Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.28 no.2 Brasília jun. 2019 Epub 27-Jun-2019
http://dx.doi.org/10.5123/s1679-49742019000200001
ORIGINAL ARTICLE
Human rabies in Brazil: a descriptive study, 2000-2017*
1Ministério da Saúde, Coordenação-Geral de Doenças Transmissíveis, Brasília, DF, Brasil
2Universidade de Brasília, Faculdade de Agronomia e Veterinária, Brasília, DF, Brasil
3Universidade de Brasília, Departamento de Saúde Coletiva, Brasília, DF, Brasil
Methods:
this is a descriptive study of human rabies cases reported in 2000-2017, with an estimate of incidence and spatial distribution.
Results:
188 cases were studied, mostly males (66.5%), rural residents (67.0%), children under 15 years (49.6%), with biting being the most frequent form of exposure (81.9%); frequency was highest in the period 2000-2008 (85.6%), with 46.6% of cases involving dogs and 45.9% bats; median incubation was 50 days, followed by, predominantly, symptoms of fever (92.6%), agitation (85.2%), paresthesia (66.7%), and dysphagia/paralysis (51.9%); the majority (70.2%) did not have prophylaxis and for the rest (29.8%) who did have prophylaxis, it was untimely and/or incomplete; 13 patients were treated according to the Recife Protocol, and two survived.
Conclusion:
human rabies incidence reduced and its epidemiological profile changed, with predominance of cases transmitted by bats; we suggest that secondary cases be investigated, and that pre-exposure prophylaxis be made available to populations at greater risk of accidents involving bats.
Keywords: Rabies; Rabies Virus; Epidemiology, Descriptive; Public Health; Public Health Surveillance
Introduction
Rabies is present in over 150 countries and approximately 59,000 people affected by this infectious disease die every year worldwide, especially in Asia and Africa.1 In Latin America, between 2013 and 2016, the incidence of human rabies transmitted by dogs reduced: cases were reported in eight countries of the subcontinent, with transmission by bats being more common.2,3 In Brazil, between 1990 and 2017, 594 cases were registered, mainly in urban environments, due to type 2 canine antigenic variants (AgV).4 In the period from 2000 to 2009, cases of human rabies transmitted by bats increased in the Brazilian territory.5
In 2009, the Pan American Health Organization (PAHO) became responsible for assisting Latin America and Caribbean countries in eliminating neglected poverty-related diseases, with rabies transmitted by dogs being selected as one of its priorities.6
Between 2013 and 2016, rabies transmitted by dogs was reported in Bolivia, Brazil, Dominican Republic, Guatemala, Haiti, Honduras, Peru and Venezuela. In 2016, 10 cases were reported: eight in Haiti and two in Guatemala. This reduction at the continental level probably happened because of dog vaccination on a large scale, greater technical cooperation between the Brazilian ministries of Health and Agriculture, and improvement in access to pre- and post-exposure prophylaxis. Nevertheless, the disease is still present in some geographic areas. The Meeting of Rabies Program Directors in the Americas usually takes place every two years. This meeting discusses and assists decision-making in the management of rabies programs in the countries involved, with the aim of eliminating rabies transmitted by dogs in the region by 2022.7
The National Program for Human Rabies Prophylaxis, established in Brazil in 1973, has reduced human and canine rabies cases, especially because of the effectiveness of canine vaccination campaigns. However, human rabies cases transmitted by animals in the sylvatic cycle, such as bats, bush dogs, foxes and non-human primates draw attention and show a change in the infection’s epidemiological profile.5
Given this scenario, this study aimed to describe the epidemiological profile of human rabies in Brazil, from 2000 to 2017.
Methods
We carried out a retrospective descriptive observational case series study, focused on human rabies cases reported in Brazil in the period from 2000 to 2017. Rabies cases confirmed either by laboratory or clinical-epidemiological criteria were included in the study.
The following variables were analyzed:
a) Sociodemographic
- sex (male; female);
- age group (in years: less than 1; 1-4; 5-9; 10-14; 15-19; 20-34; 35-49; 50-64; 65 and more);
- Federative Unit of residence;
- municipality of residence; and
- area of residence (rural, urban).
b) Epidemiological history
- incubation period;
- type of exposure to the rabies virus;
- site of wound;
- rabies prophylaxis history;
- prophylaxis completeness; and
- attacking animal species.
c) Health care and diagnosis
- hospitalization;
- hospitalization period;
- signs and symptoms;
- laboratory diagnosis; and
- viral variant typification.
Data sources used were the Notifiable Diseases Information System (SINAN) and notifications made by the Rabies Technical Group of the Ministry of Health’s Zoonoses Surveillance Technical Unit. Access to the corrected and non-nominal Health Ministry database was possible thanks to the Access to Information Act.
