Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.27 no.3 Brasília set. 2018 Epub 31-Ago-2018
http://dx.doi.org/10.5123/s1679-49742018000300013
Articles
Trends in mortality from chronic obstructive pulmonary disease in Rio de Janeiro and Porto Alegre, Brazil, 1980-2014
1Fundação Instituto Oswaldo Cruz, Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro, RJ, Brazil
Objective:
to analyze age-period-cohort (APC) effects on mortality from chronic obstructive pulmonary disease (COPD) in the municipalities of Porto Alegre, RS, and Rio de Janeiro, RJ, Brazil, between 1980 and 2014.
Methods:
this was a time series study using corrected Mortality Information System (SIM) data; APC effects were estimated by Poisson regression, in relation to the 1935 cohort.
Results:
relative risk (RR) of death due to COPD for males decreased in the most recent birth cohort (1970-1974) in Porto Alegre (RR=0.39; 95%CI 0.32;0.48) and Rio de Janeiro (RR=0.42; 95%CI 0.38;0.48); while among women an increase in risk of death due to COPD was observed in Rio de Janeiro in more recent cohorts (RR=1.41; 95%CI 1.20;1.67).
Conclusion:
risk of death due to COPD decreased among men, while risk among women in Rio de Janeiro increased.
Keywords: Pulmonary Disease, Chronic Obstructive; Mortality Registries; Time Series Studies
Introduction
Chronic obstructive pulmonary disease (COPD) is recognized as a public health problem. It is one of the major causes of chronic morbidity and mortality both in Brazil and worldwide.1,2 According to the population-based PLATINO study conducted in the municipality of São Paulo, COPD prevalence among people aged 40 years or older was 15.8% in 2005.1 Global prevalence was estimated at 11.7% for people aged 30 and over in 2010.3
COPD is defined as a preventable and treatable respiratory disease characterized by chronic airflow obstruction, usually progressive and not fully reversible, having signs and symptoms such as dyspnea, cough and expectoration. COPD diagnosis is based on clinical findings and confirmed by pulmonary function testing.4,5 The main risk factor for the development of COPD is smoking, which accounts for 80 to 90% of cases.4,5
The highest prevalence of smoking in Brazil is found in the Southern and Southeast regions of Brazil, where higher mortality rates due to COPD are also found. National surveys show that the prevalence of smoking in Brazil in people aged 18 years or older has declined substantially, from 34.3% in 1989 to 14.7% in 2013.6-8
The decrease in the prevalence of smokers is probably the result of measures implemented by the Brazilian Ministry of Health since the late 1980s, such as the National Policy on Tobacco Control, which included, among other actions, the promotion of smoke-free environments, the treatment of smokers by the Brazilian Unified Health System (SUS) in order to stop smoking, pricing and taxation policies for this sector, in addition to epidemiological surveillance actions.7,8
International studies of COPD mortality trends due to COPD in relation to the reduction in the percentage of smokers show a decline in COPD mortality rates in males over time. Among women, however, COPD mortality rates are tending to increase.9,10
In Brazil, studies of COPD mortality time trends have been performed using aggregate rates according to age groups and periods.11,12 This makes it impossible to assess the effect of birth cohorts related to exposure to long-term risk factors.13
Assessing age, period and birth cohort effects on the temporal evolution of COPD mortality contributes to the planning of public health actions and to the assessment of the impact of changes in treatment protocols, improvement of information health systems and of health policies put into practice, especially when they involve tobacco control. The main hypothesis is that cohort effects result from tobacco consumption, involving long-term exposure, whereby different generations are exposed to different risks.13
The objective of this study was to analyze the of age, period and birth cohort effect on mortality due to chronic obstructive pulmonary disease (COPD) in the municipalities of Porto Alegre and Rio de Janeiro between 1980 and 2014.
Methods
This was a time series study of COPD mortality in the municipalities of Porto Alegre and Rio de Janeiro between 1980 and 2014.
The municipality of Rio de Janeiro, capital of Rio de Janeiro state, had an estimated population of 6.5 million inhabitants in 2016. Its per capita gross domestic product (GDP) in 2014 was R$46,000. In 2014, there were 1,836 deaths due to respiratory diseases in the municipality of Rio de Janeiro. Porto Alegre, the capital of the state of Rio Grande do Sul, has a significantly smaller population, estimated at 1.5 million people in 2016 and its per capita GDP in 2014 was similar to that of Rio de Janeiro. In 2014, 1,836 deaths due to respiratory diseases in Rio de Janeiro and 1,281 such deaths in Porto Alegre were recorded.14
Data on deaths were taken from the Mortality Information System (SIM) and census data on the resident population according to the Brazilian Institute of Geography and Statistics (IBGE) were taken from the Brazilian Unified Health System IT Department (DATASUS) website, available at http://www2.datasus.gov.br/DATASUS/index.php?area=02 15
Taking these data, we selected people aged 40 years or more, as this is the age when COPD diagnosis is more common. This group was separated by sex (male; female).
The following categories were selected from the 9th revision of International Statistical Classification of Diseases and Related Health Problems (ICD-9) for the period 1980-1995: 490 (bronchitis, not specified as acute or chronic), chronic bronchitis (491), 492 (emphysema) and 496 (COPD not elsewhere classified). While for the period from 1996 to 2014 the following ICD-10 categories were selected: J40 (bronchitis, not specified as acute or chronic), J41 (simple and mucopurulent chronic bronchitis), J42 (unspecified chronic bronchitis), J43 (emphysema) and J44 (unspecified chronic obstructive pulmonary diseases). The choice of these various codes was in keeping with the variability of clinical presentation of the disease, in addition to these codes being the most widely used in epidemiological studies.
