SINAN (SINAN Net version) hantavirus database updated as at 20 September 2016 was used for this study.
In this study, a 'confirmed hantavirus case' is defined as a suspected case that is clinically compatible and laboratory confirmed, or a suspected case confirmed by clinical and epidemiological criteria. The ELISA (enzyme-linked immunosorbent assay) serological test is recommended for IgM antibody detection.3 ‘Death due to hantaviruses', in turn, is defined a confirmed hantavirus case that led to death. All cases with onset of symptoms between 1 January 2007 and 31 December 2015 were included in the study.
The studied variables were categorized as:
- sex (male, female);
- age (in years: <1, 1-4, 5-9, 10-14, 15-20, 21-34, 35-49, 50-64 and ≥80);
- area of residence (urban, rural, peri-urban areas, unknown);
- area of infection (urban, rural, peri-urban areas, unknown);
- environment where infection occurred (work, home, leisure, another, unknown);
- Brazilian region in which infection probably occurred (North, Northeast, Southeast, South, Midwest, unknown);
- year of symptoms onset (2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015);
- month of symptoms onset (January, February, March, April, May, June, July, August, September, October, November, December);
- signs and symptoms (fever, dyspnea, respiratory insufficiency, myalgia, headache, cough, hypotension, nausea/vomiting, chest pain, asthenia, abdominal pain, shock, dizziness/vertigo, lumbar pain, diarrhea, renal insufficiency, congestive heart failure, neurological symptom, petechiae and/or hemorrhagic manifestation [yes, no]);
- whether chest radiography was performed (yes, no);
- result of radiography examinations (diffuse pulmonary infiltrate, localized pulmonary infiltrate and/or pleural effusion [yes; no] );
- hospitalization (yes, no);
- use of a mechanical ventilator (yes, no);
- diagnostic criterion (unknown/blank , clinical and laboratory and/or clinical and epidemiological);
- autochthonous infection (infection occurring in the municipality where individual resides [yes, no] or undetermined) and;
- risk situations occurring up to 60 days before the onset of symptoms (exposure and cleaning of enclosed places, milling, contact with rodents, sleeping in tent, fishing/hunting, transportation of grains and/or military training [yes, no]);