The incubation period was calculated by the difference between the date of the attack and the date of onset of symptoms. Duration of hospitalization was calculated by the difference between the date of patient death or hospital discharge and their hospital admission date.
The incidence coefficient was calculated as follows: numerator (i) the quotient of the division of the study period into three equal periods of six years (2000-2005; 2006-2011; and 2011-2017); denominator (ii) the general population, calculated based on the average Brazilian population between each period’s two-year midpoint (estimated by the Brazilian Institute of Geography and Statistics - IBGE). This study considered the Brazilian Legal Amazon to be the region defined by IBGE, comprised of the states of Acre, Amapá, Amazonas, Mato Grosso, Pará, Rondônia, Roraima, Tocantins, part of Maranhão and five municipalities of Goiás.
We carried out descriptive statistics: calculation of simple and relative frequencies, measures of central tendency (mean and median) and dispersion (amplitude, standard deviation and quartile). Normality tests, such as the Kolmgorov-Smirnoff test, were not used because we did not aim to test differences between means since this is a descriptive study. The statistical software used were TabWin 32, Epi Info 7.1 and Microsoft Excel 2010.
The study project was exempt from appraisal by a Research Ethics Committee, in accordance with National Health Council Resolution No. 510, dated 7 April 2016.
Results
Out of 188 total cases of human rabies that occurred in Brazil, 125 (66.5%) were male and 126 (67.0%) lived in rural areas; age ranged from 1 to 82 years old (mean = 20.9; standard deviation = 17.1 and median = 14.5 years old; 1st quartile = 7 and 3rd quartile = 30). The under 15 years old age group was the most affected, accounting for 49.6% of the total. Animal bites were the most reported type of exposure (N=154; 81.9%), attacks on multiple parts of the body were predominant (N=40; 21.2%), followed by feet (N=38; 20.2%) and hands (32; 17.0%). Only one case reported infection by indirect contact (Table 1).
Table 1 - Sociodemographic variables distribution, types of exposure and site of wound recorded in human rabies cases (N=188), Brazil, 2000-2017
| Variables | n | % |
|---|---|---|
| Age group (in years) | ||
| <1 | - | - |
| 1-4 | 25 | 13.0 |
| 5-9 | 33 | 17.6 |
| 10-14 | 36 | 19.0 |
| 15-19 | 16 | 8.6 |
| 20-34 | 37 | 19.8 |
| 35-49 | 24 | 13.0 |
| 50-64 | 15 | 8.0 |
| >64 | 2 | 1.0 |
| Sex | ||
