Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.27 no.4 Brasília dez. 2018
http://dx.doi.org/10.5123/s1679-49742018000400001
ORIGINAL ARTICLE
Operational difficulties in the use of insecticidal dog collars for the control of visceral leishmaniasis, municipality of Montes Claros, MG, Brazil, 2012*
1Universidade Federal do Rio de Janeiro, Instituto de Estudos em Saúde Coletiva, Programa de Pós-Graduação em Saúde Coletiva, Rio de Janeiro, RJ, Brasil
2Fundação Instituto Oswaldo Cruz, Instituto Carlos Chagas, Laboratório de Biologia Celular, Curitiba, PR, Brasil
3Universidade Estadual de Montes Claros, Centro de Ciências Biológicas e da Saúde, Departamento de Saúde Mental e Saúde Coletiva, Montes Claros, MG, Brasil
4Secretaria Municipal de Saúde, Centro de Controle de Zoonoses, Programa de Controle das Leishmanioses, Montes Claros, MG, Brasil
Objective:
to describe operational difficulties in the implementation of deltamethrin-impregnated dog collars for the control of visceral leishmaniasis.
Methods:
this was a community intervention trial in the municipality of Montes Claros, MG, Brazil, comparing (i) control area - dogs without dog collars - and (ii) intervention area - use of 4% deltamethrin-impregnated collars; an initial serological survey was performed, followed by three further cycles (at 12, 18 and 24 months).
Results:
out of 4,388 dogs initially seronegative wearing collars, 36.9% were not found in the second cycle, 27.0% of them were lost owing to disappearance/given away/sale, and 22.6% because no one was at home; 56.1% of collars were lost in one year; while among dogs that stayed longer in the study, collar loss was lower.
Conclusion:
high frequencies of collar loss and no one being at home at the time of the visit are operational difficulties for the implementation of a national control program based on the strategy evaluated.
Keywords: Leishmaniasis, Visceral; Leishmania infantum; Dogs; Disease Prevention; Follow-up Studies
Introduction
In the Americas, visceral leishmaniasis (VL) is a disease caused by the protozoa of the Leishmania infantum species, transmitted by phlebotomine sandfly bites, whereby Lutzomyia longipalpis is the species of greateest epidemiological importance. It is noteworthy for its ease of adaptation to urban and peri-urban environments, as well as for the increase in its population density after rainy periods.1-3
The domestic dog has been indicated as the main reservoir host in the urban environment.3 There is evidence of spatial correlation between the incidence of human cases and the prevalence of infection in dogs from urban areas, usually with canine infection preceding human cases.4,5
VL is a neglected tropical disease, despite its importance on the global scenario.3,6 Being labeled as a typical rural disease is already outdated and it cycle has become established in urban and peri-urban areas.7 It is estimated that every year there arebetween 0.2 and 0.4 million VL cases worldwide6 and that, in the year 2015, 90% of these cases were concentrated in just seven countries: India, Kenya, Somalia, Sudan, South Sudan, Ethiopia and Brazil.8
The process of VL urbanization in Brazil began in the 1980s, notably in the cities of Teresina, PI, São Luís, MA, and Montes Claros, MG, culminating with the definitive establishment of LV in medium and large-sized cities.2,5,9
VL control in Brazil, in accordance with Ministry of Health guidelines, is based on three strategies: early diagnosis and treatment of human cases; reduction of the sandfly population; and elimination of domestic host reservoirs.5 However, these measures have shown little effectiveness in containing the spread of the disease in the national territory.10 The removal of infected dogs continues to be a controversial measure, there being insufficient scientific evidence available as to its effectiveness in reducing VL incidence.10,11 Werneck et al.11 highlight that even though the National Program for Visceral Leishmaniasis Surveillance and Control (PVCLV) has existed for more than 40 years, the elimination of dogs on a large scale is not being successful in controlling VL, thus demanding a thorough reassessment of existing strategies. Furthermore, the recommended interventions are facing serious difficulties of a logistic and financial nature for their implementation.2,12 However, there are authors who advocate systematic canine serological screening, followed by euthanasia of positive animals, because they consider this to be a good control strategy, capable of causing a sharp reduction in the prevalence of canine infection and human cases.13,14
In turn, evaluative studies of alternative control measures, such as the use of 4% deltamethrin impregnated dog collars, have demonstrated satisfactory results.15,16 This device, in addition to avoiding sandfly bites, increases the mortality of these insects, thus reducing the circulation of L. infantum in places where dogs are the main reservoir of the parasite.17 It is also a strategy that is easier to implement better accepted by the population, when compared to the elimination of infected dogs.17 However, there are few studies in the country about the effectiveness of this type of intervention. In Andradina, SP, a study showed the effectiveness of dog collars in reducing canine prevalence and incidence of human cases, when associated with existing control measures.18 Another study used simulations to compare the effectiveness of current control strategies and found that performance was better when using impregnated collars rather than euthanasia and vaccination.6,19
Although it has already shown satisfactory results, the mass deployment of deltamethrin-impregnated collars is accompanied by some operational difficulties, which, if not avoided, could undermine the impact of the intervention. Studies have pointed to low coverage and loss of collars as problems to be addressed in order to avoid reduction inthe effectiveness of the action.6,19,15 It is essential to understand the obstacles to the use of these devices as a Public Health control measure, in order to reduce the prevalence of canine visceral leishmaniasis (CVL). The objective of this study was to describe operational difficulties in the implementation of 4% deltamethrin-impregnated dog collars for the control of visceral leishmaniasis.