Brazilian regional analysis of hantavirus deaths was based on probable place of infection (PPI). In situations in which the 'PPI region' was unknown, deaths were analyzed separately.
Proportions were calculated taking the absolute number over the frequency of cases or deaths of each variable used. Distribution by month was performed taking the total of all cases and deaths per month for the entire study period. In order to calculate the proportion of hospitalizations, thenumerator was taken to be number of hospitalized cases who died, whilst the denominator was the total number of deaths. In order to calculate the lethality rate, the numerator was the total number of deaths and the denominator was the total number of cases.
TabWin 41 version 4.1.2, updated on 15 October 2015, and Microsoft Excel 2013® were used to tabulate the data.
As the study project used freely available secondary data not containing any information that could identify individuals, it was exempted from registration and evaluation by the Ethics Research Committees/National Committee for Ethics in Research (CEP/CONEP) system, in accordance with National Health Council Resolution No. 510, dated 7 April 2016, in particular Article 1 thereof.
Results
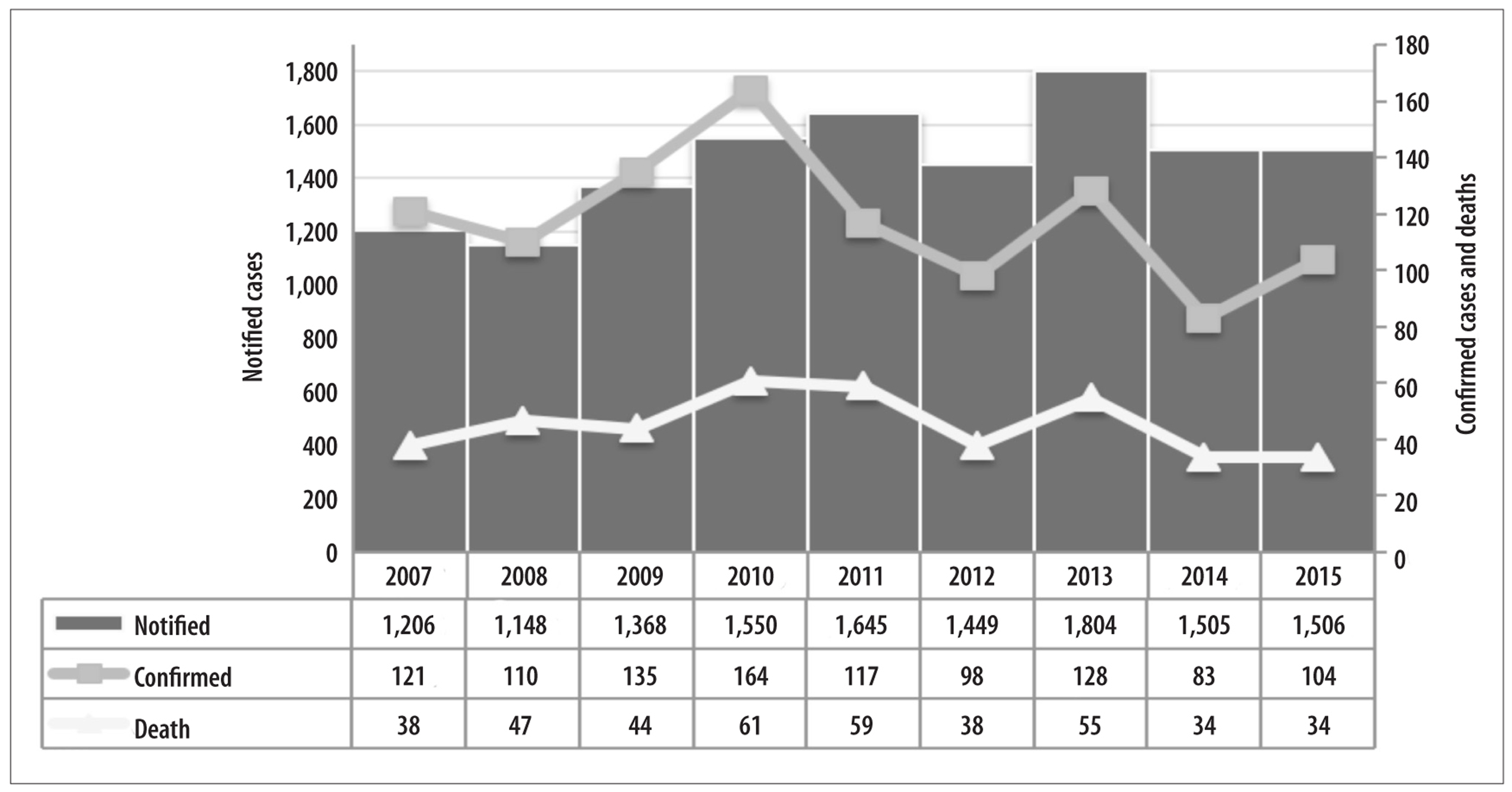
Between 2007 and 2015, there were 13,181 reported cases of hantavirus in Brazil, of which 8% (N=1,060) were confirmed and 3.1% (N=410) led to death. We found an average of 1,465 suspected cases reported per year, with the lowest number of cases reported in 2008 (N=1,148) and the highest number in 2013 (N=1,804). The average number of confirmed cases per year was 118. The proportion of confirmed cases ranged from 10.6% (N=164) in 2010 to 5.5% (N=83) in 2014. In 2013 there were 1,804 suspected cases, of which 128 were confirmed and 55 died (Figure 1).
There was a variation of between 34 and 61 deaths per year, with a mortality rate of 38.7% in the study period as a whole and an annual rate of 31% on average. Highest mortality occurred in 2011 (50.4%) (Figure1).
The Brazilian regions with the largest number of confirmed hantavirus cases were the South (n=307) and Midwest (n=304). The Midwest and Southeast concentrated the greatest numbers of deaths (n=122 and n=112, respectively). The regions with the highest mortality rates were the Northeast (50.0%) and North (46.2%) (Figure 2).