| Male | 125 | 66.5 |
| Female | 63 | 33.5 |
| Area of residence | ||
| Rural | 126 | 67.0 |
| Urban | 62 | 33.0 |
| Exposure | ||
| Biting | 154 | 81.9 |
| Scratching | 12 | 6.4 |
| Licking | 6 | 3.2 |
| Biting/scratch | 6 | 3.2 |
| Indirect Contact | 1 | 0.5 |
| Information missing | 8 | 4.3 |
| Unknown | 1 | 0.5 |
| Site of wound | ||
| Multiple locations | 40 | 21.2 |
| Feet | 38 | 20.2 |
| Hands | 32 | 17.0 |
| Lower limbs | 27 | 14.4 |
| Head | 21 | 11.2 |
| Upper limbs | 15 | 8.0 |
| Torso | 3 | 1.6 |
| Mucous membrane | 2 | 1.1 |
| Information missing | 10 | 5.3 |
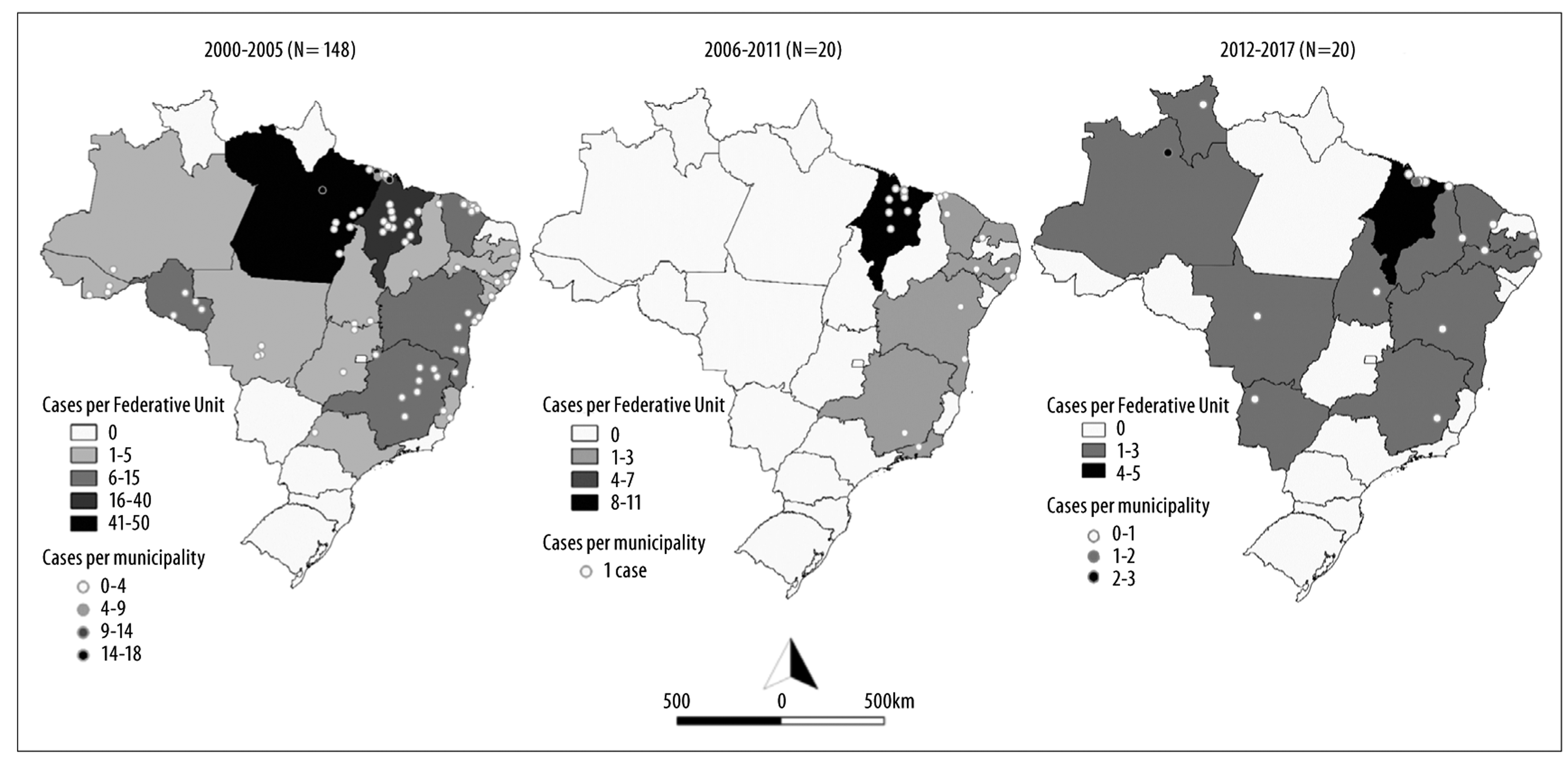
Regarding region of notification within Brazil, the Northeast predominated (N=102; 55.0%), followed by the Northern region (N=66; 34.0%). Most of the cases were in the states of Maranhão (N=55; 30.0%), Pará (N=45; 24.0%) and Ceará (N=17; 9.0%) (Figure 1). There were 68 cases (36.2%) in the Legal Amazon, all of which were in rural areas and transmitted by bats. In 2017, a rabies outbreak occurred in the Tapira riverside community, on the banks of the Unini River in the municipality of Barcelos, state of Amazonas, where three brothers under 18 years old were infected: they were residents of the rural area (extractive reserve) of the municipality, with a history of exposure to bats.
Four cases of human rabies transmitted by dogs occurred in border regions: Assis Brasil in the state of Acre in 2000; São Francisco do Pará in the state of Rondônia in 2001; Xapuri in the state of Acre in 2004; and Corumbá in the state of Mato Grosso do Sul in 2015. All four municipalities are on the border with Bolivia, while Assis Brasil in particular borders with both Bolivia and Peru.
The majority of cases (N=161; 85.6%) occurred between 2000 and 2008. Of these, 75 (46.6%) involved dogs and 74 (45.9%) bats. Sixty-three (85.1%) cases of infection by bats occurred between 2004 and 2005 and were related to three outbreaks. Between 2009 and 2017, there were 27 cases (14.4%), comprised of 11 (40.7%) cases infected by dogs, eight by bats (29.6%) and four by monkeys (14.8%); and three by cats (11.1%) with the AgV3 variant virus compatible with that found in vampire bats. In 2017, a human rabies outbreak caused by bats struck three people: two of them did not have rabies prophylaxis and died; in the third case, the patient took rabies serum and three doses of vaccine approximately 90 days after exposure. This patient was treated according to the Recife Protocol and survived with severe neurological sequelae (Figure 2).