Methods
This is a community intervention study, performed in the municipality of Montes Claros, Minas Gerais state, Brazil, from August 2012 to January 2015.
Montes Claros has a population of 361,915 inhabitants, predominantly urban (95%), according to the 2010 population census, and is located in the north of the state, 422km from the capital Belo Horizonte.20 The region's climate is hot and dry with predominance of vegetation typical of the cerrado (savanna) and the caatinga (a type of desert vegetation) regions. The local economy is based mainly on beef and dairy cattle, followed by agriculture,21 and average monthly per capita household income was R$568.00 in 2010.20
Montes Claros is an endemic VL area and between 2007 and 2013 there were 168 cases of VL among its residents, 23 of which occurred in 2013, placing the city in 19th position among the Brazilian cities with the largest number of human cases of the disease.22
As shown in Figure 1, the study was based on a comparison between two areas of the municipality with similar environmental and socioeconomic characteristics, with random allocation of two control strategies: (i) control area, without implementation of 4% deltamethrin-impregnated dog collars as a control measure; and (ii) intervention area, where 4% deltamethrin-impregnated dog collars were applied as a control measure. Both areas totaled approximately 30,000 inhabitants and 6,000 dogs. The intervention area encompassed 15 neighborhoods with records of ten human cases of VL between 2009 and 2011. With the same number of neighborhoods, the control area had recorded 12 human cases of VL in the same triennium.
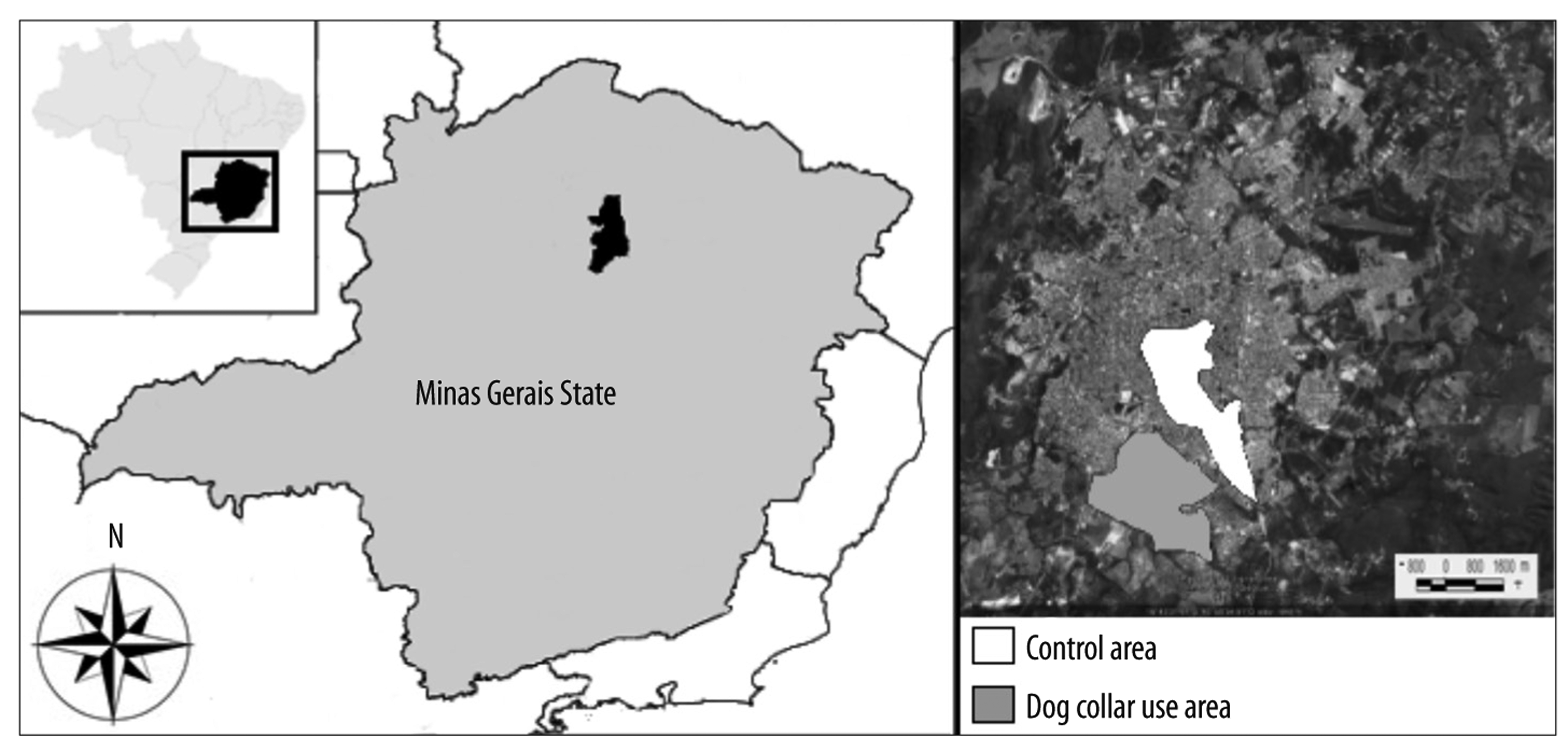
Figura 1 - Areas selected for the community intervention study for the implementation of deltamethrin-impregnated dog collars, Montes Claros, MG, 2012-2015
At the beginning of the study, in September and October 2012, a serological census-type survey of dogs kept on premises was performed in both areas (control and intervention) in order to detect CVL prevalence. Further census surveys were planned for monitoring canine infection in three cycles subsequent to the first survey, at 6, 12 and 18 months, followed by euthanasia of infected dogs. However, the second cycle was only implemented one year after the initial stage, due to operational problems with collar distribution. The monitoring of canine infection (control and intervention areas) and the replacement of collars (intervention area) took place on the basis of census surveys at 12, 18 and 24 months after the initial survey. All dogs in the areas under study were eligible to participate in the surveys, as long as their owners’ permission was obtained.
In the intervention area, 4% deltamethrin-impregnated collars were provided to be put on all dogs participating in the first and following surveys, regardless of their serological condition. In the intervention area, the collars were replaced at each cycle, but were not replaced in the periods between cycles. The company that manufactured the collars committed itself to training the study teams in the correct use of dog collars.