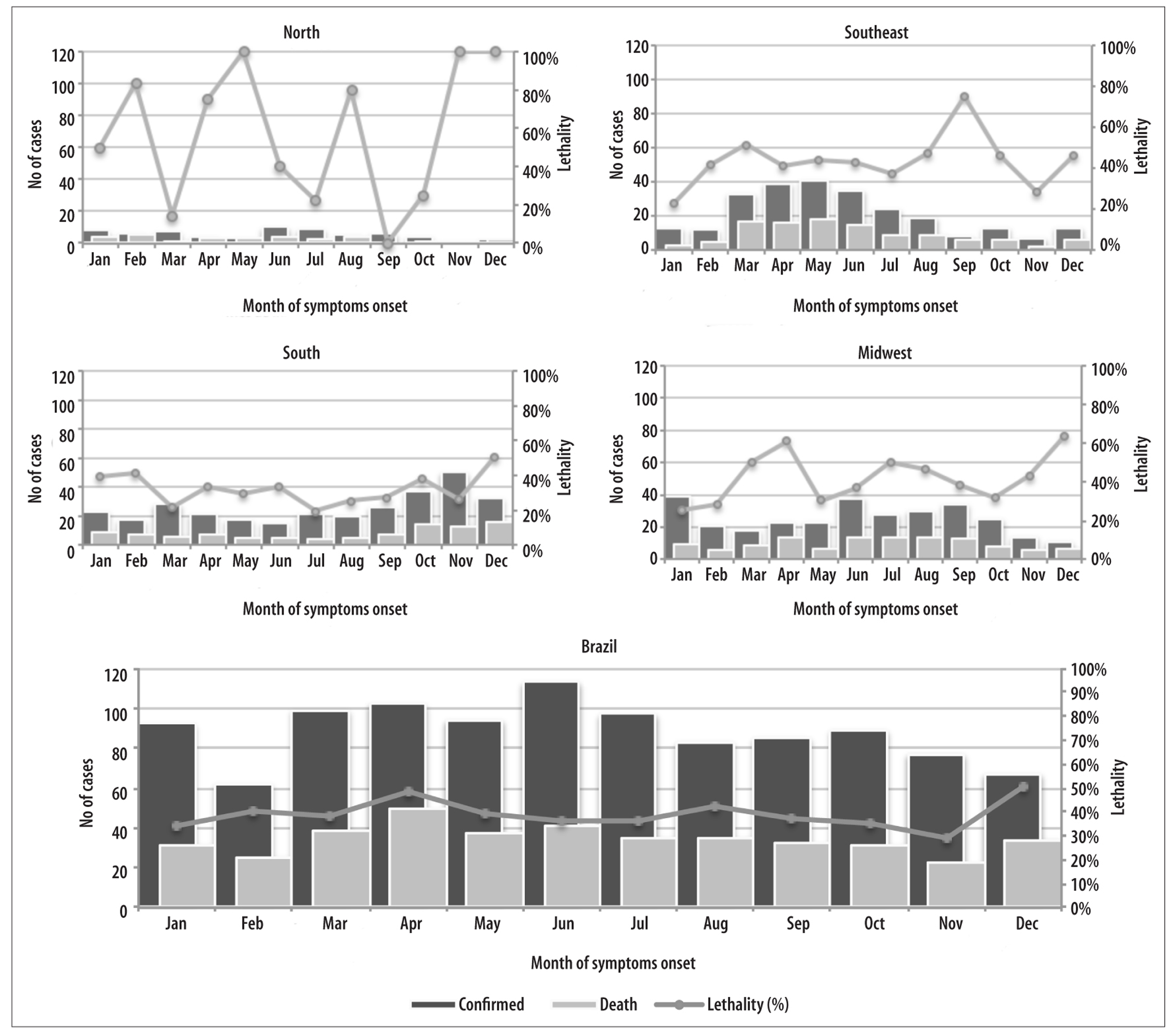
Note: 2 cases were registered in the Northeastern region (March and April) and 1 died in April; 50% lethality.
Figure 2 - Hantavirus case, death and lethalitydistribution, Brazil and regions, 2007-2015
With regard to the temporal variation of the disease in the country as a whole, there was no marked seasonality, except for a slight reduction of cases in the months of February, November and December. When analyzing the regions, seasonality was found to be more defined. In general the Northern region had few recorded cases, with a greater number (N=10) in the month of June. The Southeast region showed marked seasonality, with an increase of cases between the months of March and July (N=33 and N=35, respectively). In contrast, in the Southern region a marked increase in cases could be seen in from October to December (N=108 of 307 total cases). In the Midwest region, a slight increase of cases was observed in the month of January (N=39) and in the period from June to September (N=130 of 304 total cases) (Figure 2). The Northern region had 2 cases between March and April, and 1 died in April.
In Brazil, monthly lethality ranged from 28.6% (November) to 50.7% (December), at times showing an inverse pattern to disease seasonality, and in some regions reached high values (70%-100%) in months with a small number of cases. For example, in the Southeast region, average monthly lethality in the month of September was 75% (a month in which there was a reduced number of accumulated cases of the disease), in contrast to average monthly lethality of 45% in the months of April to June (Figure 2).
In Brazil, 75.7% of all confirmed hantavirus cases occurred in males (Figure 3). The age range with a higher proportion of cases was 20 to 34 years (39.6%), followed by 35 to 49 years (33.4%), with deaths reflecting the same pattern. The preponderance of cases in the 20 to 49 years age range was observed in both sexes, with slight differences between the country’s regions. The Southeast and Midwest followed the national pattern regarding the proportional distribution of cases by age groups, with predominance of the 20 to 34 years age group, followed by the 35 to 49 group. It is noteworthy that among females in the Southern region, the proportion of cases at age 35-49 surpassed that of the 20-34 age group (Figure 3).
