Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.28 no.2 Brasília jun. 2019 Epub 27-Jun-2019
http://dx.doi.org/10.5123/s1679-49742019000200013
ORIGINAL ARTICLE
Target therapy versus dacarbazine in first-line treatment of advanced non-surgical and metastatic melanoma: budget impact analysis from the perspective of the Brazilian National Health System, 2018-2020*
1Ministério da Saúde, Instituto Nacional de Câncer José Alencar Gomes da Silva, Rio de Janeiro, RJ, Brasil
2Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Brasília, DF, Brasil
Objective:
to estimate the incremental budget impact of target therapy for first-line treatment of advanced non-surgical and metastatic melanoma compared to dacarbazine treatment.
Methods:
budget impact analysis, from the Brazilian National Health System (SUS) perspective; based on demographic data and incidence estimates, the population over a three-year time horizon (2018-2020) was delimited and the direct medical costs were estimated; the reference scenario was treatment with dacarbazine, and the alternative scenarios were target therapy with vemurafenib, dabrafenib, vemurafenib + cobimetinib and dabrafenib + trametinib; uncertainty assessment was conducted through scenario analysis.
Results:
the incremental budget impact ranged from R$ 451,867,881.00 to R$ 768,860,968.00, representing 0.70 to 1.53% of total SUS annual outpatient drugs expenditure; in best and worst scenario, results ranged from R$ 289,160,835.00 to R$ 1,107,081,926.00.
Conclusion:
the use of target therapy compared to dacarbazine implies an excessive impact on the budget, this bring unfovorable to its possible incorporation.
Keywords: Melanoma; Molecular Targeted Therapy; Dacarbazine; Costs and Cost Analysis; Brazilian National Health System
Introduction
In Brazil, the National Policy for Health Technology Management1 and the National Policy for Cancer Prevention and Control2 determine that the incorporation, alteration and disincorporation of technologies to prevent and control cancer within the Brazilian National Health System (SUS) should be a result of recommendations made by government bodies, based on a process of health technology assessment (HTA).
According to Law No. 12,401, dated 28 April, 2011, the use of scientific evidence to guide decision makers in relation to SUS technology management should consider safety, efficacy, effectiveness and efficiency, as well as the economic, ethical, social and environmental impacts of the technology in question.3 Cost-effectiveness analysis and budget impact analysis are stages of the HTA process that estimate the efficiency and the financial consequences of adopting a particular technology.4
Indiscriminate incorporation of technologies threatens health system sustainability. The decision to allocate a particular resource means that it will be unavailable for other purposes. Budget impact analysis is indispensable to SUS cost planning, since Brazil is the only country with more than 200 million inhabitants that has adopted a public, universal and free health system.
Melanoma corresponds to less than 5% of malignant neoplasms of the skin. In Brazil, according to data from the José Alencar Gomes da Silva National Cancer Institute (INCA), it is estimated that there were 6,260 new cases in 2018.5 Despite the fact that incidence is relatively low, the disease burden is significant, due to its high metastasization potential and high lethality level. Melanoma is considered the most aggressive skin cancer and with the worst prognosis. In 2016, 1,773 people died from this disease in Brazil, resulting in a mortality rate adjusted by the world population of 0.66 per 100,000 inhabitants.6
Dacarbazine, which was standard chemotherapy for the treatment of advanced non-surgical and metastatic melanoma until 2010, does not alter the survival of patients when compared to palliative care.7 The introduction of target therapy, a systemic treatment that promotes the selective inhibition of the B-raf proto-oncogene (BRAF) gene mutation found in approximately 50% of patients, enables the activated protein kinase signaling pathway to be blocked and, consequently, inhibits tumor growth.7 Randomized clinical trials that compared isolated target therapies to conventional chemotherapy with dacarbazine showed a statistically significant reduction of 30% in risk of death within 24 months using vemurafenib, as well as a 70% reduction in risk of progression, with no reduction in mortality, when using dabrafenib.8,9 Greater overall survival and progression free survival were found for combined treatments, when compared to isolated treatments.10,11
The Ministry of Health’s Malignant Skin Melanoma Diagnostic and Therapeutic Guidelines, based on a literature review conducted in October 2012, do not recommend target therapy for advanced non-surgical and metastatic melanoma, whereby dacarbazine is the most used chemotherapy.12 According to these guidelines, in the event of the emergence of new scientific evidence, the approach they recommend should be evaluated by the National Commission on SUS Technology Incorporation (CONITEC).
This study aimed to estimate the incremental budget impact of target therapy for first-line treatment of advanced non-surgical and metastatic melanoma compared to treatment with dacarbazine.
Methods
We carried out a budget impact analysis according to recommendations made by the Methodological guidelines: budget impact analysis: a manual for the Brazilian Health System and by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).13,14
The intervention evaluated in this budget impact analysis is for advanced non-surgical and metastatic melanoma (stage III unresectable; or stage IV), with valine to glutamic acid mutation at position 600 of the protein (V600) in the BRAF gene, without previous treatment.
The technology evaluated was target therapy. Currently, two modalities of isolated target therapies for BRAF inhibition are approved in Brazil by the Brazilian Health Regulatory Agency (ANVISA): vemurafenib and dabrafenib. Regarding combined target therapy, two therapeutic regimens can be administered: vemurafenib + cobimetinib; and dabrafenib + trametinib.