Deaths from ill-defined causes were reallocated in the same proportion as deaths from chronic obstructive pulmonary disease in relation to the total number of deaths from all causes - after excluding ill-defined causes, for each category of sex, age range and year of death in both capitals, separately.16 We calculated the crude mortality rates per 100 thousand inhabitants for each year, according to sex and municipality and their respective 95% confidence intervals.
The age-period-cohort (APC) model is used to describe and predict time series of disease rates using the three different time scales: age (a); calendar period (p), when the rates were observed; and (c) cohort, or group of people observed, born in specific periods. The isolated effects of each of these factors on the evolution of COPD rates were estimated,13 In order to enable the effect of cohorts related to long-term exposure to risk factors to be estimated.13
These three factors have linear association, so that the two-way table, containing the age groups and the period (year) of death, enables the calculation of the birth cohorts (year of birth) using the following equation: c = p - a 17.18
The periods and age ranges for each sex and capital city were grouped into five-year intervals, thus generating seven periods, from 1980-1984 until 2010-2014, and nine age groups of 40-44 to 80 years or more. The total number of birth cohorts (k) is equal to the total number of age ranges (m) plus the total number of periods (n), less 1 (k=m+n-1). Therefore, the birth cohorts started in 1900 and ended in 1974, totaling 15 five-year cohorts from 1900-1904 until 1970-1974.17,18
The APC effects were estimated using the Poisson regression model, which assumes that the log of rates lap is linearly related to the effects of age, a period p and cohort c= p - a, i.e., log(l ap )= f(a) + g(p) + h(c).
Generally speaking, the effect of each of the three components can be assumed to be non-linear, thus defining the a,p c variables as categorical variables, for example, or assuming f, g and h to be non-parametric smoothing functions (splines).17,18 In the latter case, the results of the model are interpreted graphically when analyzing the trends of the smoothed rates and risks. Apart from the complete APC model, other submodels can also be defined. For example, taking age association to be linear, using the log of the rates, and assuming that f(a)= sa (drift models). The effects of age and period (AP) or age and cohort (AC) on their own can also be considered.
In this analysis, all models (drift, AP, AC and APC) were compared by means of the likelihood ratio test, using a significance level of 0.05. Residual analyses were performed on each of the final models adjusted by sex and municipality, in order to assess the quality of the data adjustments and the presence of influential points.
When interpreting the parameters of the model, the age effect (a) refers to the changes in the rates associated with the biological risk of age. The period effect (p) represents the changes related to events that occurred at any given time and that influence all age groups simultaneously. The cohort effect(c) is comprised of factors that affect a generation and cause changes in the rates in different ways, in the successive age groups, in the different periods.13
Given the linear dependence between factors of the model, there are diverse APC approaches that apply different additional restrictions, in order to solve the so-called 'non-identifiability problem', i.e. failure to estimate the complete model, due to the exact linear relationship between the temporal effects (age-period and birth cohort). In this article, we considered the parameterization proposed by Clayton & Shilfflers18 (1987), Holford13 (1991) and Cartensen17 (2007).
This approach considers estimable functions, in which one of the effects was restricted in order to achieve zero mean, with zero inclination. The cohort effects were prioritized and thus we applied this restriction to the period effects. In this case, the graphical interpretation of the effects estimated by the models occurs in such a way that, once a reference cohort has been defined, the longitudinal effects for age are interpreted as the rates observed for the cohort, and the cohort effect is interpreted as RR in relation to cohort c0. The 1935 cohort was adopted as the reference cohort in this study as it was the midway cohort.17 Confidence intervals of 95% for the rates and RR were generated automatically by the APC functions in the statistical program. The rate logarithm (log) was adopted in Figures 2 and 3 because the logarithmic scale allows a better graphical representation of the rates which may show great variability.
The APC modeling and descriptive graphics were performed using the apc.fit function and the ggplot2, gridExtra and Epi 2.0 packages (R statistical program, version 3.1.3).19
This study used aggregated secondary data, with no identification of subjects, and was therefore conducted in accordance with the ethical principles established by National Health Council (CNS) Resolution No. 510, dated April 7th 2016.
Results
Expanding mortality by redistributing deaths due to ill-defined causes in the municipality of Rio de Janeiro, between 1993 to 2007, resulted in an increase above 10% (maximum of 12% in 2003) in deaths due to COPD. In Porto Alegre, the increases were less important (maximum 2%).
Generally speaking, COPD mortality rates were higher among men, in both capitals. They were also higher in Porto Alegre than in Rio de Janeiro, regardless of sex. In both sexes, there was a general trend of increasing rates until the end of the 1990s. A decrease began after this period and was more intense among males. However, in the municipality of Porto Alegre, between the years 2000 and 2005, a new increase in mortality rates was observed. In the case of women in Porto Alegre there was oscillation between increased rates elevation and a significant decrease between 2000 and 2014.Tthe higher variability observed in the rates for Porto Alegre may possibly be attributed to a smaller number of deaths per year (Figure 1).