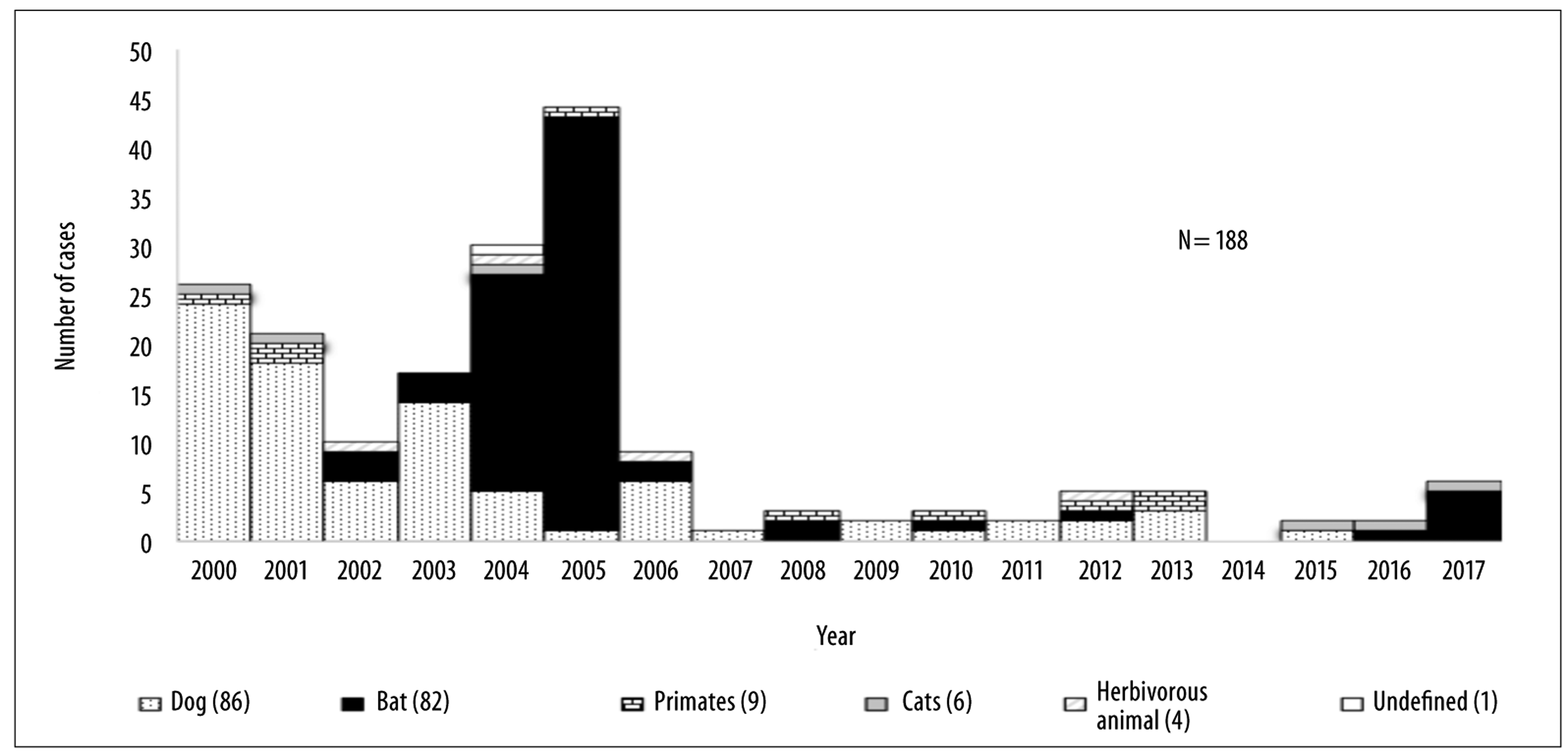
Figure 2 - Notified human rabies case distribution (N=188), per animal species involved and year, Brazil, 2000-2017
The incidence coefficients for the three periods analyzed were: for the period 2000-2005, 0.842 rabies cases/100,000 inhabitants; 2006-2011, 0.0105/100,000 inhab.; and 2012-2017, 0.0098/100,000 inhab. (Figure 3).