The study was carried out from the perspective of SUS as the funding body of public health services. Given the dynamic context and the high dissemination of oncology technologies, we considered it sufficient to adopt a time horizon of three years (2018-2020) for this analysis.
The reference scenario corresponds to the standard treatment of the target population on SUS: chemotherapy using an intravenous 1,000mg/m² dose of dacabazin every 21 days, until there is disease progression or uncontrollable treatment intolerance.
The alternative scenarios included the diverse modalities of target therapy, used until the patient shows disease progression or uncontrollable treatment intolerance:
Alternative scenario 1
Vemurafenib, with one 960mg dose (4 240mg tablets) administered orally every 12 hours.
Alternative scenario 2
Dabrafenib, with one 150mg dose (two 75mg capsules; or three 50mg capsules) administered orally every 12 hours.
Alternative scenario 3
Vemurafenib (same dose prescribed for alternative scenario 1) + cobimetinib, with one 60mg dose (three 20mg tablets) administered orally, once a day, for 21 days a month.
Alternative scenario 4
Dabrafenib (same dose prescribed for alternative scenario 2) + trametinib, with one 2mg dose (one 2.0mg tablet; or four 0.5mg tablets) once a day.
The design of the reference scenario and the alternative scenarios was modeled with three health stages related to advanced non-surgical and metastatic melanoma: no progression; with progression; and death (Figure 1).

Figure 1 - Analytical model of decarbazine as an alternative target therapy for first-line treatment of advanced non-surgical and metastatic melanoma
The diffusion rate of new technology in SUS and the market-share rate in relation to the standard therapy and between different modalities of target therapies, over the study time horizon, were not considered, given the inherent uncertainty of these suppositions: there are gaps in the scientific evidence as to the most appropriate methodology to determine these rates.15 Pharmaceutical innovations that are more efficacious compared to other drugs available usually become widespread quickly within SUS.15 Moreover, according to INCA clinical oncologists, consulted through a semi-structured interview, target therapy, once it has been incorporated, could become a substitute within the first year of use.
The study population consisted of previously untreated patients with advanced non-surgical and metastatic melanoma (stage III unresectable; or stage IV), with BRAF V600 gene mutation.
Annual incidence of melanoma cases at stages IIIc and IV with BRAF mutation cared for by SUS, valid for the first year of the defined time horizon, both in the reference and the alternative scenarios, was defined from robust and stratified population and epidemiologic data, obtained from information made available by the Brazilian Institute of Geography and Statistics (IBGE), and from the Population-Based Cancer Registries.16,17 We used the Brazilian population forecast by sex and age (18 or more) for the years 2018-2020.16 In 2013, the median melanoma incidence rate in Brazil, after being adjusted by the world population, was 4.84 per 100,000 in males and 3.22 per 100,000 in females; 26.2% of cases were diagnosed at stages IIIc and IV, and 74.7% of these cases were treated on the SUS.17 Prevalence of BRAF V600 mutation in patients with advanced non-surgical and metastatic melanoma was estimated to be 48%, based on the data from the scientific literature.18
Additionally, in order to calculate the population size in the second and third years analyzed, survival dynamics needed to be taken into consideration, i.e. the percentage of surviving patients with no disease progression in the previous years, and who, consequently, would continue to receive treatment. These data were obtained from a survey of the scientific literature.19,20 For the reference scenario with dacarbazine, progression-free survival in the first year was 10.7%; in the second year it was 5.2%; while in the third year, all patients progressed or died, based on a Phase III randomized double-blind multicenter clinical trial, that used dacarbazine for comparison.19 For the alternative scenarios with target therapy, we used the progression hazard ratios (HR) in relation to dacarbazine, reported in a network meta-analysis: 0.38 for vemurafenib; 0.37 for dabrafenib; 0.22 for vemurafenib + cobimetinib; and 0.21 for dabrafenib + trametinib.20
Therefore, in the reference scenario in which only dacarbazine would be available: (i) in the first year, the population was comprised only of incident patients; (ii) for the incidents of the second year, it was necessary to add the survivors who did not progress in the first year; and (iii) for the incidents of the third year, we had to add the survivors who did not progress in the first and second years. The same logic was applied to alternative scenarios; however, incident patients were stratified as being “without mutation” and “with mutation”, thus generating a number of survivors without progression unequal in both groups, given the difference in treatment efficacy.
Only direct medical costs were considered in the model. Health resource identification and measurement were done based on a review of the scientific literature,20 reading the recommendations of dacarbazine and target therapy manufacturers, recommendations contained in the Ministry of Health’s Malignant Skin Melanoma Diagnostic and Therapeutic Guidelines,12 consultation with INCA clinical oncologists through a semi-structured interview, and data from the SUS Outpatient Information System (SIA/SUS), made available by the Brazilian National Health System Information Technology Department (DATASUS). The resources included the cobas® 4800 BRAF V600 test, indispensable for the identification of patients with BRAF V600 mutations, oncologic therapies, medical appointments, laboratory tests, imaging exams and exams for monitoring adverse events, all needed for patient follow-up.