Trained professionals under the supervision of a veterinary surgeon visited the households and, when there was a dog, the asked the owner’s permission for the animal's participation in the project. If they owner agreed, they signed a Free and Informed Consent form.
The first time a dog took part in the study, a form was filled out with the following data: sex (male; female), age (in years), breed (purebred; not purebred) and how long the dog have lived at the household ( up to one year; between one and two years; more than two years). In the intervention area, the collars were put on the dogs. In the subsequent surveys, forms were filled out containing the following information: if the owner had agreed to participate in the study; and if the animal was wearing the collar at the time of the visit (intervention area). At the time of the visit, if there was no one at home, a second attempt was made, on another day and at a different time. New dogs could be incorporated into the study in any of the four cycles, and could also leave the study ; and could also rejoin the study in a later cycle. Seropositive dogs not collected for euthanasia could stay in the study and, in the case of those in the intervention area, they could receive the collar. Collars were not replaced between cycles.
Screening for L. infantum infection involved drawing a blood sample by puncture from the distal ear flap region for on-site performance of an immunochromatographic Dual-Path Platform test (DPP®) (Bio-Manguinhos/Fiocruz, Rio de Janeiro, RJ, Brazil), with the result obtained in approximately 15 minutes. Animals with reactive DPP results underwent cephalic or jugular venipuncture to collect approximately 2mL of blood which was stored in a tube without anticoagulant. The refrigerated samples were transported to the macro-regional laboratory of the Montes Claros Regional Health Superintendency. The serum was separated by centrifugation (at a speed of 3,000 rpm for 10 minutes) and stored at -20ºC until the time the confirmatory serology test was performed. The ELISA (enzyme-linked immunosorbent assay) test, carried out in accordance with the manufacturer's recommendations, was used to confirm positive results for L. infantum infection in serum samples. Dogs were considered to be infected when they had a positive DPP result followed by a reactive ELISA result, in accordance with Ministry of Health recommendations.5
The infected animals were collected and sent for euthanasia which was carried out under the supervision of the municipality’s Center for Zoonosis Control (CZC), according to the ethical norms recommended by the Federal Council of Veterinary Medicine. The procedures for conducting canine serological tests and for the removal of seropositive dogs were performed according to the CZC’s routine, including the use of personal protective equipment and muzzles for dogs. In cases in which the owner of the infected animal refused to send it for euthanasia, the dog was not excluded from the study and could wear a collar (if it belonged to the intervention area) and participate in the subsequent surveys.