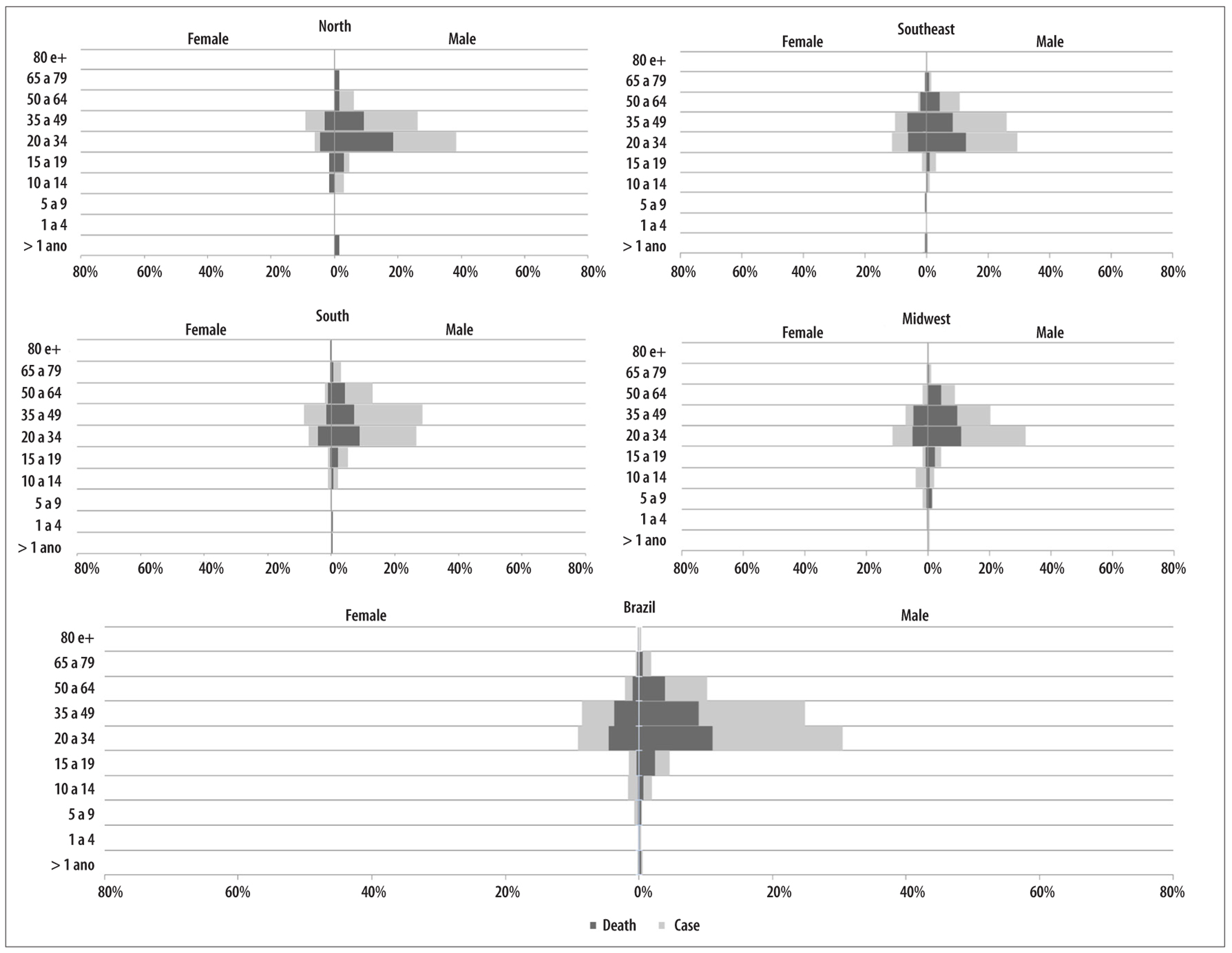
Note: All the proportions of cases and deaths were calculated taking the total number of cases in Brazil in the study period as the denominator (N=1,060). In the Northeast region, there were 2 cases: 1 female (17 years); and 1 male (52 years) who evolved to death.
Figure 3 - Proportion of hantavirus cases and deaths, according to sex and age, Brazil and regions, 2007-2015
With regard to deaths occurring in males, proportional distribution by age followed - in general - the distribution pattern of confirmed cases; i.e., we found the highest proportions of deaths in age groups with a greater proportion of confirmed cases. Particularly in relation to the 20-34 years age groups, the lethality rate remained relatively constant, ranging from 32.0% to 48.0% in all regions of Brazil (Figure 3).
With regard to the female sex, in general the proportional distribution of deaths was inversely proportional to the distribution of confirmed cases, particularly for the 20-49 age group, i.e., among females the proportion of deaths was higher in groups with a lower proportion of confirmed cases. For example, in the Southern region of the country, there was a greater proportion of cases in women aged 35-49 years and a higher proportion of deaths in the 20-34 age group. This fact leads to greater variations in lethality among women, particularly in these two age groups (variation between 42% and 75% for 20-24 years; and between 19% and 63% for 34-49 years), in different regions of Brazil (Figure 3).
Fever and dyspnea were the most frequently reported symptoms (>85.0%); the exception was the Northern region, where headache stood out in third place among the symptoms observed, ahead of respiratory insufficiency. In Brazil as a whole, among the cases that progressed to death and underwent chest radiography, 68.0% had diffuse pulmonary infiltrate. Among the regions, diffuse pulmonary infiltrate also stood out, in particular in the Midwest where 90% of deaths had this radiographic finding (Table 1).