We used the macro-costing method to attribute values to health resources. This method allows generalization of results for other oncologic care institutions in Brazil.21 We used SIA/SUS data and also retrieved reimbursement and cost amounts from the Procedures, Medicines, Orthoses, Prostheses and Special Materials Price List Management System (SIGTAP), as well as prices available on the Health Price Bank, both of which are Brazilian National Health System information sources.22,23 We also consulted the ANVISA Drug Market Regulation Chamber (CMED) price list in order to estimate costs of target therapies not available on SUS.24 The price suggested for the cobas® 4800 BRAF V600 test, also unavailable on SUS, was obtained through a telephone price quotation from a private clinical analysis laboratory. In this way, we estimated the annual cost per patient. We did not find national data referring to the cost of adverse event management, emergency services or hospitalization. Costs were estimated in BRL (R$), based on 2017 currency levels. In accordance with the methodological guidelines, we did not apply discount or adjustment for inflation.13
The budget impact analysis was carried out by a cost calculator, developed on a deterministic electronic spreadsheet created using Microsoft Excel® 2016 software. We used a static model, which consisted of multiplying the individual cost of each technology per patient by the number of individuals for whom its use was recommended.
The incremental budget impact was calculated by the difference between the alternative treatment scenario costs and the reference cost, considering the scenario’s target population in each year of the time horizon. We also estimated the percentage of total expenditure on SUS outpatient drugs that might be used for target therapy, because this segment comprises the specialized component of the Pharmaceutical Assistance Financing Package, which includes general medication for outpatients with high cost rare and chronic diseases. According to analysis by the Institute for Applied Economic Research (IPEA) of the Ministry of Economy, SUS outpatient drug expenditure was R$18.6 billion in 2016. Their research was based on the budget spent by the Ministry of Health, the Health Departments of the 26 Brazilian states and the Federal District as well as the municipal Health Departments, using data from public access information systems: Siga Brasil; and the Public Health Budgets Information System (SIOPS).25
Apparent validity was established by reviewing the structure of the model, the assumptions and the parameter values checked with specialists, by means of semi-structured individual interviews.
Internal validation of the model consisted of reviewing data transcription and software programming syntax.
The impact of uncertainties related to the estimates and assumptions adopted on the results of the budget impact analysis was evaluated by scenarios. The study population size and the costs of the scenarios were selected for variation, as they are key issues in budget impact analysis. In accordance with the Brazilian guideline recommendation, the variables were modified randomly, increasing them by 20% (worst scenario) and decreasing them by 20% (best scenario).13