Each owner was instructed to identify possible allergic reactions after the collar had been put on the dog. If such reactions did occur, the owner was to get in touch immediately by toll-free telephone with the customer call center service of the company that manufactured the product in order to get guidance from a veterinary surgeon. The household visits occurred between August 2012 and January 2015.
The data were consolidated in tables describing the characteristics of the study areas, participation of dogs in the different cycles, frequency and reasons for loss to follow-up, in addition to the frequency of loss of collars between cycles. We evaluated the differences in the distribution of the characteristics of the dogs according to the area (intervention or control), through the Pearson Qui-squared test (proportions) or Student's t test, (means), with a significance level of 5%.
The project was approved by the Oswaldo Cruz Foundation Ethics Committee on Animal Use (License LW-70/12) and deemed exempt from the need for ethical evaluation for studies in humans by the Ethics Review Committee of the Pan American Health Organization (reference: PAHO-2012-11-0024. As provided for in the study protocol, in the last cycle of visits collars were put on all dogs including those from the control area.
Results
20,477 dogs participated in at least one of the study cycles, 54.2% of which were in the intervention area. More than half of the dogs participated in only one cycle, 17.5% of the dogs took part in two consecutive cycles, and 12.7% in three consecutive cycles (Table 1).
Table 1 - Distribution of the dogs according to participation in cycles and collar use area, Montes Claros, MG, 2012-2015
| Number of Cycles | Participation in cycles | Control | Intervention | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | n | % | n | % | N | % | |
| 1 cycle | X | . | . | . | 1,675 | 17.9 | 1,815 | 16.3 | 10,555 | 51.5 |
| . | X | . | . | 981 | 10.5 | 1,063 | 9.6 | |||
| . | . | X | . | 840 | 9.0 | 1,055 | 9.5 | |||
| . | . | . | X | 1,467 | 15.6 | 1,659 | 14.9 | |||
| 2 cycles | X | X | . | . | 483 | 5.1 | 603 | 5.4 | 4,213 | 20.6 |
| . | X | X | . | 528 | 5.6 | 506 | 4.6 | |||
| . | . | X | X | 584 | 6.2 | 876 | 7.9 | |||
| X | . | X | . | 107 | 1.1 | 94 | 0.9 | |||
| X | . | . | X | 61 | 0.6 | 59 | 0.5 | |||
| . | X | . | X | 140 | 1.5 | 172 | 1.5 | |||
| 3 cycles | X | X | X | . | 477 | 5.1 | 516 | 4.6 | 3,205 | 15.7 |
| . | X | X | X | 717 | 7.7 | 887 | 8.0 | |||
| X | X | . | X | 166 | 1.8 | 200 | 1.8 | |||
| X | . | X | X | 136 | 1.5 | 106 | 1.0 | |||
| 4 cycles | X | X | X | X | 1,009 | 10.8 | 1,495 | 13.5 | 2,504 | 12.2 |
| Total | 9,371 | 100.0 | 11,106 | 100.0 | 20,477 | 100.0 | ||||
Legend:
X = participated in the cycle.
. = did not participate in the cycle.
Of the 9,002 dogs that participated in the original survey, 54.3% were in the intervention area. In the group wearing collars, there was greater presence of short-haired animals (p=0.019), mixed-breed dogs (p<0.001) and having lived for a shorter time at the household (p<0.001). In the control area, the average number of dogs per household was 1.62, this being close to the average number of 1.59 dogs per household (p=0.403) in the intervention area. The areas showed no difference in regard to the mean age of the dogs and proportion of females. The average time interval between the first and second visits was slightly greater in the area without intervention, 357 days (standard deviation = 11), when compared to the corresponding time interval in the area with dogs wearing collars, 349 days (standard deviation=14) (p<0.001). There was no significant difference between the two areas regarding the prevalence of infection in the original survey (p=0.732), with almost 10% of seropositive animals (Table 2).