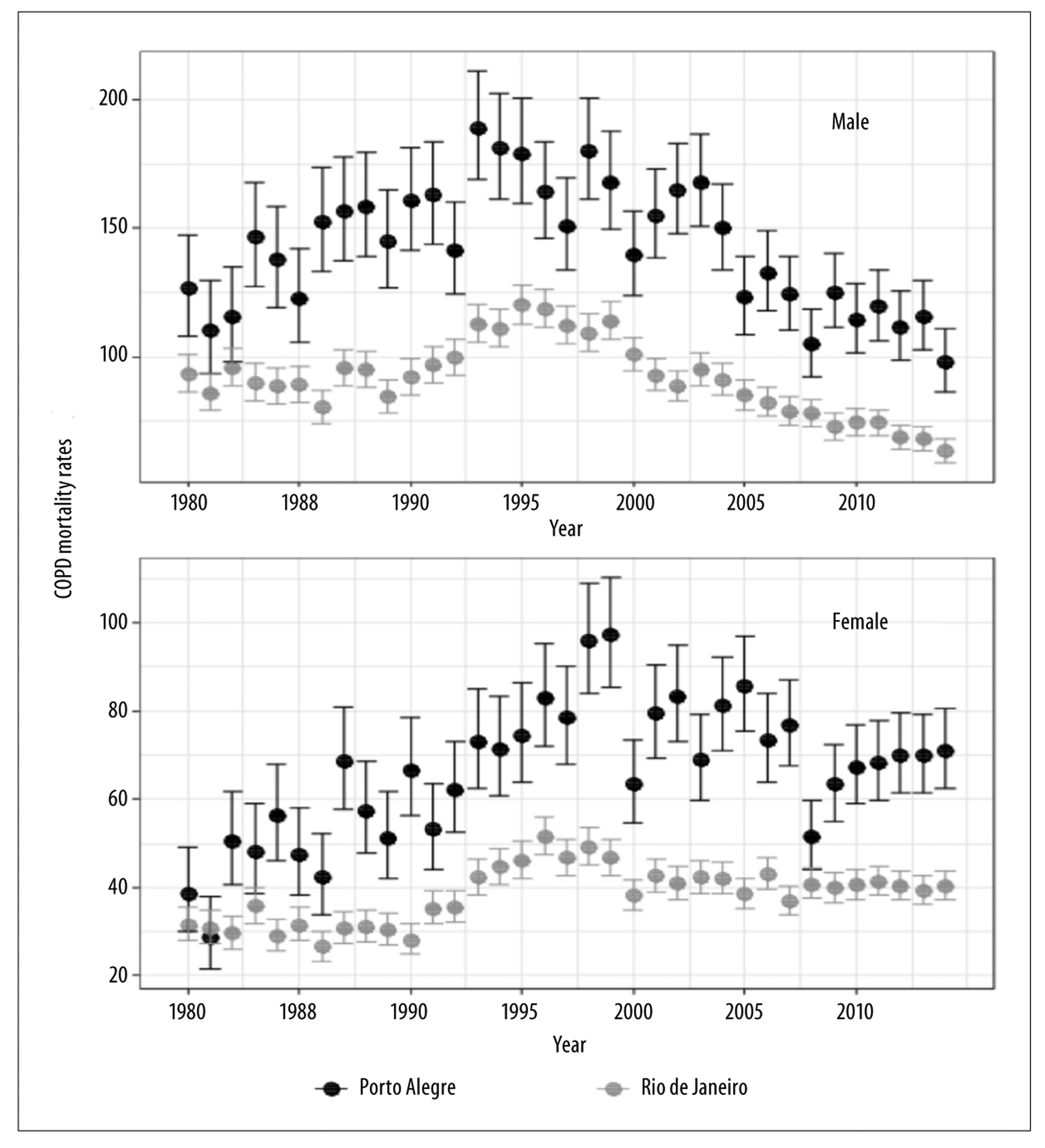
Figure 1 - Chronic obstructive pulmonary disease (COPD) mortality rates (per 100.000 inhabitants) and their 95% confidence intervals, according to sex, Rio de Janeiro and Porto Alegre, 1980-2014
Mortality rates increased with age, in all periods and all cohorts. In both sexes in the 65 and over age range, we observed an increase in mortality rates until the period 1995-1999, when they began to decline, more markedly among men (Figures 2 and 3).
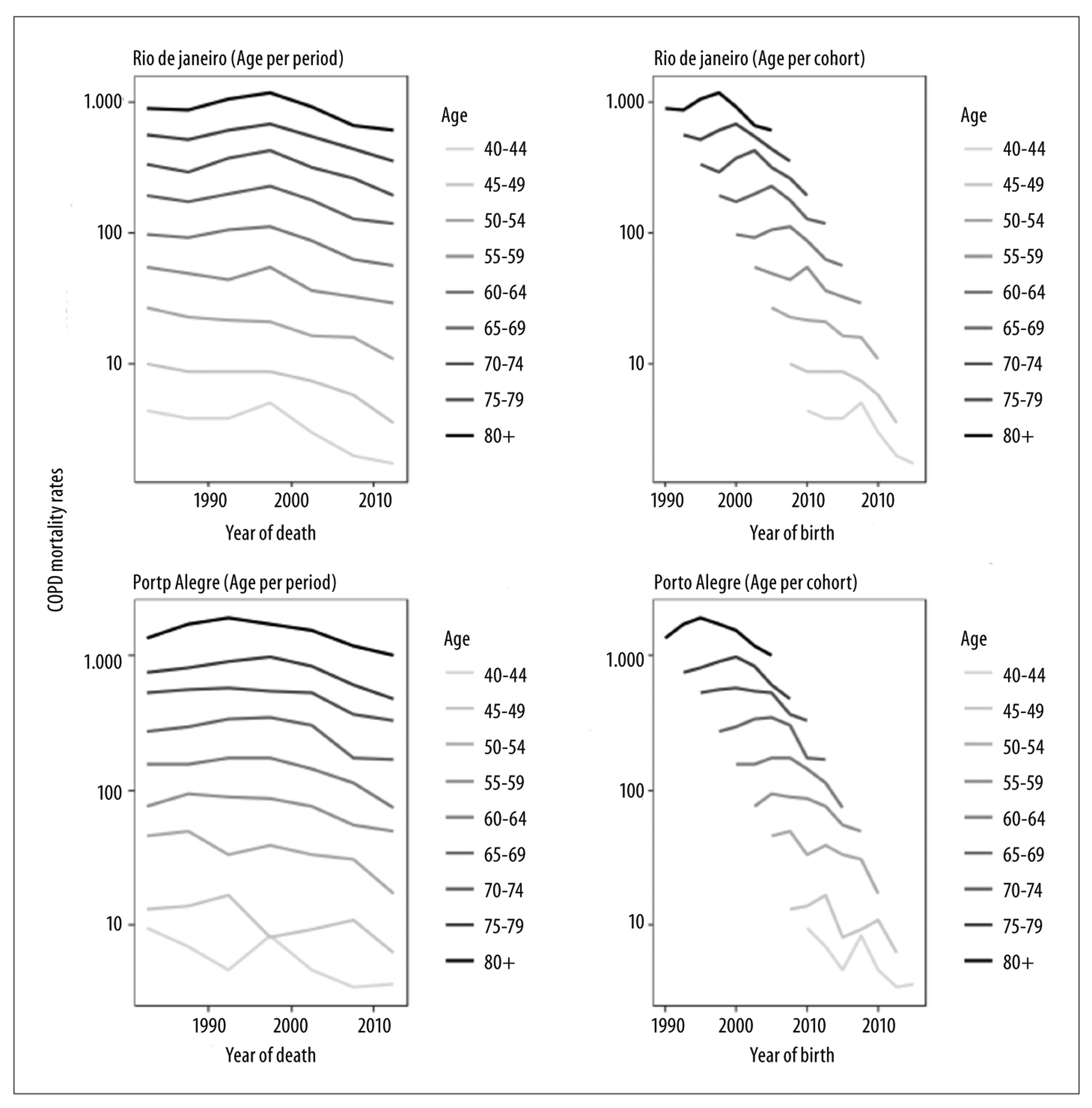
Figure 2 - Mortality rate log for chronic obstructive pulmonary disease (COPD) in males, according to age and year of death, age and year of birth, Rio de Janeiro and Porto Alegre, 1980-2014