The study was based on public access and public domain information retrieved from databases containing aggregated information which does not permit individual identification of patients. It was also based on a review of scientific texts. Therefore, in accordance with National Health Council (CNS) Resolution No. 510, dated 7 April 2016, this study project was exempt from appraisal by an institutional Research Ethics Committee or by the National Committee for Ethics in Research (CONEP).
Results
Based on the epidemiologic data, we estimated the initial cohorts of patients with melanoma at stages IIIc and IV attending SUS services in 2018, totalizing 1,204 cases, both in the reference scenario and the alternative scenarios; in the scenarios based on target therapies, the population was stratified between “without mutation” (626) and “with mutation” (578) (Figure 2). Considering estimated incidence and the dynamics of survival in 2019 and 2020, we included 1,350 and 1,429 patients, respectively, in the reference scenario. In 2019 and 2020, in the alternative scenarios, the population was comprised of 693 and 725 individuals without mutation, and 678-688 and 725-742 individuals with mutation.
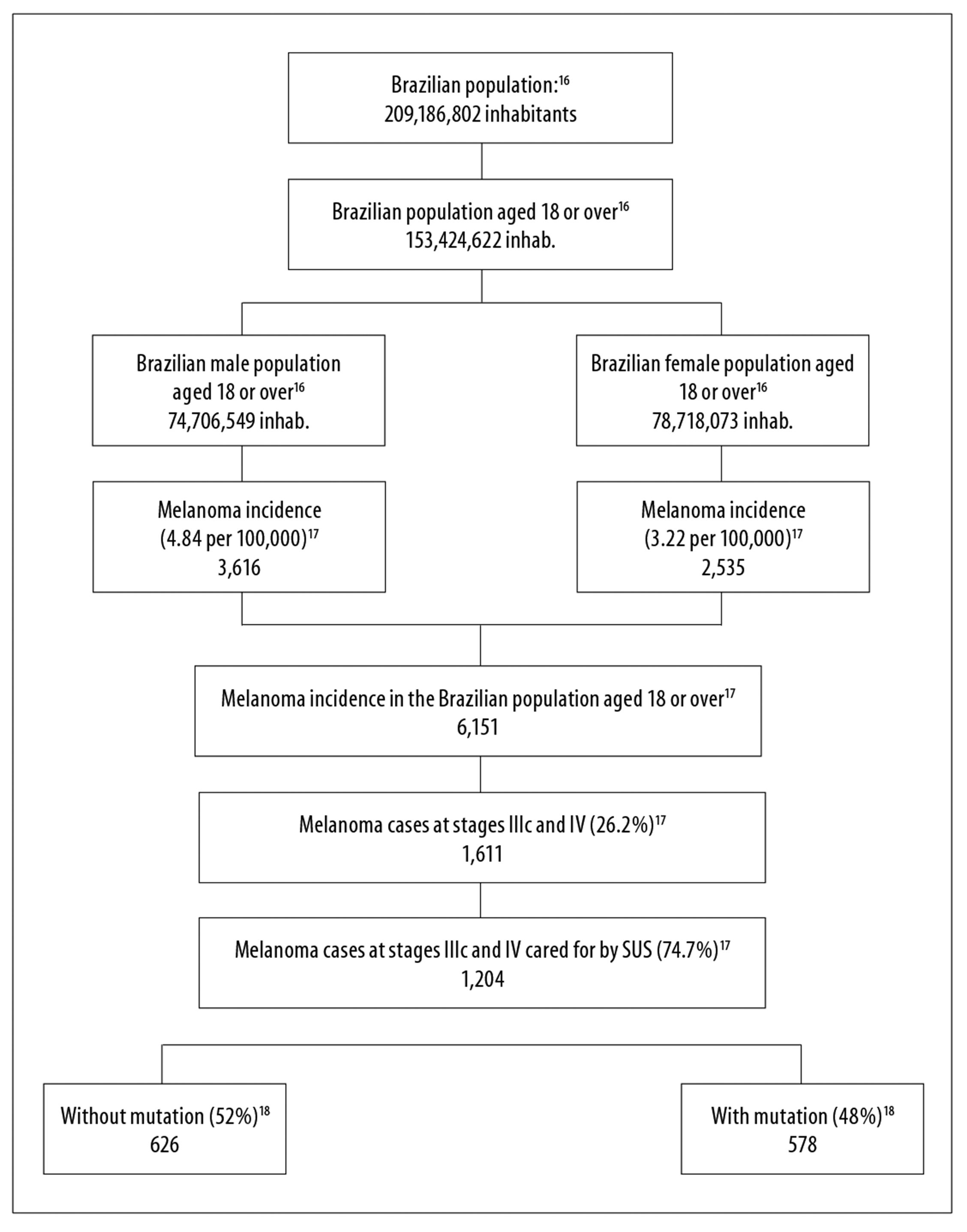
Figure 2 - Estimate of advanced non-surgical and metastatic skin melanoma (stages IIIc and IV) attending the Brazilian National Health System in the first year of the reference and alternative scenarios, Brazil, 2018
The test cost per patient was R$ 1,053.00 (Table 1). Annual cost of treatment was R$ 13,212.60 for dacarbazine, R$ 257,673.60 for vemurafenib, R$ 238,528.80 for dabrafenib, R$ 393,291.81 for vemurafenib + cobimetinib and R$ 375,631.20 for dabrafenib + trametinib. The cost of monitoring survivors with no progression ranged from R$ 2,861.76 to R$ 3,408.48.
Table 1 - Estimated annual cost per patient with advanced non-surgical and metastatic skin melanoma (stages IIIc and IV), Brazil, 2018-2020
| Component | Cost (R$) | Sources |
|---|---|---|
| Screening test | ||
| V600/BRAF mutation test | 1,053.00 | Price quotation from a private laboratory |
| Treatment | ||
| Dacarbazine | 13,212.60 | SIA/SUSa in 2014 |
| Vemurafenib | 257,673.60 | CMEDb / package insert |
| Dabrafenib | 238,528.80 | CMEDb / package insert |
| Vemurafenib + cobimetinib | 393,291.81 | CMEDb / package insert |
| Dabrafenib + trametinib | 375,631.20 | CMEDb / package insert |
| Monitoring of survivors with no progression | ||
| Dacarbazine | 2,861.76 | SIGTAPc / package insert / guidelinesd / specialist |
| Vemurafenib | 3,043.80 | SIGTAPc / package insert / guidelinesd / specialist |
| Dabrafenib | 2,999.40 | SIGTAPc / package insert / guidelinesd / specialist |
| Vemurafenib + cobimetinib | 3,408.48 | SIGTAPc / package insert / guidelinesd / specialist |
| Dabrafenib + trametinib | 3,364.08 | SIGTAPc / package insert / guidelinesd / specialist |
a) SIA/SUS: Brazilian National Health System Outpatient Information System
b) CMED: Drug Market Regulation Chamber
c) SIGTAP: Procedures, Medicines, Orthoses, Prostheses and Special Materials Price List Management System
d) Malignant Skin Melanoma Diagnostic and Therapeutic Guidelines18
The therapeutic regimens accounted for 82% of the total cost of treatment of the study population over the three-year time horizon in the reference scenario, and between 97 and 98% in the alternative scenarios. Monitoring of survivors with no progression ranged from 1.5% to 2.3% of the costs in the alternative scenarios and up to 18% in the reference scenario. The BRAF V600 mutation test accounted for between 0.46 and 0.74% of total costs in the alternative scenarios.