Table 2 Characteristics of dogs, prevalence of infection and the proportion of seropositive dogs collected by collar use area, Montes Claros, MG, 2012-2015
| Variables | Control | Intervention | P -valuea |
|---|---|---|---|
| Sex | |||
| Female | 2,277 (55.3) | 2,772 (56.7) | 0.194 |
| Male | 1,837 (44.7) | 2,116 (43.3) | |
| Purebred (%) | |||
| No | 2,259 (54.9) | 3,056 (62.5) | <0.001 |
| Yes | 1,855 (45.1) | 1,832 (37.5) | |
| Hair (%) | |||
| Long | 1,246 (30.3) | 1,353 (27.7) | 0.019 |
| Short | 2,866 (69.7) | 3,534 (72.3) | |
| Time living in household in years (%) | |||
| <1 | 855 (20.8) | 1,450 (29.7) | <0.001 |
| 1-2 | 1,163 (28.3) | 1,007 (20.6) | |
| >2 | 2,095 (50.9) | 2,427 (49.7) | |
| Positive dog removed (%) | |||
| No | 102 (25.3) | 103 (21.8) | 0.225 |
| Yes | 301 (74.7) | 369 (78.2) | |
| Infection (%) | 403 (9.9) | 472 (9.7) | 0.732 |
| Mean age in years (SDb) | 3.4 (3.2) | 3.4 (3.0) | 0.673 |
| Average number of dogs per household (SDb) | 1.6 (1.1) | 1.6 (1.0) | 0.403 |
a) Pearson Qui-squared test (categorical variables) or Student's t test, (mean difference for continuous variables).
b) SD: standard deviation.
Of the 875 positive animals resulting from the initial survey, 670 (76.6%) were euthanized by CZC: 301 from the control area (74.7%) and 369 from the intervention area (78.2%) (p=0.225) (Table 3).
Table 3 - Destination of seroreactive dogs in first cycle by area of collar use, Montes Claros, MG, Brazil, 2012-2015
| Seroreactive dogs | Control | Intervention | ||
|---|---|---|---|---|
| n | % | n | % | |
| Removed for euthanasia | 301 | 74.7 | 369 | 78.2 |
| Refusal | 65 | 16.1 | 69 | 14.6 |
| Death | 10 | 2.5 | 23 | 4.9 |
| Disappeared/given away/sold | 3 | 0.7 | 4 | 0.8 |
| Owner moved house | 1 | 0.3 | - | 0.0 |
| Not informed | 23 | 5.7 | 7 | 1.5 |
| Total | 403 | 100.0 | 472 | 100.0 |
As shown in Table 3, out of the 403 seropositive dogs identified in the control area, 102 (25.3%) were not euthanized by CZC for various reasons: primarily because of the owner’s refusal to have their dog put down, or death of the animal before removal for euthanasia. In the intervention area, out of 472 seropositive dogs, 103 (21.8%) were not taken to be put down. Similarly to the control area, the main reason for this was the owner’s refusal to have their dog put down or death of the animal.
39.6% of seronegative dogs comprising the study baseline did not take part in the second cycle; of these, 29.8% did not participate because the owner claimed they had died, and 27.2% because there was no one at home at the time of the visit. In the intervention area, death of the dog - from any cause - was the most frequent reason for its absence from the study in the second cycle (30.1%); followed by 27.0% of cases in which the owner claimed that the dog had disappeared, had been given away or sold. While in the control area, no one being at home was the main reason for the discontinuity in the dog's participation in the project (31.8%), followed by the death of the animal (29.5%) (Table 4).
Table 4 - Loss of follow-up of seronegative dogs between the first and second cycles, Montes Claros, MG, 2012-2015
| Loss of seronegative dogs | Control | Intervention | ||
|---|---|---|---|---|
| n | % | n | % | |
| No one at home | 498 | 31.8 | 367 | 22.6 |
| Death | 462 | 29.5 | 488 | 30.1 |
| Disappeared/given away/sold | 314 | 20.1 | 437 | 27.0 |
| Owner moved house | 166 | 10.6 | 226 | 13.9 |
| Refusal | 52 | 3.3 | 30 | 1.9 |
| Removed for another reason | 10 | 0.6 | 10 | 0.6 |
| Hard to control | 6 | 0.4 | 10 | 0.6 |
| Other | 6 | 0.4 | 14 | 0.9 |
| Not informed | 51 | 3.3 | 39 | 2.4 |
| Total | 1,565 | 100.0 | 1,621 | 100.0 |
In the first cycle, 99.9% of the dogs in the intervention area wore a collar. Even after an interval of approximately one year, collar loss between the first and second cycles of the study was 56.1%. This is only slightly higher than the losses of 51.2% in the consecutive. Collar loss between the first two (consecutive) assessments of dogs was 54.0%; between the second and the third it was 40.8%; and between the third and fourth it was 35.3%. Collar loss between the first two cycles was higher in dogs aged less than or equal to 1 year (65.7%), when compared to those over the age of one year (53.5%) (p<0.001).