Table 1 - Clinical characteristics at first healthcare consultationa of the hantavirus cases leading to death, according to probable region of infection, Brazil, 2007-2015
| Variables | North (N=30) | Northeast (N=1) | Southeast (N=112) | South (N=98) | Midwest (N=122) | Unknown (N=47) | Brazil (N=410) | |
|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | N | N | % | |
| Signs and Symptoms | ||||||||
| Fever | 26 | 1 | 103 | 87 | 105 | 44 | 366 | 89.3 |
| Dyspnea | 26 | 1 | 100 | 86 | 109 | 40 | 362 | 88.3 |
| Respiratory insufficiency | 17 | 1 | 93 | 79 | 92 | 37 | 319 | 77.8 |
| Myalgia | 17 | 1 | 69 | 74 | 77 | 35 | 273 | 66.6 |
| Headache | 22 | 1 | 62 | 75 | 81 | 28 | 269 | 65.6 |
| Cough | 20 | 1 | 70 | 58 | 81 | 28 | 258 | 62.9 |
| Hypotension | 18 | - | 70 | 69 | 58 | 25 | 240 | 58.5 |
| Nausea/vomiting | 19 | 1 | 59 | 68 | 69 | 24 | 240 | 58.5 |
| Chest Pain | 14 | 1 | 45 | 51 | 65 | 16 | 192 | 46.8 |
| Asthenia | 15 | 1 | 42 | 55 | 6 | 21 | 185 | 45.1 |
| Abdominal pain | 9 | 1 | 32 | 61 | 50 | 16 | 169 | 41.2 |
| Shock | 3 | 1 | 60 | 48 | 22 | 17 | 151 | 36.8 |
| Dizziness/vertigo | 15 | 1 | 33 | 45 | 44 | 10 | 148 | 36.1 |
| Lumbar pain | 13 | 1 | 27 | 43 | 27 | 10 | 121 | 29.5 |
| Diarrhea | 5 | - | 23 | 26 | 30 | 9 | 93 | 22.7 |
| Renal Insufficiency | 6 | 1 | 22 | 25 | 16 | 11 | 81 | 19.8 |
| Cardiac insufficiency | 3 | 1 | 22 | 14 | 13 | 8 | 61 | 14.9 |
| Neurological Symptom | 1 | 1 | 10 | 11 | 4 | 6 | 33 | 8.0 |
| Petechiae | 4 | - | 6 | 10 | 51 | 1 | 27 | 6.6 |
| Hemorrhagic manifestation | - | - | - | 2 | 1 | - | 3 | 0.7 |
| Radiography | ||||||||
| Radiography performed | 23 | 1 | 94 | 83 | 96 | 40 | 337 | 82.2 |
| Diffuse pulmonary infiltrate | 18 | 1 | 70 | 69 | 87 | 34 | 279 | 68.0 |
| Localized pulmonary infiltrate | 1 | - | 10 | 11 | 5 | 3 | 30 | 8.9 |
| Pleural effusion | 2 | 1 | 9 | 12 | 5 | 6 | 35 | 10.4 |
| Hospitalization | 28 | 1 | 104 | 95 | 116 | 45 | 389 | 94.9 |
| Use of mechanical ventilator | 11 | 1 | 87 | 71 | 82 | 38 | 290 | 74.6 |
| Clinical form | ||||||||
| Unknown/blank | - | 2 | 2 | 1 | 1 | 6 | 1.5 | |
| Cardiopulmonary Syndrome per hantavirus | 27 | 1 | 93 | 80 | 111 | 37 | 349 | 85.1 |
| Prodromal or nonspecific | 3 | - | 17 | 16 | 10 | 9 | 55 | 13.4 |
| Diagnostic Criteria | ||||||||
| Unknown/blank | - | - | 2 | 2 | 1 | 1 | 6 | 1.5 |
| Clinical-laboratorial | 26 | 1 | 105 | 96 | 112 | 44 | 384 | 93.7 |
| Clinical-epidemiological | 4 | - | 5 | - | 9 | 2 | 20 | 4.9 |
a) Data collected routinely during the first consultation. Exceptionally, information may have been obtained during the hospitalization period.
Of the 410 deaths due to hantavirus recorded in the country, 94.9% were hospitalized. In all the country’s regions approximately 90% of cases that died were hospitalized. 74.6% of all cases that progressed to death in Brazil as a whole used mechanical ventilators. This percentage was also found in the country’s regions, with the exception of the Northern region, where only 30.0% of individuals evolving to death used this equipment (Table 1).
Cardiopulmonary syndrome was the most frequently found clinical condition among those who died from hantavirus in Brazil as a whole and was found in more than 80% of the cases that progressed to death. Clinical and laboratory criteria were used to confirm diagnosis of almost all (93.7%) hantavirus deaths in the study period (Table 1).
The majority of individuals who died lived in urban areas (58.3%), although in the Southern region only 28.6% of deaths related to those living in urban areas. In Brazil as a whole, hantavirus infection occurred in rural areas among more than 70% of those who died. This pattern was observed in all regions, especially the North and the South, which recorded, respectively, 90.0% and 82.7% of deaths having rural areas as their probable place of infection. In the country as a whole, the probable place of infection was above all the 'workplace' and 'home', accounting for 39.0% and 31.7% of recorded deaths, respectively. This pattern occurred in almost all regions. It should be noted that in Northern region, 60.0% of hantavirus deaths had the workplace as their probable place of infection (Table 2).