Figure 3a illustrates the annual and total budget impact of the standard therapy and of the target therapies. The incremental budget impact in the alternative scenarios using target therapies was as follows:
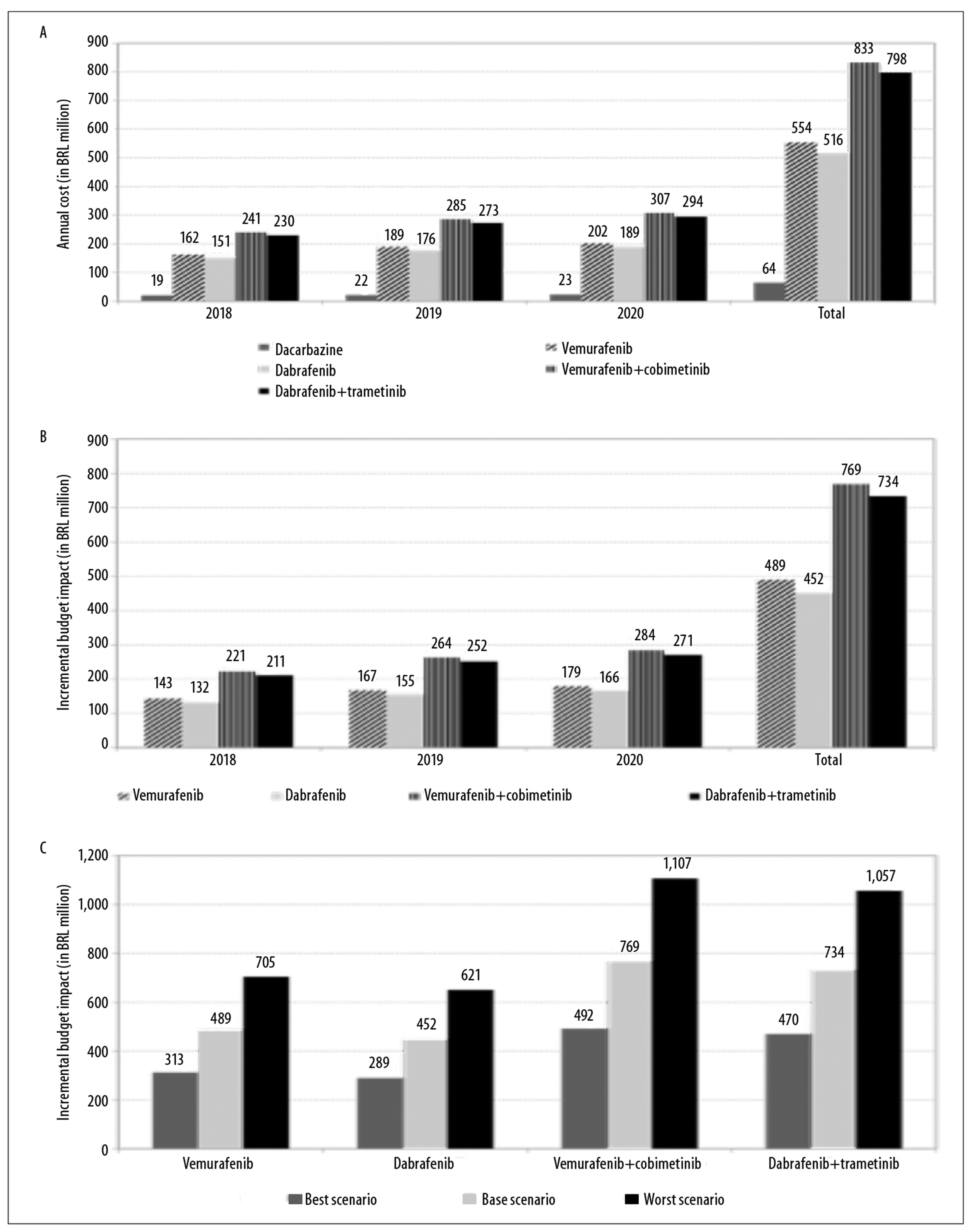
Figure 3 - Estimates of (a) annual and total budget impact of standard therapy and target therapies, estimates of (b) annual and total incremental budget impact of target therapies, and sensitivity analysis (best and worst scenario) of (c) incremental budget impact, for treatment of advanced non-surgical and metastatic skin melanoma (stages IIIc and IV), Brazil, 2018-2020
First year
R$ 142,621,428.00 (vemurafenib)
R$ 131,533,975.00 (dabrafenib)
R$ 221,191,862.00 (vemurafenib + cobimetinib)
R$ 210,961,970.00 (dabrafenib + trametinib)
Second year
R$ 167,461,943.00 (vemurafenib)
R$ 154,620,120.00 (dabrafenib)
R$ 263,594,092.00 (vemurafenib + cobimetinib)
RS251,650,125.00 (dabrafenib + trametinib)
Third year
R$ 179,399,382.00 (vemurafenib)
R$ 165,713,785.00 (dabrafenib)
R$ 284,075,014.00 (vemurafenib + cobimetinib)
R$ 271,298,039.00 (dabrafenib + trametinib)
Total
R$ 489,482,753.00 (vemurafenib)
R$ 451,867,881.00 (dabrafenib)
R$ 768,860,968.00 (vemurafenib + cobimetinib)
R$ 733,910,134.00 (dabrafenib + trametinib) (Figure 3b).
The annual incremental budget impact for alternative scenarios with target therapies was 0.70% to 1.19% in the first year, 0.83% to 1.41% in the second year and 0.89% to 1.53% of total SUS annual outpatient drug expenditure.
Modifying the parameters, for the best and worst scenarios, caused variation in results of the incremental budget impact calculation: from R$ 313,268,962.00 to R$ 704,855,164.00 for vemurafenib; from R$ 289,160,835.00 to R$ 650,611,880.00 for dabrafenib; from R$ 492,036,411.00 to R$ 1,107,081,926.00 for vemurafenib + cobimetinib; and from R$ 469,667,877.00 to R$ 1,056,752,724.00 for dabrafenib + trametinib (Figure 3c).