Discussion
Based on a community intervention study, this article describes operational difficulties in implementing 4% deltamethrin-impregnated dog collars to control visceral leishmaniasis. Standing out among the main issues identified are the high frequencies of collar loss and no one being at home on the occasion of the visit made by health professionals. These difficulties are central aspects to be considered, in the event of implementing this type of intervention on a large scale in Brazil.
76.6% of the animals found to be infected in the first cycle were euthanized. This data is consistent with the results of other studies that also identified euthanasia as the main reason for loss to follow-up of seropositive dogs.23-25 In addition to euthanasia, Morais23 pointed to claims of the animal dying from other causes, this being the case of 5.2% of seropositive dogs in that study, as another reason for the dog being missing from the place where it lived. This rate was higher than that found in our study (2.5% in the control area and 4.9% in the intervention area).
Of the 4,053 dogs that left the study after the first cycle, 78.6% were seronegative. The claim that the animal had died was the most frequent reason for uninfected dogs (29.8%) leaving the study. This rate is slightly lower than the 34% identified by Lopes in the municipality of Juatuba, MG,24 also in an interval of one year. After a 16-month interval, Coura-Vital26 reported loss by death among seronegative dogs of approximately 6%.
Finding no one at home was the main reason for loss to follow-up of seronegative dogs in the control area (31.8%) and the third most frequent reason in the intervention area (22.6%). This is an important limitation for dog collar implementation. After a 16-month interval, Coura-Vital26 obtained a lower percentage of loss for this reason (19%), probably because unoccupied households were visited by three times before being considered lost. It is necessary to rethink the visiting strategy, in a manner that takes into account that dwellers may be away from home during business hours; otherwise, they will remain on the sidelines of recommended control actions.
It should be noted that the loss ratio in this study was described based on the dog owner's account. Part of these claims may be subterfuge to stop taking part in the research. This unspoken refusal could be attributed the fact of blood samples being taken from apparently healthy animals, causing the owner to anticipate the possibility of a positive test result and potential need for euthanasia.
Collar loss was 56% when considering only the first two (consecutive) time dog collars were put on, regardless of the cycle in which the animal had joined the study. This rate was higher than the 41% found by Reithinger et al.15 with follow-up after just five months . Foglia-Manzillo et al.27 reported 30% collar loss in one year, although the dogs under study were restricted to kennels. Monitoring of dogs domiciled in the municipality of Andradina, SP, found much lower loss percentages with half-yearly assessments, namely 5.5% loss at six months and 6.4% at twelve months.18 Reithinger et al.15 listed some reasons, reported by owners, for the loss of collars: the fact of the dog managing to undo the collar buckle shortly after application; the collar itself becoming worn; and adverse events, such as skin irritation. It is worth mentioning that lower losses were recorded as the time spent by the dog in the study increased. Data suggest that the passing of time favors a better adaptation by the animal to the collar. This is a particularly advantageous aspect for a future control program using deltamethrin collars. Strategies need to be discussed regarding more rapid replacement of collars, as large-scale collar losses may impair the performance of this measure. With regard to reactions to collar use, only 74 dogs (0.7%) had collar allergy reported and in 14.8% of these cases, allergy was reported on the occasion of the first visit, even before the collar had been used.
The impact of the use of collars on CVL transmission depends on coverage rates (ideally, above 90%)6,19 and collar loss,15 which implies timely collar replacement dogs new to the location also having collars put on them. In addition to the high percentage of lost collars, one of the difficulties capable of compromising the effectiveness of the intervention was the fact that more than 2,600 dogs joined the study at the second cycle in the intervention area (almost half of the animals that participated in this cycle). This means that part of those dogs could already have been living in this area and would have been without a collar until the second cycle began. 46% of the dogs in the intervention joining the study at the second stage had been living there for at least a year.
Although no difference in infection prevalence in the two areas studied - control and intervention - was found in the initial survey, suggesting similar strength of transmission, significant variation was identified for some variables: breed, type of hair and length of residence. These differences should be considered when assessing the effectiveness of collar use, because by independently influencing individual risk of CVL, they could lead to errors in the estimates of effectiveness.
When this study was initially planned, the use of collars was to have been assessed in conjunction with the euthanasia of positive dogs and spraying of the home with insecticide. However, spraying occurred in approximately 1% of households, thus making it infeasible to include it in the analysis. The use of insecticide in combating sandflies reduces the risk of seroconversion in dogs from endemic areas and is a feasible measure for disease control.28 Barata et al.,29 in a study conducted in the municipality of Governador Valadares, MG, identified an increase of canine seropositivity in areas with higher density of L. longipalpis. This finding points to the need for more rigorous implementation of control measures, including the use of residual insecticides.