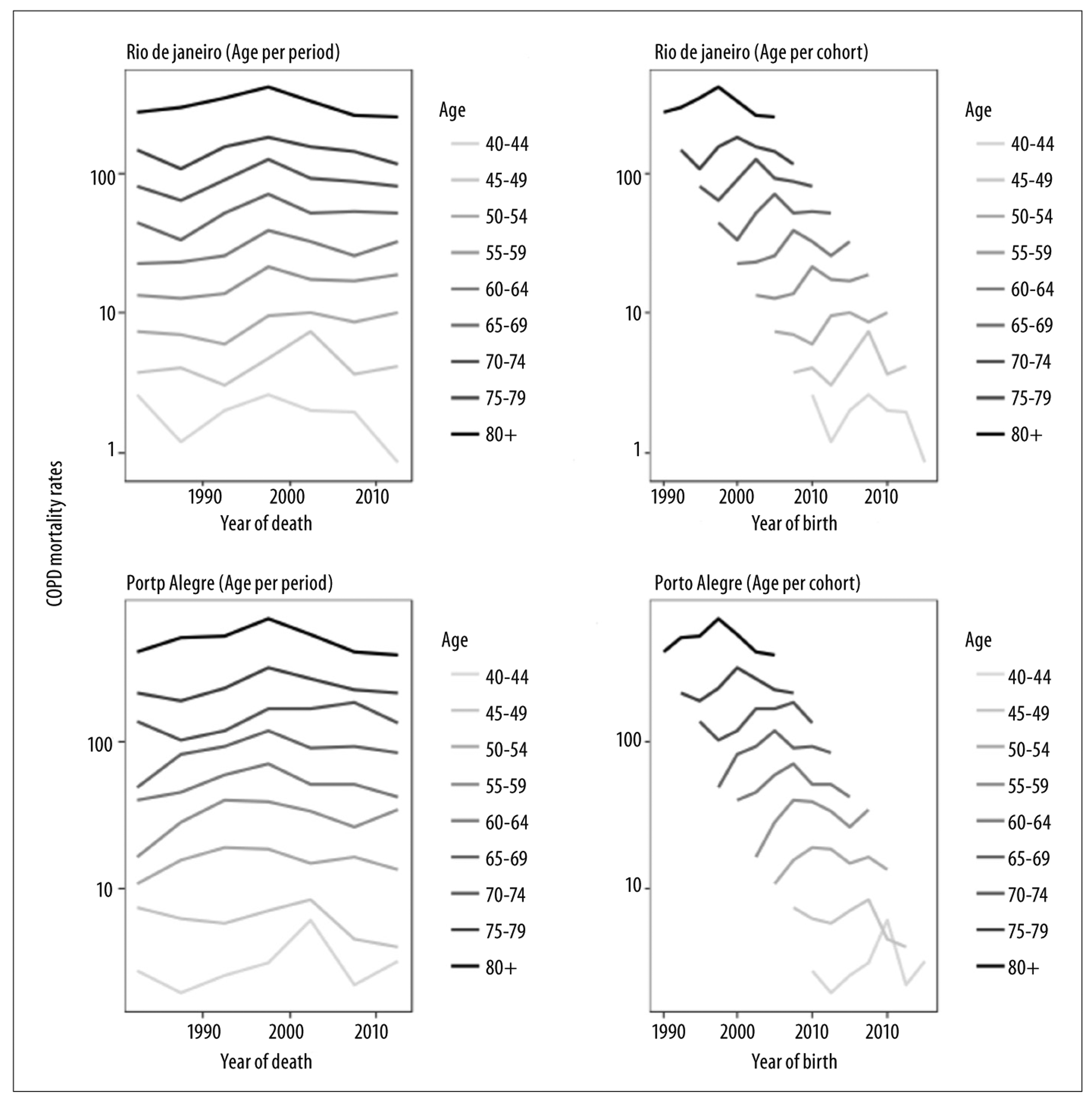
Figure 3 - Mortality rate log for chronic obstructive pulmonary disease (COPD) in females according to age and year of death, age, and year of birth, Rio de Janeiro and Porto Alegre, 1980-2014
In relation to the male cohorts, in most age categories, mortality rates were higher in the earlier cohorts, in comparison to the more recent cohorts (Figure 2). There was a more significant increase in mortality rates in recent years among women aged between 45 and 60 years in the municipality of Rio de Janeiro. In Porto Alegre, an increase in the mortality rate in recent cohorts was only observed in women aged 40 to 44 and 55 to 59 years (Figure 3).