Discussion
The costs obtained through this budget impact analysis for potential incorporation of target therapy in treating advanced non-surgical and metastatic melanoma on SUS are over R$ 700 million over a three-year time horizon. Although there are no defined thresholds for budget impact, these costs can be considered high (over R$85 million) for the system, based on the distribution of costs resulting from the historical analysis of drug recommendation reports evaluated by CONITEC.26 The high incremental budget impact can be explained by the exponential increase in costs of new oncology technologies. Indeed, in this study, therapeutic regimens accounted for the largest percentage of costs in both the reference scenario and the alternative scenarios, with annual target therapy costing 18 to 30 times more when compared to the cost of dacarbazine.
The study population was estimated based on population and epidemiological data, since we did not find robust data measuring demand that might have been closer to reality. The Brazilian melanoma incidence rates used in this study included all age groups; however fewer than 1% of patients were under 18 years old. Additionally, the probabilities of progression free survival used in the calculation of the population size over the time horizon used for our analysis were based on efficacy results found in randomized clinical trials that were not conducted in Brazil. For this reason they might not be generalizable for Brazil and are probably overestimated. BRAF V600 mutation prevalence was not estimated based on national data. Brazilian research has not reported results disaggregated according to cancer staging, and positivity has ranged from 39% to 70%.27 We also did not consider market-share between the different modalities of target therapies in relation to standard therapy, as well as in relation to immunotherapy, which could eventually be evaluated for incorporation. As such, the population size may not be reliable. Nonetheless, the variation in these assumptions and estimates when analyzing the best scenario with a 20% smaller population revealed that the incremental budget impact would still be considerable and would lead to an excessive increase in expenditure.
We considered all costs relevant to the system, despite the costs of hospitalization owing to complications of the disease or resulting from treatment not having been estimated, given that target therapy accounted for up to 98% of total costs in the alternative scenarios. Be that as it may, in the network meta-analysis that used direct and indirect evidence related to isolated or combined target therapies, all of which were compared with dacarbazine, no statistically significant differences were found in the occurrence of severe adverse events.19 We were unable to conduct probabilistic sensitivity analysis given the lack of results of precision measures and variability of cost-related parameters.
The estimates we obtained are consistent with results of economic evaluations using budget impact analysis conducted in other countries. In Norway, a health technology assessment conducted in 2015 concluded that the budget impact for the public health system resulting from the incorporation of target therapy could be substantial; although price negotiation might possibly reduce this impact by between 63% and 84%, depending on the therapeutic regimen adopted.19 In Italy, a more recent budget impact analysis carried out in 2017 reported that incorporation of target therapy by the public health system would require almost 200% more resources for the treatment of patients with metastatic melanoma with BRAF mutation over the three years considered.28 With effect from 2015 in the United Kingdom, the National Institute for Health and Care Excellence (NICE) has recommended treatment of unresectable melanoma or metastatic melanoma found to be positive for BRAF V600 mutation with vemurafenib or dabrafenib only if the manufacturer provides these drugs with the discount agreed with the Patient Access Schemes project, which aims to ensure patient access to high cost medication not otherwise considered to be cost-effective.29 In the United States, although from the perspective of private health plans rather than public services, important financial consequences of introducing target therapy in clinical practice have been pointed out, especially because of drug costs.30
In Brazil, target therapy, when compared to the use of dacarbazine in patients with advanced non-surgical and metastatic melanoma, entails a significant increase in expenditure that is unfavorable to its possible incorporation.
Acknowledgments
To Andreia Cristina de Melo, head of the Clinical Trials and Technological Development Division of the José Alencar Gomes da Silva National Cancer Institute, for the consultancy services and epidemiological data provided. To the SUS Institutional Development Support Program, which funds the Master of Business Administration Degree Course in Economics and Health Technology Evaluation, at the Health Science Education Department Postgraduate Program / Oswaldo Cruz German Hospital.