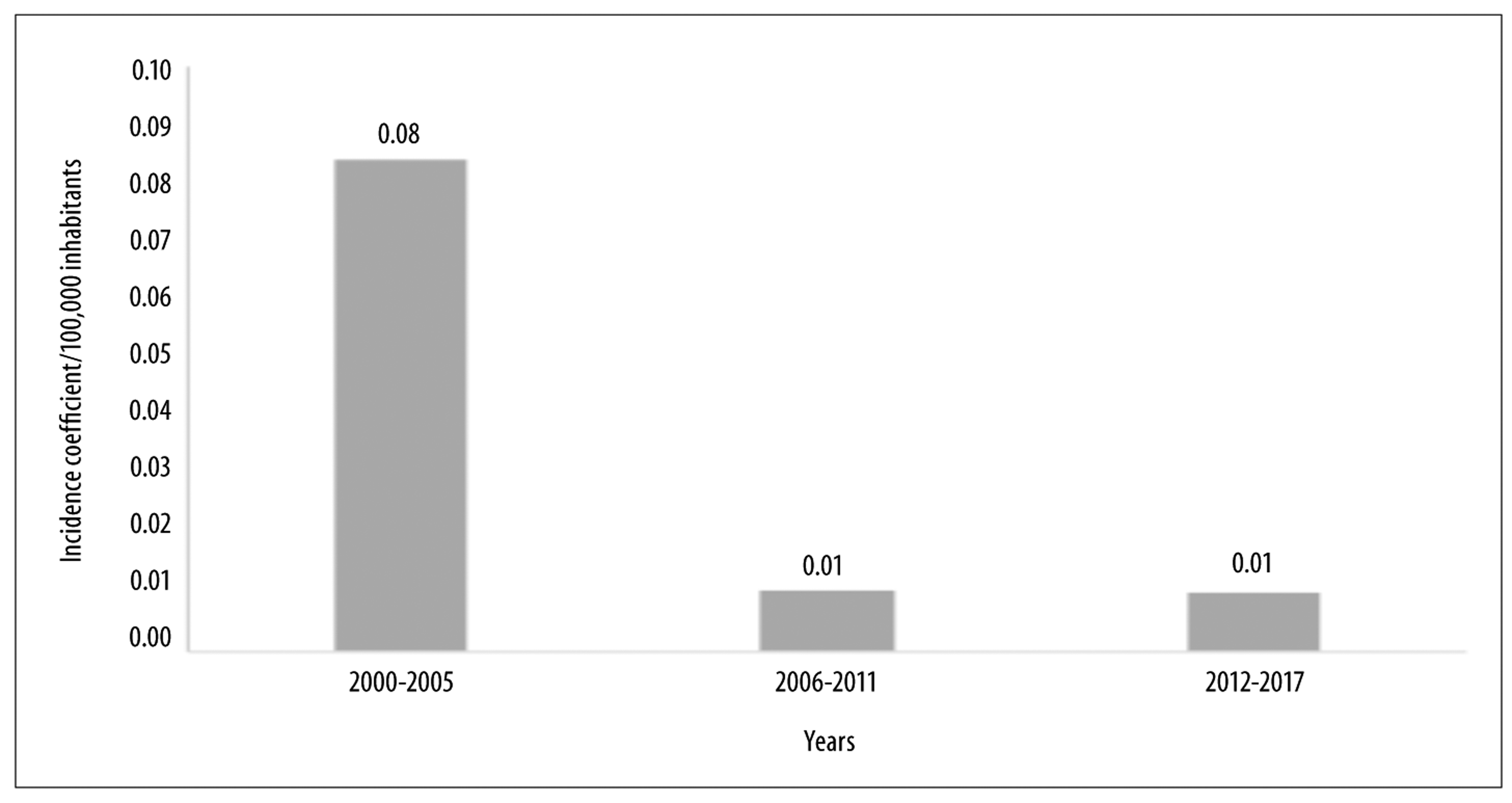
Figure 3 - Incidence coefficient (per 100,000 inhabitants) of notified human rabies cases, Brazil, 2000-2017
The human rabies signs and symptoms predominating in Brazil were fever (92.6%), agitation (85.2%), paresthesia (66.7%) and dysphagia/paralysis (51.9%) (Table 2). Median incubation time was 50 days (minimum = 11; maximum = 290); infection transmitted by dogs had a median of 57 incubation days (minimum = 11; maximum = 290), while for infection by bats it was 39 days (minimum = 16; maximum = 244).
Table 2 - Notified human rabies case distribution (N=188) according to diagnosis type, viral variant and signs and symptoms, Brazil, 2000-2017
| Variables | n | % |
|---|---|---|
| Diagnosis type | ||
| Laboratory tests | 150 | 79.8 |
| Clinical-epidemiological | 20 | 10.6 |
| Clinical | 18 | 9.6 |
| Antigenic variant(AgV)a | ||
| AgV3 (Desmodus rotundus) | 27 | 59.0 |
| AgV2 (Canis lupus) | 15 | 32.6 |
| AgVnC (Callithrix jacchus) | 3 | 6.5 |
| AgV1 (Canis lupus) | 1 | 2.2 |
| Signs and symptomsb | 3 | 6,5 |
| Fever | 25 | 92.6 |
| Agitation | 23 | 85.2 |
| Paresthesia | 18 | 66.7 |
| Dysphagia/paralysis | 14 | 51.9 |
| Hydrophobia | 9 | 33.3 |
| Aerophobia | 6 | 22.2 |
| Vomiting | 6 | 22.2 |
| Difficulty walking | 5 | 18.5 |
| Sialorrhea | 5 | 18.5 |
| Headache | 5 | 18.5 |
| Local pain | 4 | 14.8 |
| Hallucination | 3 | 11.1 |
| Photophobia | 2 | 7.4 |
a) 46 cases with viral variant typification.
b) More than one symptom per person may be recorded; signs and symptoms were only available with effect from notifications made in 2007; these data relate to 30 cases.