In both sexes the analysis of the likelihood ratio test showed that the complete model (PCA) showed a better fit to the data (p<0.0001) than the models including only age, age-drift or just two factors (AP and AC).
The mortality rates adjusted by the APC model increased sharply with advancing age, in longitudinal perspective of the 1935 cohort, in both sexes and both municipalities and were higher in Porto Alegre than in Rio de Janeiro, with a mean difference of 1.65 per 100.000 in men, and almost twice (1.86 per 100.000) in women (Figures 4 and 5).
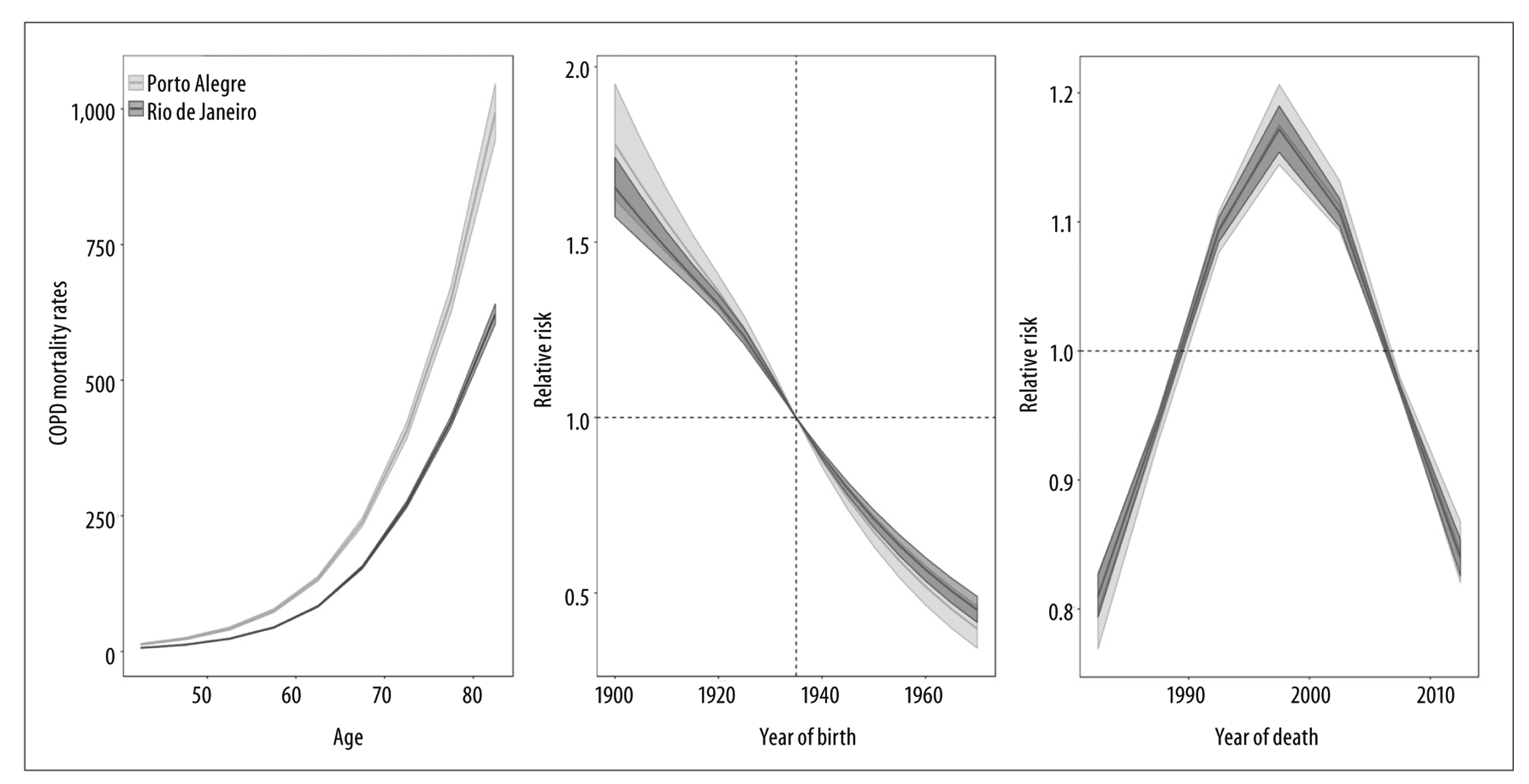
Figure 4 - Model adjusted for age-period-cohort (APC) for mortality due to chronic obstructive pulmonary disease (COPD) and 95% confidence intervals for males, Rio de Janeiro and Porto Alegre, 1980-2014
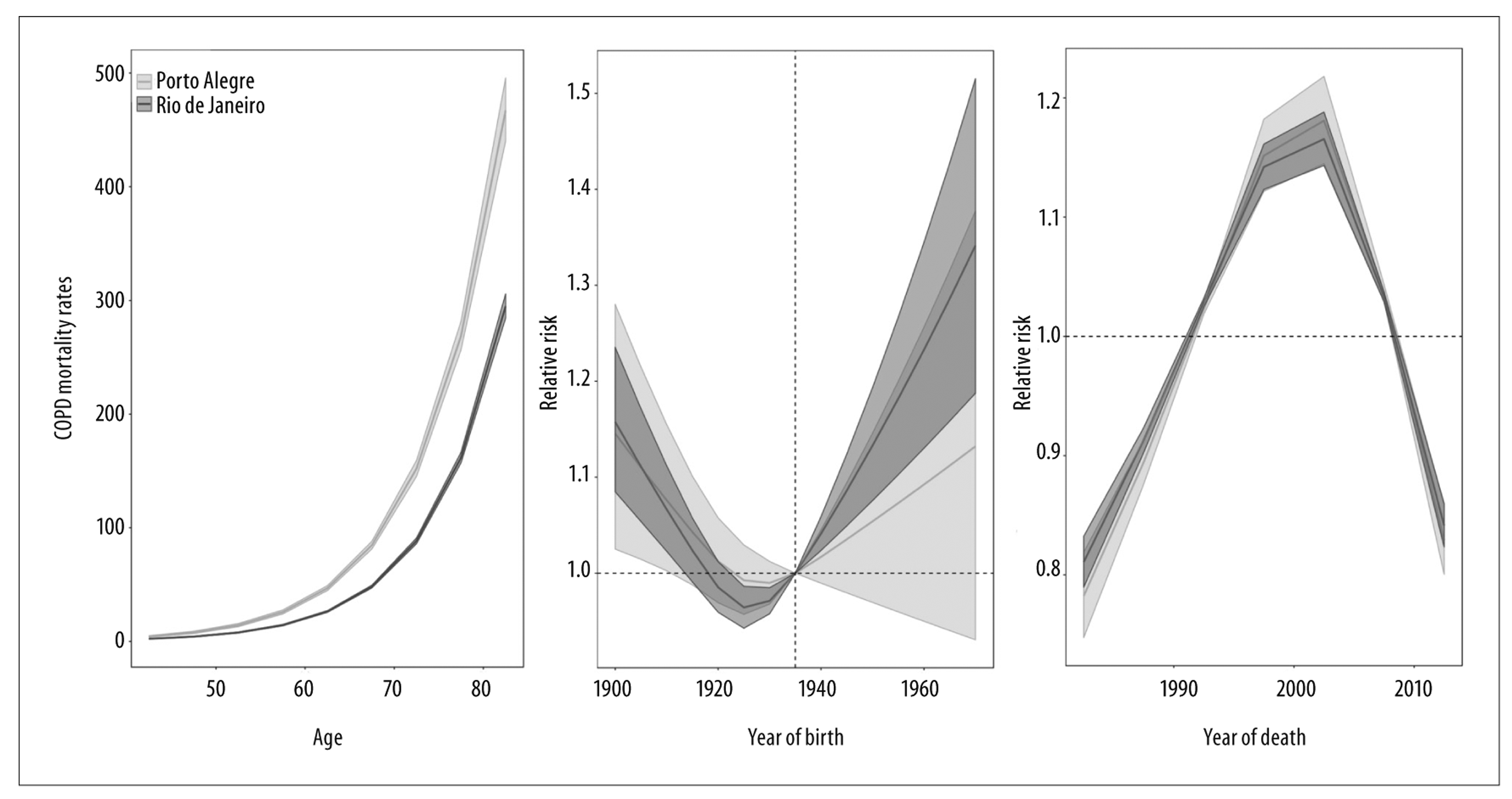
Figure 5 - Model adjusted for age-period-cohort (APC) for mortality due to chronic obstructive pulmonary disease (COPD) and respective 95% confidence intervals for females, Rio de Janeiro and Porto Alegre, 1980-2014
Similar trends in the period and cohort effects among men were observed in both capitals. There has been a steady decline in the risk of death from COPD over the successive generations (Figure 4). The risk ratio (RR) for death among men in the oldest cohort, in relation to the 1935 cohort risk, was 1.81 (95%CI 1.63; 2.00) and 