REFERENCES
1. Brasil. Ministério da Saúde. Portaria GM/MS nº 2690, de 05 de novembro de 2009. Institui, no âmbito do Sistema Único de Saúde (SUS), a Política Nacional de Gestão de Tecnologias em Saúde [Internet]. Diário Oficial da da União, Brasília (DF), 2009 nov 6; Seção 1:61. Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2009/prt2690_05_11_2009.html [ Links ]
2. Brasil. Ministério da Saúde. Portaria GM/MS no 874, de 16 de maio de 2013. Institui a Política Nacional para a Prevenção e Controle do Câncer na Rede de Atenção à Saúde das Pessoas com Doenças Crônicas no âmbito do Sistema Único de Saúde (SUS) [Internet]. Diário Oficial da União, Brasília (DF), 2013 maio 17; Seção 1:129. Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt0874_16_05_2013.html [ Links ]
3. Brasil. Presidência da República. Casa Civil. Lei nº 12.401, de 28 de abril de 2011. Dispõe sobre a assistência terapêutica e a incorporação de tecnologia em saúde no âmbito do Sistema Único de Saúde - SUS [Internet]. Diário Oficial da União, Brasília (DF), 2011 abr 29; Seção 1:1. Disponível em: http://www.planalto.gov.br/ccivil_03/_Ato2011-2014/2011/Lei/L12401.htm [ Links ]
4. Silva MT, Silva EN, Pereira MG. Budget impact analysis. Epidemiol Serv Saúde [Internet]. 2017 Apr-Jun [citado 2019 Apr 4];26(2):421-4. Disponível em: Disponível em: http://www.scielo.br/pdf/ress/v26n2/en_2237-9622-ress-26-02-00421.pdf . doi: 10.5123/S1679-49742017000200020 [ Links ]
5. Ministério da Saúde (BR). Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. Estimativa 2018: incidência de câncer no Brasil [Internet]. Rio de Janeiro: Instituto Nacional de Câncer José Alencar Gomes da Silva; 2017 [citado 2018 jun 05]. 128 p. Disponível em: Disponível em: http://www1.inca.gov.br/inca/Arquivos/estimativa-2018.pdf [ Links ]
6. Ministério da Saúde (BR). Departamento de Informática do SUS. Informações de saúde (TABNET) [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2018 jun 26]. Disponível em: Disponível em: http://datasus.saude.gov.br/informacoes-de-saude/tabnet [ Links ]
7. Davey RJ, van der Westhuizen A, Bowden NA. Metastatic melanoma treatment: combining old and new therapies. Crit Rev Oncol Hematol [Internet]. 2016 Feb [citado 2019 Apr 4];98:242-53. Disponível em: Disponível em: https://www.sciencedirect.com/science/article/pii/S1040842815300809?via%3Dihub . doi: 10.1016/j.critrevonc.2015.11.011 [ Links ]
8. McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol [Internet]. 2014 Mar [citado 2019 Apr 4];15(3):323-32. Disponível em: Disponível em: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70012-9/fulltext . doi: 10.1016/S1470-2045(14)70012-9 [ Links ]
9. Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet [Internet]. 2012 Jul [citado 2019 Apr 4];380(9839):358-65. Disponível em: Disponível em: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60868-X/fulltext . doi: 10.1016/S0140-6736(12)60868-X [ Links ]
10. Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med [Internet]. 2014 Nov [citado 2019 Apr 4];371(20):1867-76. Disponível em: Disponível em: https://www.nejm.org/doi/full/10.1056/NEJMoa1408868 . doi: 10.1056/NEJMoa1408868 [ Links ]
11. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet [Internet]. 2015 Aug [citado 2019 Apr 4];386(9992):444-51. Disponível em: Disponível em: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)60898-4/fulltext . doi: 10.1016/S0140-6736(15)60898-4 [ Links ]
12. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Protocolos clínicos e diretrizes terapêuticas em oncologia [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2018 jun 10]. 356 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/protocolos_clinicos_diretrizes_terapeuticas_oncologia.pdf [ Links ]
13. Ministério da Saúde (BR). Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Ciência e Tecnologia. Diretrizes metodológicas: análise de impacto orçamentário: manual para o Sistema de Saúde do Brasil [Internet]. Brasília: Ministério da Saúde; 2012. [citado 2018 jun 10]. 76 p. Disponível em: Disponível em: http://rebrats.saude.gov.br/diretrizes-metodologicas [ Links ]
14. Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health [Internet]. 2014 Jan-Feb [citado 2019 Apr 4];17(1):5-14. Disponível em: Disponível em: https://www.ncbi.nlm.nih.gov/pubmed/24438712 . doi: 10.1016/j.jval.2013.08.2291 [ Links ]
15. Schneiders RE, Ronsoni RM, Sarti FM, Nita ME, Bastos EA, Zimmermann IR, et al. Factors associated with the diffusion rate of innovations: a pilot study from the perspective of the Brazilian Unified National Health System. Cad Saúde Pública [Internet]. 2016 Sep [citado 2019 Apr 4];32(9):e00067516. Disponível em: Disponível em: http://www.scielo.br/pdf/csp/v32n9/1678-4464-csp-32-09-e00067516.pdf . doi: 10.1590/0102-311x00067516 [ Links ]
16. Ministério do Planejamento, Orçamento e Gestão (BR). Instituto Brasileiro de Geografia e Estatística. Diretoria de Pesquisas. Coordenação de População e Indicadores Sociais. Projeção da população do Brasil por sexo e idade para o período 2000-2060. [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2013 [citado 2018 jan 3]. Disponível em: Disponível em: https://ww2.ibge.gov.br/home/estatistica/populacao/projecao_da_populacao/2013/default.shtm [ Links ]