Of the total number of cases, 150 (79.8%) were confirmed by laboratory criteria; in 46 (24.0%) of them it was possible to identify the viral variant, mainly with effect from 2005. The most detected virus variant was Agv3 from the Desmodus rotundus bat, present in 27 cases (59.0%), including three cases transmitted by cats. Median hospital stay was 6.5 days (minimum = 1; maximum = 120).
One hundred and thirty-two (70.2%) rabies case patients did not have prophylaxis and 56 (29.8%) had inappropriate and/or incomplete prophylactic treatment. The time elapsed before seeking the first rabies vaccine dose was, on average, 44 days after the attack (standard deviation: 37 days). As of 2008, 13 rabies cases were treated in Brazil and the Recife Protocol was used for all of them: two patients undergoing treatment survived with neurological sequelae, resulting in a success rate of 2/13.
Discussion
Human rabies incidence reduced between 2006 and 2017, although cases involving bats still occurred. The North and Northeast regions accounted for the majority of the cases, most frequently among men, children and adolescents living in rural areas. Biting was the most frequent form of exposure, with wounds in multiple parts of the body. Wild animals corresponded to approximately half of rabies transmission to humans. The occurrence of cases in humans in border regions is an important indicator for continuing to vaccinate dogs and cats against rabies as a form of prevention.
This study has information bias limitations, given that it is based on secondary data sources and this can lead to inaccurate estimates. Data relating to signs and symptoms were available as of 2007; the previous information system reporting form did not include this variable.
The results of this study converge with those of studies described in Ecuador in 2016,8 and in Peru in 2009,9 in that they indicate reduction of urban human rabies cases, in addition to reporting cases related to bats. The challenges to dealing with rabies impacts are evident when one looks at the results of studies conducted in India in 2016,10 in China in 2013,11 and in South Africa in 2011,12 in view of the difficulties which their authors report in controlling the disease even when there is continuous incidence reduction.
We observed a higher frequency of rabies cases in children and adolescents, and in males, resembling the profile reported in India in 2017,10 and in the United States in 1998,13 where children and men seem to be more exposed to the rabies virus, probably because of more contact with domestic animals. However, these data differ from a study conducted in Sri Lanka in 201314 which found median age above 40 years old, possibly related to risk factor aspects in local settings, such as the priority given to prophylaxis in children/adolescents and health education in schools.
In our study, cases in rural areas predominated, probably because of outbreaks with exposure to bats in the Brazilian Amazon in 2006 and 2009,15,16 as also reported in an outbreak in Peru in 2009.9
The reduction of cases of human rabies transmitted by dogs in Brazil is in line with the efforts made by Latin America countries to control this.7,17 On the other hand, continuing cases of rabies transmitted by dogs, especially in Asia and Africa, reveal the difficulties and challenges for health programs to succeed,10,11 whereby effective canine rabies vaccination coverage and timely and complete post-exposure prophylaxis are fundamental for reducing the incidence of the disease.16,17
The predominant signs and symptoms we found were similar to those reported in a study conducted in 2008-2009 in the Congo with 21 patients;18 but differed from research done between 1983-2009 in South Africa with 353 cases,12 and a study in 1980-1996 in the United States with 32 participants.13 According to both these studies, hydrophobia and sialorrhea were more frequent. It would be important for future studies to check whether there is a relationship between signs and symptoms and the viral variant.
The incubation period we found was longer than the one presented in a study conducted in India in 2011,10 similar to the one in South Africa in 2011 (54 days),12 and shorter than the one in the United States in 1998 (84 days).13
The majority of cases were confirmed by laboratory criteria; a quarter of these cases had a viral variant typified, more so in the last ten years, thus showing improved laboratory support; nevertheless, we suggest that further efforts to improve the strengthening of laboratory surveillance in Brazil should be made. Viral variant characterization is important for better rabies epidemiological knowledge.3 In addition, environmental monitoring is a surveillance tool that contributes to understanding animal rabies, mainly in secondary cases, such as dogs and cats infected with a bat variant of rabies, which can help in decision-making for disease control, intensifying passive surveillance of bats and monitoring of contact dogs and cats.