1.65 (95%CI% 1.55;1.75) in Porto Alegre and in Rio de Janeiro, respectively. And in the most recent cohort, RR was 0.39 (95%CI 0.32;0.48) in Porto Alegre and 0.42 (95%CI 0.38;0.48) in Rio de Janeiro (Figure 4).
In relation to the period effect, we found higher relative risk of death from 1990 until the year 2005, with the highest risk detected in the period 1995-1999, both in Porto Alegre (RR=1.16; 95%CI 1.13;1.19) and in Rio de Janeiro (RR=1.14; 95%CI 1.13;1.16) (Figure 4).
Among women, the cohort effects differed between the two capitals. In Rio de Janeiro, risk of death decreased in older cohorts and increased in more recent cohorts, in relation to the 1935 cohort. The biggest risk ratio for death among women was observed in the 1970 cohort (RR=1.41; 95%CI 1.20;1.67). In Porto Alegre, the older cohorts presented a higher risk of death for women (RR=1.30; 95%CI 1.14;1.47); and the more recent cohorts (RR<1) showed no statistically significant differences in relation to the 1935 cohort (Figure 5). The period effects were similar for the male sex in both capital cities (Figures 4 and 5).
The residual analysis of the four adjusted APC models (Porto Alegre and Rio de Janeiro, for both sexes) showed that all were adjusted adequately by the age, period and cohort variables. Residual analysis versus leverages showed no points that could compromise the results found.