The main aim of this work was to describe a reference panorama for the incorporation of collars in the routine of visceral leishmaniasis control services, based on existing municipal infrastructure, identifying the real difficulties of their adoption, while taking into account that the efficacy of collars in preventing sandfly bites among dogs has already been demonstrated in other studies.17,30 Camargo-Neves et al.18 stress that the implementation of a VL control program, whether or not it uses this new tool, depends on the municipality creating structure in this sense and the strict planning of actions. David et al.17 emphasize the field work allows one to determine the effectiveness of the collar in the dogs’ natural habitat. However, in order to be effective, the measure must result in a large reduction in the incidence of canine infection in endemic areas.
An additional question to be considered in relation to our study refers to the six-monthly periodicity of the surveys, unlike the annual interval recommended by the National Program for Visceral Leishmaniasis Surveillance and Control - PVCLV.5 Given that the collar validity period between six and eight months, the logistics in the implementation of control measures would be more complex than the logistics used in our study: visits should occur more frequently, but this implies increased cost of the program. Considering the importance of maintaining a high level of collar use, it would be fundamental to identify feasible strategies for replacing lost collars.
Nevertheless, the operational difficulties and limitations identified in this study, notably the high frequencies of collar loss and unattended households on the occasion of the visits, can be overcome in the event of PVCLV adopting the use of collars as a visceral leishmaniasis control measure in Brazil.
REFERENCES
1. Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Mem Inst Oswaldo Cruz. 2005 Dec;100(8):811-27. [ Links ]
2. Harhay MO, Olliaro PL, Costa DL, Costa CH. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 2011 Sep;27(9):403-9. [ Links ]
3. World Health Organization. Control of the Leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases [Internet]. Geneva: World Health Organization; 2010 [cited 2017 Sep 26]. Available from: Available from: http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf?ua=1 [ Links ]
4. Oliveira CL, Assunção RM, Reis IA, Proietti FA. Spatial distribution of human and canine visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994-1997. Cad Saúde Pública. 2001 Sep-Oct;17(5):1231-9. [ Links ]
5. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância e controle da leishmaniose visceral [Internet]. Brasília: Ministério da Saúde; 2006 [citado 2018 jun 21]. 120 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral.pdf [ Links ]
6. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012 May;7(5):e35671. [ Links ]
7. Dantas-Torres F, Brandão-Filho SP. Visceral leishmaniasis in Brazil: revisiting paradigms of epidemiology and control. Rev Inst Med Trop. 2006 May-Jun; 48(3):151-6. [ Links ]
8. World Health Organization. Global health observatory (GHO) data. Leishmaniasis, 2015. [Internet]. 2015 [cited 2017 Sep 26]. Available from: Available from: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ [ Links ]
9. Maia-Elkhoury ANS, Alves WA, Sousa-Gomes ML, Sena JM, Luna EA. Visceral leishmaniasis in Brazil: trends and challenges. Cad Saúde Pública . 2008 Dec;24(12):2941-7. [ Links ]
10. Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America - a systematic review. PLoS Negl Trop Dis. 2010 Jan;4(1):e584. [ Links ]
11. Werneck GL, Costa CHC, Carvalho FAA, Pires e Cruz MS, Maguire JH, Castro MC. Effectiveness of insecticide spraying and culling of dogs on the incidence of Leishmania infantum infection in humans: a cluster randomized trial in Teresina, Brazil. PLoS Negl Trop Dis. 2014 Oct 30;8(10):e3172. [ Links ]
12. Zuben APB, Donalísio MR. Dificuldades na execução das diretrizes do Programa de Vigilância e Controle da Leishmaniose Visceral em grandes municípios brasileiros. Cad Saúde Pública. 2016 jun;32(6):e00087415. [ Links ]
13. Ashford DA, David JR, Freire M, David R, Sherlock I, Eulalio MC, et al. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyg. 1998 Jul;59(1):53-7. [ Links ]
14. Palatnik-de-Sousa CB, Santos WR, França-Silva JC, Costa RT, Reis AB, Palatnik M, et al. Impacto of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am J Trop Med Hyg. 2001 Nov;65(5):510-7. [ Links ]
15. Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impregnated dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil? Int J Parasitol. 2004 Jan;34(1):55-62. [ Links ]
16. Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002 Aug 3;360(9330):374-9. [ Links ]
17. David JR, Stamm LM, Bezerra HS, Souza RN, Killick-Kendrick R, Lima JW. Deltamethrin-impregnated dog collars have a potent anti-feeding and insecticidal effect on Lutzomyia longipalpis and Lutzomyia migonei. Mem Inst Oswaldo Cruz. 2001 Aug;96(6):839-47. [ Links ]
18. Camargo-Neves VLF, Rodas LAC, Pauliquevis Junior C. Avaliação da efetividade da utilização de coleiras impregnadas com deltametrina a 4% para o controle da leishmaniose visceral americana no Estado de São Paulo: resultados preliminares. Boletim Epidemiol Paulista. 2004 dez;1(12):7-14. [ Links ]
19. Sevá AP, Ovallos FG, Amaku M, Carrillo E, Moreno J, Galati EAB, et al. Canine-based strategies for prevention and control of visceral leishmaniasis in Brazil. PLos One. 2016 Jul;11(7):e0160058. [ Links ]
20. Instituto Brasileiro de Geografia e Estatística. Cidades, Montes Claros [Internet]. 2010 [citado 2017 set 26]. Disponível em: Disponível em: https://cidades.ibge.gov.br/brasil/mg/montes-claros/panorama [ Links ]
21. Prefeitura de Montes Claros (MG). Aspectos gerais [Internet]. 2017 [citado 2017 set 26]. Disponível em: Disponível em: http://www.montesclaros.mg.gov.br/cidade/aspectos_gerais.htm [ Links ]
22. Ministério da Saúde. Secretaria de Vigilância em Saúde. Datasus. Leishmaniose visceral [Internet]. 2015. [citado 2017 set 26]. Disponível em: Disponível em: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/leishvbr.def [ Links ]
23. Morais MHF. Avaliação das atividades de controle da leishmaniose visceral na Regional Noroeste de Belo Horizonte, 2006 a 2010 [tese]. Belo Horizonte (MG): Universidade Federal de Minas Gerais; 2011. [ Links ]
24. Lopes EGP. Dinâmica da transmissão da leishmaniose visceral em uma coorte de cães em Juatuba-MG, de 2010 a 2011 [tese]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, 2013. [ Links ]
25. Gonçalves SA. Controle do reservatório canino para leishmaniose visceral, na regional noroeste de Belo Horizonte, Minas Gerais, 2006-2011 [dissertação]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, 2013. [ Links ]
26. Coura-Vital W. Estudo epidemiológico prospectivo em cães assintomáticos infectados por Leishmania (Leishmania) infantum e identificação de biomarcadores de infecção, 2011 [tese]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, 2011. [ Links ]
27. Foglia Manzillo V, Oliva G, Pagano A, Manna L, Maroli M, Gradoni L. Deltamethrin-impregnated collars for the control of canine leishmaniasis: evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Vet Parasitol. 2006 Nov;142(1-2):142-5. [ Links ]
28. Coura-Vital W, Reis AB, Fausto MA, Leal GG, Marques MJ, Veloso VM, et al. Risk factors for seroconversion by Leishmania infantum in a cohort of dogs from an endemic area of Brazil. PLoS One. 2013 Aug;8(8):e71833. [ Links ]
29. Barata RA, Peixoto JC, Tanure A, Gomes ME, Apolinário EC, Bodevan EC, et al. Epidemiology of visceral leishmaniasis in a reemerging focus of intense transmission in Minas Gerais State, Brazil. Biomed Res Int. 2013 Aug;2013:405083. [ Links ]
30. Killick-Kendrick R, Killick-Kendrick M, Focheux C, Dereure J, Puech MP, Cadiergues MC. Protection of dogs from bites of phlebotomine sandflies by deltamethrin collars for control of canine leishmaniasis. Med Vet Entomol. 1997 Apr;11(2):105-11. [ Links ]
*This article is derived from the Doctoral thesis entitled 'Assessment of the effectiveness of the use of insecticidal dog collars for the control of visceral leishmaniasis, municipality of Montes Claros, MG, Brazil", defended in 2016 by Erika Barretto Alves at the Postgraduate Program in Collective Health, Federal University of Rio de Janeiro (UFRJ). The research project was funded by the Brazilian Ministry of Health (Agreement No. 01234/2010) and by the Pan American Health Organization (Agreement Letter BR/LOA/1300025.001). To carry out the research, the author Guilherme Loureiro Werneck received a productivity scholarship from the National Council for Scientific and Technological Development (CNPq: Process No. 311507/2014-0.).
Received: January 02, 2017; Accepted: May 28, 2018











 texto em
texto em 