17. Melo AC, Wainstein AJA, Buzaid AC, Thuler LCS. Melanoma signature in Brazil: epidemiology, incidence, mortality and trend lessons from a continental mixed population country in the last 15 years. Melanoma Res [Internet]. 2018 Dec [citado 2019 Apr 4];28(6):629-36. Disponível em: Disponível em: https://insights.ovid.com/pubmed?pmid=30204684 . doi: 10.1097/CMR.0000000000000511 [ Links ]
18. Carlino MS, Haydu LE, Kakavand H, Menzies AM, Hamilton AL, Yu B, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br J Cancer [Internet]. 2014 Jul [citado 2019 Apr 4];111(2):292-9. Disponível em: Disponível em: https://www.nature.com/articles/bjc2014287 . doi: 10.1038/bjc.2014.287 [ Links ]
19. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med [Internet]. 2011 Jun [citado 2019 Apr 4];364(26):2517-26. Disponível em: Disponível em: https://www.nejm.org/doi/full/10.1056/Nejmoa1104621 . doi: 10.1056/NEJMoa1104621 [ Links ]
20. Pike E, Torkilseng EB, Sæterdal I, Jimenez E, Odgaard-Jensen J, Harboe I, et al. A health technology assessment of the new drugs for inoperable or metastatic malignant melanoma patients. Report from Kunnskapssenteret no 22-2015 [Internet]. Oslo: Norwegian Knowledge Centre for the Health Services; 2015 [citado 2018 Jan 03]. 118 p. Disponível em: Disponível em: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0092997/pdf/PubMedHealth_PMH0092997.pdf [ Links ]
21. Oliveira ML, Santos LMP, Silva EN. Bases metodológicas para estudos de custos da doença no Brasil. Rev Nutr [Internet]. 2014 set-out [citado 2019 abr 4];27(5):585-95. Disponível em: Disponível em: http://www.scielo.br/pdf/rn/v27n5/1415-5273-rn-27-05-00585.pdf . doi: 10.1590/1415-52732014000500007 [ Links ]
22. Ministério da Saúde (BR). Departamento de Informática do Sistema Único de Saúde (Datasus). Sistema de gerenciamento da tabela de procedimentos, medicamentos e órteses, próteses e materiais especiais [Internet]. Brasília: Ministério da Saúde; 2017 [citado 2017 jun 10]. Disponível em: Disponível em: http://sigtap.datasus.gov.br/tabela-unificada/app/sec/inicio.jsp [ Links ]
23. Ministério da Saúde (BR). Banco de preços em saúde [Internet]. Brasília: Ministério da Saúde; 2017 [citado 2017 jun 10]. Disponível em: Disponível em: http://portalms.saude.gov.br/gestao-do-sus/economia-da-saude/banco-de-precos-em-saude [ Links ]
24. Agência Nacional de Vigilância Sanitária (BR). Câmara de regulação mercado de medicamentos [Internet]. Brasília: Agência Nacional de Vigilância Sanitária; 2017 [citado 2017 jun 10]. Disponível em: Disponível em: http://portal.anvisa.gov.br/cmed [ Links ]
25. Vieira FS. Evolução do gasto com medicamentos do Sistema Único de Saúde no período de 2010 a 2016 [Internet]. Rio de Janeiro: Instituto de Pesquisa Econômica Aplicada; 2018 [citado 2019 abr 4]. Disponível em: Disponível em: http://www.ipea.gov.br/portal/index.php?option=com_content&view=article&id=32195 [ Links ]
26. Zimmermann IR, Oliveira EF, Vidal AT, Santos VCC, Petramale CA. A qualidade das evidências e as recomendações sobre a incorporação de medicamentos no Sistema Único de Saúde: uma análise retrospectiva. Rev Eletrônica Gest Saúde [Internet]. 2015 out [citado 2019 abr 4];6(Suppl 4):3043-65. Disponível em: Disponível em: https://dialnet.unirioja.es/servlet/articulo?codigo=5560368 [ Links ]
27. Inumaru JS, Gordo KI, Fraga Júnior AC, Silva AM, Leal CB, Ayres FM, et al. Analysis of the BRAF V600E mutation in primary cutaneous melanoma. Genet Mol Res [Internet]. 2014 Jan [citado 2019 Apr 4];13(2):2840-8. Disponível em: Disponível em: https://www.geneticsmr.com/articles/2800 . doi: 10.4238/2014.January.22.8 [ Links ]
28. Pompilio G, Campanella P, Integlia D. Cost-effectiveness and budget impact of new therapies for metastatic melanoma in Italy. Value Health [Internet]. 2017 Oct-Nov [citado 2019 Apr 4];20(9):A425. Disponível em: Disponível em: https://www.valueinhealthjournal.com/article/S1098-3015(17)30493-X/fulltext . doi: 10.1016/j.jval.2017.08.159 [ Links ]
29. National Institute for Heath and Care Excellence (NICE). Melanoma: assessment and management. NICE guideline [Internet]. London: National Institute for Heath and Care Excellence; 2015 [citado 2018 Jun 10]. 61 p. Disponível em: Disponível em: https://www.nice.org.uk/guidance/ng14/resources/melanoma-assessment-and-management-pdf-1837271430853 [ Links ]
30. Li Z, Whisman T, Tang J, Grzegorzewski K, Quadri S, Mahmood S, et al. Evaluating expected medication costs and budget impact of systemic therapies for unresectable/metastatic melanoma. Value Health [Internet]. 2016 May [citado 2019 Apr 4];19(3):A143. Disponível em: Disponível em: https://www.valueinhealthjournal.com/article/S1098-3015(16)01622-3/abstract . doi: 10.1016/j.jval.2016.03.1554 [ Links ]
*Article derived from the final assignment of the Master of Business Administration Degree course in Economy and Health Technology Assessment, entitled “Budget impact of target therapy compared to dacarbazine in first-line treatment of advanced non-surgical and metastatic melanoma”, defended by Flávia de Miranda Corrêa at the Health Science Education Department Postgraduate Program / Oswaldo Cruz German Hospital, on December 14, 2017.
Received: December 03, 2018; Accepted: March 20, 2019











 texto em
texto em 