Discussion
This study confirmed the exponential effect of age on mortality due to COPD expected for chronic diseases.8 Porto Alegre presented the highest COPD mortality rates, in all age ranges and for both sexes, over the period considered. Regardless of the municipality and period, the rates were higher in men than in women. However, whereas among men the relative risk of death due to COPD decreased over the successive cohorts, in both municipalities, among women there was increased risk in more recent years in the municipality of Rio de Janeiro.
Age is a relevant factor for these trends. Aging produces a complex set of structural alterations to the body, such as cell aggression and repair mechanisms, immune deficiency and systemic inflammatory processes. Functional respiratory changes occur, including alveolar dilation, increased air spaces, reduction in the gas exchange surface and the loss of respiratory tract support tissue. Aging also makes respiratory centers less sensitive, which may explain the deregulation of the respiratory system and organic stress situations, such as infections and exacerbations due to COPD.20
Pulmonary aging associated with cumulative exposure to pollutants, in addition to the development of other morbidities with advancing age, significantly increase risk of mortality.4,21
The highest COPD mortality rates in the cities of Rio de Janeiro and Porto Alegre occur in their respective macroregions. Higher COPD mortality rates in Porto Alegre are possibly related to higher prevalence of regular use of cigarettes by both sexes. This same prevalence is also found in Brazil as a whole. In 2004, this prevalence was 25.2% in Porto Alegre, while in the municipality of Rio de Janeiro it was 17.5% in the same year.22
The state of Rio Grande do Sul is Brazil’s largest leaf tobacco producer. Tobacco is grown on small farms located near to tobacco processing industries. This may have influenced higher tobacco consumption in this population.23 Related to this is the fact that although the population of Porto Alegre was less than one quarter of that of Rio de Janeiro in 2016, the number of deaths due to respiratory diseases in the period is approximately the same in both municipalities.14
Smoking is the main factor related to COPD. Other factors may have influenced the COPD mortality trends and cohort effects observed, such as, for example, changes in lifestyle related to sedentary habits, stress and inadequate diet, changes in health policies associated with improved care for patients with chronic diseases and improved access to health care, changes in population survival and exposure to environmental pollution, in addition to the occurrence of chronic diseases and infectious diseases.21,24 Open access data about environmental pollution available for conducting studies in the two cities are scarce. The improvement in living conditions, resulting from advances in economic, cultural and political development, produces both period and cohort effects, making it more difficult to distinguish the results of each temporal effect.24
The higher COPD mortality rates among males are thought to be related to the history of tobacco consumption, which in principle is a predominantly male behavior.4,5 This situation changed with the rise in female smokers, above all with effect from the second half of the 20th century. Although the prevalence of smoking among adult women appears to be decreasing in several countries, in Brazil this reduction is less pronounced in comparison to men. In particular, the prevalence of young women smokers has increased in such a way that in many places it is now close to that found among men, as is the case in Porto Alegre and Rio de Janeiro.6,22
This change of behavior, influenced by the act of smoking as a status symbol and as a sign of independence in society, as well as being encouraged by tobacco industry marketing, may possibly be reflected in the trend of increasing COPD mortality rates among women in recent cohorts, as seen in Rio de Janeiro, in contrast to the reduction or stabilization of rates among men, thus corroborating the results of several studies worldwide.8,9,25,26
Increased risk of mortality from chronic obstructive pulmonary disease among women in Rio de Janeiro is also in agreement with conclusive studies of greater female susceptibility to early onset of severe COPD, when subject to the same level of exposure to tobacco as men.27 This finding may be revealed not only in the more accentuated decline of female pulmonary function, but also in worse clinical evolution, characterized by greater presence of symptoms such as dyspnea, higher occurrence of exacerbations and reduced quality of life when compared to the same degree of pulmonary impairment among men.28
Studies show that the increase in the risk of developing COPD and severity of the disease in females, in relation to males, can be explained by higher levels of reactivity of the respiratory tract to tobacco smoke and greater biological alterations in pulmonary function over time, in addition to inhalation patterns: women smokers tend to inhale deeply and hold tobacco smoke for longer , thus inducing more severe lesions in the lungs.28,29
Several studies of COPD mortality trends based on APC models used different statistical methods with the aim of solving the problem of non-identifiability, so that each of them generated a set of parameters for specific effects depending on the approach taken.9,24-26 Therefore, caution needs to be taken when comparing estimates, since the linear trends found in this study, when compared to other studies, are not just a feature of the model but also of the method chosen, arbitrarily, to define the trends.17 The complete model APC sought to measure the contribution of each one of these effects.13 In this study, the restriction adopted to solve linear dependence tended to give greater weight to the cohort effect than to the period effect. This choice was based on the cumulative effect of smoking and when it starts, usually during adolescence; and the long COPD latency period, which is reflected in COPD mortality rates several decades, or at least 20 years, after prolonged exposure to smoking.4,5
The reduction in mortality observed in recent generations of men reflects the success of the actions of the National Program for Tobacco Control since 1988, which appeared decades later.7 Declining COPD mortality rates in the male population, associated with a decrease in the prevalence of smoking, are described in several studies, with specific intensity, periods and decreasing proportions according to gender.9,10,24-26 In Brazil, a corresponding generational decrease was found in relation to lung cancer mortality, this also being a disease associated with smoking.30
The reduction in the prevalence of smoking and COPD mortality suggests that the various strategies for tobacco control have been effective, especially for men. However, the increased risk of death due to COPD among women in more recent cohorts in Rio de Janeiro highlights the importance of strengthening these strategies among females.
It is recommended that studies of tobacco consumption trends in Brazil be conducted in order to analyze future impact on mortality, capable of assessing to what extent the prevalence of the habit of smoking is responsible for the magnitude of mortality from chronic obstructive pulmonary disease.