Table 2 - Characteristics of deaths due to hantavirus, according to probable region of infection, Brazil, 2007-2015
| Variables | North (N=30) | Northeast (N=1) | Southeast (N=112) | South (N=98) | Midwest (N=122) | Unknown (N=47) | Brazil (N=410) | |
|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | N | N | % | |
| Residence Area | ||||||||
| Unknown/blank | - | - | 5 | 3 | 5 | 1 | 14 | 3.4 |
| Urban | 17 | 1 | 82 | 28 | 67 | 44 | 239 | 58.3 |
| Rural | 13 | - | 24 | 64 | 46 | 2 | 149 | 36.3 |
| Peri-urban areas | - | - | 1 | 3 | 4 | - | 8 | 2.0 |
| Autochthonous to municipality of residence | ||||||||
| Yes | 25 | 1 | 92 | 81 | 98 | - | 297 | 72.4 |
| No | 5 | - | 20 | 15 | 22 | - | 62 | 15.1 |
| Unspecified | - | - | - | 2 | 2 | 47 | 51 | 12.4 |
| Aof infection | ||||||||
| Unknown/blank | 0 | - | 11 | 5 | 7 | 26 | 49 | 12.0 |
| Urban | 2 | - | 22 | 6 | 11 | 4 | 45 | 11.0 |
| Rural | 27 | 1 | 68 | 81 | 96 | 15 | 288 | 70.2 |
| Peri-urban areas | 1 | - | 11 | 6 | 8 | 2 | 28 | 6.8 |
| Place of infection | ||||||||
| Unknown/blank | 2 | - | 18 | 9 | 15 | 25 | 69 | 16.8 |
| Household | 8 | - | 39 | 39 | 42 | 2 | 130 | 31.7 |
| Work | 18 | 1 | 42 | 35 | 53 | 11 | 160 | 39.0 |
| Leisure | 1 | - | 12 | 8 | 8 | 7 | 36 | 8.8 |
| Others | 1 | - | 1 | 7 | 4 | 2 | 15 | 3.7 |
| Risk situation/activity | ||||||||
| Exposure/cleaning | 15 | 1 | 37 | 75 | 48 | 1 | 185 | 45.1 |
| Rodents | 9 | 1 | 36 | 43 | 49 | 39 | 145 | 35.4 |
| Deforestation | 14 | 1 | 31 | 54 | 39 | 48 | 144 | 35.1 |
| Milling | 10 | 1 | 26 | 42 | 29 | 48 | 112 | 27.3 |
| Transportation | 5 | - | 15 | 14 | 13 | 22 | 57 | 13.9 |
| Fishing/Hunting | 7 | 1 | 9 | 17 | 30 | 13 | 75 | 18.3 |
| Slept in tent | 7 | 1 | 9 | 31 | 22 | 30 | 6 | 1.5 |
| Military Training | 1 | - | 2 | - | 1 | 49 | 78 | 19.0 |
In Brazil as a whole, more than 70% of hantavirus deaths were due to autochthonous infections in the municipality of residence of the individual. In relation to risk situation or activity, 'exposure to or cleaning' places favorable to the presence of rodents was reported in 45.1% of deaths investigated, followed by direct contact with or seeing wild dead or alive rodents or their remains (35.4%) and land deforestation or plowing , crop planting, crop harvesting and cutting firewood, among other similar procedures (35.1%). The Southern and Southeastern regions followed the same pattern observed for Brazil as a whole, while the Northern region showed a slight difference between activities, with deforestation being more prevalent there (46.7%) than contact with rodents (30.0%) (Table 2).
Discussion
In Brazil, from 2007 to 2015, almost two fifths of hantavirus cases progressed to death. The fatality rate for the disease varied according to the month (higher in December, lower in November), sex (higher in females in relation to males), age (higher in the elderly and children) and regions (higher in the North, lower in South). The majority of individuals who died lived in urban areas and were infected in rural areas. The workplace was the most probable place of infection.
This study corroborates the findings of Willemann & Oliveira,12 who observed regional differences in hantavirus lethality in a case-control study with secondary data from 2007 to 2010, relating to the regions of Brazil, with the objective of evaluating risk factors for death from hantavirus. With regard to the groups most vulnerable to death from hantavirus, in a descriptive study conducted in Brazil’s Distrito Federal between 2004 and 2013, Dusi et al.13 also found higher lethality in females than in males. Menezes et al.,14 in a cross-sectional study conducted in the state of Goias between 2007 and 2013, and Kaya et al.,15 in a review on prognostic factors for hantavirus infection, also identified higher lethality in most vulnerable age groups - such as children - as well as in other groups traditionally considered to be less exposed to infection.
Based on these findings, the hypothesis is raised that there were difficulties in clinical suspicion of the disease in individuals belonging to groups less vulnerable to hantavirus infection (women, children and elderly people from non-endemic regions and living in urban areas), which may have contributed to the delay in the differential diagnosis of the disease and to the delay in adequate clinical management, consequently increasing the probability of evolution to death. This hypothesis should be addressed in future studies, by recovering the clinical course of patients and assessing the quality of care offered to them, trying, for example, to identify and prevent avoidable deaths among these unfavorable but sometimes unavoidable hantavirus outcomes.