With effect from 2005, experimental treatment of rabies has taken place worldwide based on the Milwaukee Protocol.3.19 In Brazil this has been adopted via the Recife Protocol.19 The first human cure achieved in this way in Brazil happened in 2008, in the state of Pernambuco.20.21 In view of experiences like this, or the successful treatment of a case in the state of Amazonas in 2017, it is important to discuss improvements to Brazil’s protocol. Notwithstanding, adequate prevention and timely and complete prophylaxis continue to be the main strategies to be used. Alongside this the following continue to be essential actions: (i) canine vaccination campaigns in the states where they are held annually, and (ii) the environmental monitoring of variants, as an instrument of epidemiological surveillance and the actions thereof.3
Human rabies incidence reduced in Brazil in the period studied. Wild animals, especially bats, accounted for almost half of the cases. More investigation of human rabies cases via secondary transmission is necessary. The viability of pre-exposure prophylaxis in populations at higher risk of accidents involving bats should be evaluated.
REFERENCES
1. World Health Organization. Rabies. Epidemiology and burden of disease [Internet]. Geneva: World Health Organization; 2013 [cited 2018 Nov 21]. Disponível em: Disponível em: http://www.who.int/rabies/epidemiology/en/ [ Links ]
2. Devleesschauwer B, Aryal A, Sharma BK, Ale A, Declercq A, Depraz S, et al. Epidemiology, impact and control of rabies in Nepal: a systematic review. PLoS Negl Trop Dis [Internet]. 2016 Feb [cited 2019 Feb 26];10(2):e0004461. Disponível em: Disponível em: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.000446 . doi: 10.1371/journal.pntd.0004461 [ Links ]
3. World Health Organization. WHO expert consultation on rabies. Third report [Internet]. Geneva: World Health Organization ; 2018 [cited 2018 Nov 21]. 184 p. Disponível em: Disponível em: http://apps.who.int/iris/bitstream/handle/10665/272364/9789241210218-eng.pdf?ua=1 [ Links ]
4. Ministério da Saúde (BR). Situação epidemiológica. Raiva Humana [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2018 out 08]. Disponível em: Disponível em: http://portalms.saude.gov.br/saude-de-a-z/raiva/situacao-epidemiologica [ Links ]
5. Wada MY, Rocha SM, Maia-Elkhoury ANS. Situação da raiva no Brasil, 2000 a 2009. Epidemiol Serv Saúde [Internet]. 2011 out-dez [citado 2019 fev 26];20(4):509-18. Disponível em: Disponível em: http://scielo.iec.gov.br/pdf/ess/v20n4/v20n4a10.pdf . doi:10.5123/S1679-49742011000400010 [ Links ]
6. Schneider MC, Aguilera XP, Silva Júnior JB, Ault SK, Najera P, Martinez J, et al. Elimination of neglected diseases in Latin America and the Caribbean: a mapping of selected diseases. PLoS Negl Trop Dis [Internet]. 2011 Feb [cited 2019 Feb 26];5(2). Disponível em: Disponível em: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0000964 . doi:10.1371/journal.pntd.0000964 [ Links ]
7. Freire de Carvalho M, Vigilato MAN, Pompei JA, Rocha F, Vokaty A, Molina Flores B, et al. Rabies in the Americas: 1998-2014. PLoS Negl Trop Dis [Internet]. 2018 Mar [cited 2019 Feb 26];12(3). Disponível em: Disponível em: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0006271 . doi:10.1371/journal.pntd.0006271 [ Links ]
8. Ortiz-Prado E, Ponce-Zea J, Ramirez D, Stewart-Ibarra AM, Armijos L, Yockteng J, et al. Rabies epidemiology and control in Ecuador. Glob J Health Sci [Internet]. 2016 May [cited 2019 Feb 26];8(3):113-21. Disponível em: Disponível em: http://www.ccsenet.org/journal/index.php/gjhs/article/view/48912 . doi:10.5539/gjhs.v8n3p113 [ Links ]
9. Salmón-Mulanovich G, Vásquez A, Albújar C, Guevara C, Laguna-Torres A, Salazar M, et al. Human rabies and rabies in vampire and nonvampire bat species, southeastern Peru, 2007. Emerg Infect Dis [Internet]. 2009 Aug [cited 2019 Feb 26];15(8):1308-10. Disponível em: Disponível em: https://wwwnc.cdc.gov/eid/article/15/8/08-1522_article . doi: 10.3201/eid1508.081522 [ Links ]
10. Mani RS, Anand AM, Madhusudana SN. Human rabies in India: an audit from a rabies diagnostic laboratory. Trop Med Int Heal [Internet]. 2016 Apr [cited 2019 Feb 26];21(4):556-63. Disponível em: Disponível em: https://onlinelibrary.wiley.com/doi/full/10.1111/tmi.12669 . doi: 10.1111/tmi.12669 [ Links ]