Referências
1. Menezes AM, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005; 366(9500):1875-81. [ Links ]
2. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-71. doi: 10.1016/S0140-6736(14)61682-2. [ Links ]
3. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E,et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5:2020415. doi: 10.7189/ jogh.05-020415. [ Links ]
4. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [ Links ]
5. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4):347-65. DOI: 10.1164/rccm.201204-0596PP. [ Links ]
6. Fundação Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa Nacional de Saúde 2013: percepção do estado de saúde, estilos de vida e doenças crônicas: Brasil, grandes regiões e unidades da federação [Internet]. 2014 [citado 2016 nov 22]. Disponível em: Disponível em: http://www.ibge.gov.br/home/estatistica/populacao/pns/2013/default.shtm [ Links ]
7. Levy D, de Almeida LM, Szklo A. The Brazil SimSmoke policy simulation model: the effect of strong tobacco control policies on smoking prevalence and smoking-attributable deaths in a middle income nation. PLoS Med. 2012;9(11). doi: 10.1371/journal.pmed.1001336. [ Links ]
8. Valente JG, Malta DC. Tendências do tabagismo na população adulta das capitais brasileiras: uma análise dos dados de inquéritos telefônicos de 2006 a 2009. Rev Bras Epidemiol. 2011; 14(Supl 1), S103-14. [ Links ]
9. Ford ES. Trends in mortality from COPD among adults in the United States. Chest. 2015; 148(4):962-70. DOI: 10.1378/ chest.14-2311. [ Links ]
10. Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-Year Trends in Smoking-Related Mortality in the United States. N Engl J Med. 2013; 368(4):351-64. [ Links ]
11. Campos HS. Mortalidade por DPOC no Brasil, 1980-1998. Pulmão RJ. 2003; 12(4):217-25. [ Links ]
12. Graudenz GS, Gazotto GP. Mortality trends due to chronic obstructive pulmonary disease in Brazil. Rev Assoc Med Bras. 2014 mai-jun;60(3):255-61. [ Links ]
13. Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Publ Health. 1991; 12(1):425-57. [ Links ]
14. Ministério da Saúde, Brasil. Estatística Vitais. Departamento de Informática do SUS. Informações de Saúde. [citado 2017 04 set]. Disponível em: Disponível em: http://www2.datasus.gov.br/DATASUS/index.php [ Links ]
15. Instituto Brasileiro de Geografia e Estatística, Brasil [Internet]. Cidades@ [citado 2017 04 set]. Disponível em: Disponível em: http://cidades.ibge.gov.br/xtras/home.php [ Links ]
16. Gadelha AMJ, Leite IC, Valente JG, Schramm JMA, Portela MC, Campos MR. Relatório final do Projeto estimativa da carga de doença do Brasil - 1998. Rio de Janeiro: Fiocruz; 2002. [ Links ]
17. Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007; 26(15):3018-45. [ Links ]
18. Clayton D, Schifflers E. Models for temporal variation in cancer rates I: Age-period and Age-Cohort models. Stat Med. 1987; 6(4):449-67. [ Links ]
19. R Core Team. R: A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing: Vienna, Austria. 2016 [citado 2017 04 set]. Disponível em: Disponível em: https://www.R-project.org/ [ Links ]
20. Miller M. Structural and Physiological Age-Associated Changes in Aging Lungs. Seminars in Respiratory and Critical Care Medicine. 2010; 31(05):521-7. [ Links ]
21. Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J. 2001; 17(5):1024-33. [ Links ]
22. Ministério da Saúde. Inquérito Domiciliar sobre Comportamentos de Risco e Morbidade Referida de Doenças e Agravos não Transmissíveis. Rio de Janeiro: Instituto Nacional de Câncer / Secretaria de Vigilância em Saúde, 2004. [ Links ]
23. Cargnin AP, Bertê AMA, Lemos BO, Oliveira SB. Atlas socioeconômico do Rio Grande do Sul: quinze anos acompanhando as transformações do estado. Geo UERJ. 2014; 2(24) [ Links ]
24. Yang Y. Trends in US adult chronic disease mortality, 1960-1999: Age, period, and cohort variations. Demography. 2008; 45(2):387-416. [ Links ]
25. Janssen F, Kunst AE. Cohort patterns in mortality trends among the elderly in seven European countries, 1950-99. Int J Epidemiol. 2005; 34(5):1149-59. [ Links ]
26. Pham TM, Ozasa K, Kubo T, Fujino Y, Sakata R, Grant EJ, Matsuda S, Yoshimura T. Age-period-cohort analysis of chronic obstructive pulmonary disease mortality in Japan, 1950-2004. J Epidemiol. 2012; 22(4):302-7. [ Links ]
27. Silverman EK, Weiss ST, Drazen JM, et al: Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000; 162:2152. [ Links ]
28. Ohar J, Fromer L, Donohue JF. Reconsidering sex-based stereotypes of COPD. Primary Care Respiratory Journal [Internet]. 2011; 20(4):370-8. Disponível em: http://www.nature.com/articles/pcrj201170 http://www.datasus.gov.br [ Links ]
29. Sin DD, Cohen SB-Z, Day A, Coxson H, Paré PD. Understanding the biological differences in susceptibility to chronic obstructive pulmonary disease between men and women. Proc Am Thorac Soc. 2007; 4(8):671-4. [ Links ]
30. Souza MC, Vasconcelos AG, Cruz OG. Trends in lung cancer mortality in Brazil from the 1980s into the early 21st century: age-period-cohort analysis. Cad Saude Publica. 2012; 28(1):21-30. [ Links ]
Received: August 17, 2017; Accepted: May 10, 2018











 texto en
texto en 
 Curriculum ScienTI
Curriculum ScienTI