Indeed, access to good quality health care services with health professionals trained, able and agile in defining suspected diagnosis and establishing early and appropriate hantavirus case management can influence disease evolution decisively. Campos et al.16 report on an important support measure to be considered: restricted fluid intake by individuals with hantavirus, as if this therapeutic measure is not respected, it is associated with a higher probability of death. This usually occurs due to the absence of appropriate differential diagnosis, whereby the medical recommendation consists of hydrating the patient, this being the recommendation for patients diagnosed with dengue, influenza and pneumonia, for example. The high incidence of emerging arboviroses (dengue fever, chikungunya and Zika virus infection) in areas with low detection of hantavirus cases can lead to underdiagnosis of the disease. In Ceará, in 2008, during a period of increased dengue cases in that state, researchers identified positive serology for hantavirus in samples of patients with suspicion of dengue. This provides evidence of disease co-circulation.17
The signs and symptoms that stood out among hantavirus cases in Brazil (fever, dyspnea and respiratory insufficiency) did not differ from those found in individuals resident in other Latin American and South American countries, as described in studies conducted in Chile and Argentina.18-20 Some of the most prevalent signs and symptoms - fever, myalgia and headache - confirm the insidious onset of hantavirus with benign and unspecific clinical presentation, contributing to the low demand for health services in the initial phase of infection.21 However, according to the results of this study, when there are signs of hypotension and shock when patients are attended to for the first time, this indicates hantavirus infection and at times the disease can develop rapidly and severely. The mechanisms determining this broad spectrum of hantavirus clinical presentation and evolution are not fully understood. Studies of the susceptibility of mankind to the virus and studies of the pathogenesis of the disease associated with viral variation are still insufficient.22
Radiography is an important diagnostic examination to be carried out in individuals with suspected hantavirus. The identification of diffuse pulmonary infiltrate - indicating the cardiopulmonary phase of the disease - helps diagnosis, while also alerting as to its severity.5 In fact, in this study, the majority of individuals who progressed to death had diffuse pulmonary infiltrate. This finding was also observed by Insaurralde and Páez9 in an analysis of prognostic factors for death in Brazil between 1993 and 2006. Ferreira et al.23 found similar results when reported cases occurred in 1996, 1998 and 1999. In addition to these researchers, Figueiredo et al.18 found similar results from a review of hantavirus cases described in South America.
Sometimes, due to the diffuse pulmonary infiltrate and respiratory insufficiency caused by hantavirus, mechanical ventilators are used. In this study, the use of this type of technology appears in about 70% of individuals who died, thus emphasizing the severity of the disease. Elkhoury et al.10 investigated prognostic factors for death in Brazil between 1993 and 2006 and identified that the need to use respiratory support is a marker of unfavorable prognosis and progression to death owing to hantavirus. However, lack of access to this technology could be decisive for case evolution.
The relatively low proportion of mechanical ventilator use among the cases that died in the North (36.7%) and Midwest regions (67.2%) found here deserves reflection: it can be an indicator of (i) the milder form of the disease or (ii) difficulties in accessing this technology. It is worth remembering that among the deaths due to hantavirus in these two regions, the North and Midwest, high proportions of respiratory insufficiency and diffuse pulmonary infiltrate were found, in addition to more than 20% of them never having had a chest x-ray. These are two regions of the country - and coincidentally the largest that have the highest hantavirus lethality rates. The hypothesis of suspected or confirmed hantavirus cases having significant difficulties in accessing adequate healthcare in Brazil’s Midwest and Northern regions is plausible, but needs to be studied in more depth.
There are limitations in this study. The use of secondary data from SINAN does not eliminate the possibility of hantavirus cases and deaths being underreported. However, Oliveira et al.22 and Menezes et al., when using the same sources, demonstrated adequate consistency14, as well as wide case coverage. Even so, data recording errors or recall bias may occur, since certain information, such as exposure to risk situations, collected during the investigation, relates to a period of up to 60 days prior to infection. Another aspect to be considered is the validity and completeness of the information collected on the SINAN notification form. Thus, the potential exists for more severe cases or those that evolve to death having greater likelihood of being notified on SINAN, compared to cases with benign evolution, and this could lead to a kind of selection (or notification) bias. Therefore, exclusive use of this information system may overestimate lethality, given that is underestimates its denominator. To overcome such limitations, future studies should consider the possibility of incorporating other databases - for example the Mortality Information System (SIM), the Brazilian National Health System Hospital Information System (SIH/SUS) and the Laboratory Environment Manager (GAL) -, as well as outpatient medical records.
Despite these limitations, the results of this research offer input for addressing the characteristics of hantavirus cases and deaths and for discussing shortcomings in caring for the sick, thus opening a field of fundamental debate related to the quality of care provided to people with hantavirus in Brazil.