11. Guo D, Zhou H, Zou Y, Yin W, Yu H, Si Y, et al. Geographical analysis of the distribution and spread of human rabies in China from 2005 to 2011. PLoS One [Internet]. 2013 Aug [cited 2019 Feb 26];8(8):e72352. Disponível em: Disponível em: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0072352 . doi:10.1371/journal.pone.0072352 [ Links ]
12. Weyer J, Szmyd-Potapczuk AV, Blumberg LH, Leman PA, Markotter W, Swanepoel R, et al. Epidemiology of human rabies in South Africa, 1983-2007. Virus Res [Internet[. 2011 Jan [cited 2019 Feb 26];155(1):283-90. Disponível em: Disponível em: https://www.sciencedirect.com/science/article/abs/pii/S0168170210003825?via%3Dihu . doi: 10.1016/j.virusres.2010.10.023 [ Links ]
13. Noah DL, Drenzek CL, Smith JS, Krebs JW, Orciari L, Shaddock J, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med [Internet]. 1998 Jun [cited 2019 Feb 26];128(11):922-30. Disponível em: Disponível em: https://annals.org/aim/article-abstract/711459/epidemiology-human-rabies-united-states-1980-1996?volume=128&issue=11&page=922 . doi: 10.7326/0003-4819-128-11-199806010-00012 [ Links ]
14. Matsumoto T, Ahmed K, Karunanayake D, Wimalaratne O, Nanayakkara S, Perera D, et al. Molecular epidemiology of human rabies viruses in Sri Lanka. Infect Genet Evol [Internet]. 2013 May [cited 2019 Feb 26];18:160-7. Disponível em: Disponível em: https://www.sciencedirect.com/science/article/pii/S1567134813002074?via%3Dihub . doi:10.1016/j.meegid.2013.05.018 [ Links ]
15. Rosa EST, Kotait I, Barbosa TFS, Carrieri ML, Brandão PE, Pinheiro AS, et al. Bat-transmitted human rabies outbreaks, Brazilian amazon. Emerg Infect Dis [Internet]. 2006 Aug [cited 2019 Feb 26];12(8):1197-202. Disponível em: Disponível em: https://wwwnc.cdc.gov/eid/article/12/8/05-0929_article . doi: 10.3201/1208.050929 [ Links ]
16. Mendes WDS, Silva AAM, Neiva RF, Costa NM, Assis MS, Vidigal PMO, et al. An outbreak of bat-transmitted human rabies in a village in the Brazilian Amazon. Rev Saúde Pública [Internet]. 2009 Dec [cited 2019 Feb 26];43(6):1075-7. Disponível em: Disponível em: http://www.scielo.br/pdf/rsp/v43n6/21.pdf . doi: 10.1590/S0034-89102009005000073 [ Links ]
17. Vigilato MAN, Clavijo A, Knobl T, Silva HMT, Cosivi O, Schneider MC, et al. Progress towards eliminating canine rabies: policies and perspectives from Latin America and the Caribbean. Philos Trans R Soc Lond B Biol Sci [Internet]. 2013 Jun [cited 2019 Feb 26];368(1623):20120143. Disponível em: Disponível em: https://royalsocietypublishing.org/doi/full/10.1098/rstb.2012.0143?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed& . doi: 10.1098/rstb.2012.0143 [ Links ]
18. Muyila DI, Aloni MN, Lose-Ekanga MJ, Nzita JM, Kalala-Mbikay A, Bongo HL, et al. Human rabies: a descriptive observation of 21 children in Kinshasa, the Democratic Republic of Congo. Pathog Glob Health [Internet]. 2014 Nov [cited 2019 Feb 26];108(7):317-22. Disponível em: Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4241783/ . doi: 10.1179/2047773214Y.0000000161 [ Links ]
19. Ministério da Saúde (BR). Departamento de Vigilância Epidemiológica. Secretaria de Vigilância em Saúde. Protocolo para tratamento de raiva humana no Brasil. Epidemiol Serv Saúde [Internet]. 2009 out-dez [citado 2019 fev 26];18(4):385-94. Disponível em: Disponível em: http://scielo.iec.gov.br/pdf/ess/v18n4/v18n4a08.pdf [ Links ]
20. Willoughby RE, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, et al. Survival after treatment of rabies with induction of coma. N Engl J Med [Internet]. 2005 Jun [cited 2019 Feb 26];352(24):2508-14. Disponível em: Disponível em: https://www.nejm.org/doi/10.1056/NEJMoa050382?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov . doi: 10.1056/NEJMoa050382 [ Links ]
21. Caicedo Y, Paez A, Kuzmin I, Niezgoda M, Orciari LA, Yager PA, et al. Virology, immunology and pathology of human rabies during treatment. Pediatr Infect Dis J [Internet]. 2015 May [cited 2019 Feb 26];34(5):520-8. Disponíel em: Disponíel em: https://insights.ovid.com/pubmed?pmid=25405805 . doi: 10.1097/INF.0000000000000624 [ Links ]
Received: November 24, 2018; Accepted: February 05, 2019











 texto em
texto em