REFERENCES
1. Organización Panamericana de la Salud, Organización Mundial de la Salud. Hantavirus en las Américas: guía para el diagnóstico, el tratamiento, la prevención y el control [Internet]. Washington, D.C: Organización Panamericana de la Salud,; 1999 [citado 2018 jan 22]. 66 p. Disponible: Disponible: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=20157&Itemid=270&lang=en [ Links ]
2. Santos ED, Garrett DO. Avaliação do sistema de vigilância de hantavírus no Brasil. Epidemiol Serv Saúde. 2005 jan-mar;14(1):15-31. [ Links ]
3. Ministério da Saúde (BR), Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Hantavirose [Internet]. In: Guia de vigilância em saúde. Brasília: Ministério da Saúde; 2016 [citado 2018 jan 22]. 607-17 p. Disponível em: Disponível em: http://portalarquivos.saude.gov.br/images/pdf/2016/agosto/25/GVS-online.pdf [ Links ]
4. Melo-Silva CR, Maranhão AQ, Nagasse-Sugahara TK, Bisordi I, Suzuki A, Brigido MM. Characterization of hantaviruses circulating in Central Brazil. Infect Genet Evol. 2009 Mar;9(2):241-7. [ Links ]
5. Ministério da Saúde (BR), Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Manual de vigilância, prevenção e controle das hantaviroses [Internet].Brasília: Ministério da Saúde; 2013 [citado 2018 jan 22]. 94 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_prevencao_controle_hantaviroses.pdf [ Links ]
6. Pan American Health Organization. Zoonoses and communicable diseases common to man and animals: chlamydioses, rickettsioses, and viroses [Internet]. 3rd ed. Washington, D.C.: Pan American Health Organization; 2003 [cited 2017 Jan 22]. v. 2. (Scientific and Technical Publication n, 580). Available in: http://new.paho.org/hq/dmdocuments/2010/ZoonosesVol-2.pdf [ Links ]
7. Riquelme R, Rioseco ML, Bastidas L, Trincado D, Riquelme M, Loyola H, et al. Hantavirus pulmonary syndrome, Southern Chile, 1995-2012. Emerg Infect Dis. 2015 Apr;21(4):562-8. [ Links ]
8. Martinez VP, Bellomo CM, Cacace ML, Suárez P, Bogni L, Padula PJ. Hantavirus pulmonary syndrome in Argentina, 1995-2008. Emerg Infect Dis. 2010 Dec;16(12):1853-60. [ Links ]
9. Insaurralde A, Páez M. Situación epidemiológica del síndrome pulmonar por hantavirus (SPH). Paraguay 2000-2004. Mem Inst Investig Cienc Salud. 2008 jun;6(1):28-33. [ Links ]
10. Elkhoury MR, Mendes WS, Waldman EA, Dias JP, Carmoe EH, Vasconcelos PFC. Hantavirus pulmonary syndrome: prognostic factors for death in reported cases in Brazil. Trans R Soc Trop Med Hyg. 2012 May;106(5):298-302. [ Links ]
11. Oliveira RC, Sant’ana MM, Guterres A, Fernandes J, Hillesheim NL, Lucini C, et al. Hantavirus pulmonary syndrome in a highly endemic area of Brazil. Epidemiol Infect. 2016 Apr;144(5):1096-106. [ Links ]
12. Willemann MCA, Oliveira SV. Risk factors associated with hantavirosis fatality: a regional analysis from a case-control study in Brazil. Rev Soc Bras Med Trop. 2014 Jan-Feb;47(1):47-51. [ Links ]
13. Dusi RM, Bredt A, Freitas DRC, Bofill MIR, Silva JAM, Oliveira SV, et al. Ten years of a hantavirus disease emergency in the Federal District, Brazil. Rev Soc Bras Med Trop. 2016 Jan-Feb;49(1):34-40. [ Links ]
14. Menezes Filho HR, Moreli ML, Sousa ALL, Costa VG. Estudo transversal da letalidade da hantavirose no estado de Goiás, 2007-2013. Epidemiol Serv Saúde. 2016 jul-set;25(3):519-30. [ Links ]
15. Kaya S. Prognostic factors in hantavirus infections. Mikrobiyol Bul. 2014 Jan;48(1):179-87. [ Links ]
16. Campos GM, Borges AA, Badra SJ, Figueiredo GG, Souza RL, Moreli ML, et al. Pulmonary and cardiovascular syndrome due to hantavirus: clinical aspects of an emerging disease in southeastern Brazil. Rev Soc Bras Med Trop. 2009 May-Jun;42(3):282-9. [ Links ]
17. Lima DM, Sabino-Santos Junior G, Oliveira AC, Fontes RM, Colares JK, Araújo FM, et al. Hantavirus infection in suspected dengue cases from State of Ceará, Brazil. Rev Soc Bras Med Trop. 2011 Nov-Dec;44(6):795-6. [ Links ]
18. Figueiredo LT, Souza WM, Ferrés M, Enria DA. Hantaviruses and cardiopulmonary syndrome in South America. Virus Res. 2014 Jul;187:43-54. [ Links ]
19. Bayard V, Kitsutani PT, Barria EO, Ruedas LA, Tinnin DS, Muñoz C, et al. Outbreak of hantavirus pulmonary syndrome, Los Santos, Panama, 1999-2000. Emerg Infect Dis. 2004 Sep;10(9):1635-42. [ Links ]
20. Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994 Apr;330(14):949-55. [ Links ]
21. Raboni SM, Probst CM, Bordignon J, Zeferino A, Santos CN. Hantaviruses in central South America: phylogenetic analysis of the S segment from HPS cases in Paraná, Brazil. J Med Virol. 2005 Aug;76(4):553-62. [ Links ]
22. Oliveira SV, Fonseca LX, Silva PMRB, Pereira SVC, Caldas EP. Análise do perfil epidemiológico da hantavirose no brasil no período de 2007 a 2012. Rev Patol Trop. 2014 abr-jun;43(2):131-42. [ Links ]
23. Ferreira MS, Nishioka SA, Santos TL, Santos RP, Santos PS, Rocha A. Hantavirus pulmonary syndrome in Brazil: clinical aspects of three new cases. Rev Inst Med Trop S Paulo. 2000 Feb;42(1):41-6. [ Links ]
This article is based on the dissertation entitled “Epidemiological profile and factors associated with death due to hantavirus in Brazil, 2007-2015”, Lidsy Ximenes Fonseca, submittted in 2017, to the University of Brasília (UnB) Postgraduate Collective Health Program as a partial requirement for obtaining the title of Master of Collective Health, in the focus area of Epidemiology.
Received: June 09, 2017; Accepted: December 28, 2017











 texto en
texto en