Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.1 Brasília 2020 Epub 27-Jan-2020
http://dx.doi.org/10.5123/s1679-49742020000100009
PROFILE OF NATIONAL HEALTH DATABASES
Notifiable Diseases Information System (SINAN): main features of tuberculosis notification and data analysis
1Secretaria de Vigilância em Saúde, Programa Nacional de Controle da Tuberculose, Brasília, DF, Brasil
2Governo do Distrito Federal, Secretaria de Saúde do Distrito Federal, Brasília, DF, Brasil
3Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis, Brasília, DF, Brasil
4Universidade Federal do Rio de Janeiro, Instituto de Estudos de Saúde Coletiva, Rio de Janeiro, RJ, Brasil
The Notifiable Diseases Information System (SINAN) enables knowledge of the profile of people with active tuberculosis (TB) in a country of continental dimensions such as Brazil. Available in all Brazilian municipalities and states, the system enables continuous consolidation of data, evaluation and monitoring of actions related to TB control in the country. The purpose of this paper is to present the specificities of SINAN-Net related to TB, including the follow-up screen, the record linkage and the follow-up report. Additionally, we describe the main variables and indicators and the challenges and limitations of the system.
Keywords: Health Information Systems; Epidemiological Monitoring; Tuberculosis
Introduction
Knowing the profile of people with active tuberculosis (TB) in a country of continental dimensions such as Brazil is only possible thanks to the existence of the Notifiable Diseases Information System (SINAN). Available in all Brazilian municipalities and states, SINAN enables continuous data consolidation, monitoring and evaluation of actions related to TB control nationwide, as well as providing support, indirectly, for purchasing medication and supplies.
This health information system was developed with the aim of standardizing collection and processing of data on notifiable diseases and conditions throughout the national territory, providing information for analysis of the morbidity profile of the country’s residents, in order to contribute to decision making at municipal, state and federal level.1 The system’s objectives also include monitoring the population’s health and anticipating the occurrence of events, identifying the epidemiological reality of a given geographic area and assisting with health service planning, defining intervention priorities and evaluating the impact of control actions undertaken.2
SINAN is responsible for notification, investigation and, in the case of communicable diseases, follow-up and treatment as well. The diseases and conditions notified on SINAN are defined by the National Compulsory Notification List of diseases.3 The List is common in the entire national territory, but is flexible to enables state and municipal health departments to include diseases and conditions of interest at state, regional or local level. Notification is usually made by citizens or health professionals working at different levels of the health system, primary health facility, municipal and state health departments, through to the Ministry of Health. The system enables the characteristics of the event of interest to be monitored and its distribution and trends to be verified in space and over time.2
Background
SINAN was created with the purpose of correcting the difficulties faced by the Compulsory Disease Notification System (SNCD), which was developed from the establishment of the institution of the National Epidemiological Surveillance System by Law No. 6.259, dated 30/10/1975,4 and by Decree No. 78.231, dated 12/08/1976.5 SNDC had underreporting problems and, because of this, did not achieve the objective of providing for proper morbidity profile analysis, nor did it stimulate surveillance actions at the local level.6 Moreover, the collection instruments were excessively unspecific and in addition some fundamental variables were not collected.6
SINAN was conceived and developed in 1993 in the DOS operating system format. At that time, implementation occurred heterogeneously in the Federation Units and municipalities, without any coordination or follow-up by health service managers at the three government levels.7,2 Use of SINAN was regulated by Ministerial Ordinance,8 published on December 18th 1997, which made it obligatory for its national database to be regularly updated by the federative entities. From then on, regular data input and updating of the system became one of the strategies for controlling the transfer of federal resources allocated specifically to surveillance.1,2 Between 1998 and 2000 the SINAN version for Windows - SINAN Windows - was developed and strategies were defined for its immediate implementation throughout the entire national territory.2
The TB module has been present on SINAN ever since the first DOS versions of the system. From then on, case notification, investigation and follow-up have taken place via the system. System updates over the years have led to adaptations and improvements to TB data routines and analysis.
The first great impact on data analysis occurred when the system migrated from the DOS version to the Windows version;7 at that time incompatibility between the systems was so great that it resulted in database discontinuity and analysis had to be done separately for data prior to the year 2000 and after this period.
In 2007, the system underwent structure updating and incorporation of new technologies, including the development of an application for performing routines via internet (table updating, data transfer and return flow); as well as to enable greater flexibility in its operationalization and use right from primary health facility. For this reason, as well as to differentiate it from the previous version, the updated system received the name SINAN-Net.1,9
The Net version1,9 required alterations to the notification/investigation form and to the TB follow-up report. However, in order to avoid database discontinuity, which would have hindered analysis, prior preparation of TB data was carried out. The TB database was migrated from one system to the other, keeping the database intact, without interfering with data analysis. The only impact on analysis resulted from changes to the variables that were either added or altered on the notification/investigation form and the TB follow-up report.
The most recent large-scale updating of the notification/investigation form and the TB follow-up report occurred in 2015 when SINAN-Net version 5.0 was implemented.10 This version included fields for special populations (prison population; homeless population; health professionals; immigrants), associated diseases and conditions (illegal drug use; tobacco smoking), beneficiary of a governmental cash transfer program, antiretroviral therapy during TB treatment, Xpert MTB/RIF test and sensitivity test.
The following categories were also added: post-death, in the ‘type of entry’ variable; and change of treatment regimen, failure and primary loss to follow-up, in the ‘treatment outcome’ variable. The following fields were excluded: institutionalized; tuberculin test; bacilloscopy with other material; culture with other material; drugs; indicated for observed treatment; and work-related disease.11
The purpose of this paper is to present SINAN-Net version 5.0 specificities in relation to TB, including the follow-up screen, the record linkage routine and the follow-up report.
Characteristics of the SINAN-Net Tuberculosis system
Data collection
In order to assist health professionals working with epidemiological surveillance of TB, instruments were developed for all stages of their actions, from active finding of symptomatic cases to treatment follow-up (Figure 1).

Source: Manual of Recommendations for Tuberculosis Control in Brazil.11
Figure 1 - Flow and recording instruments used in tuberculosis epidemiological surveillance in Brazil
TB surveillance begins with active finding of respiratory symptomatic cases: those with a cough, whether productive or not, for three weeks or more. This criterion regarding length of time with a cough differs, depending on the population to be investigated for TB and the risk of becoming ill, as described in the TB Control Recommendations Manual.11
When identifying a respiratory symptomatic case (suspected case of pulmonary TB), health professionals must arrange for laboratory examinations to confirm or rule out TB and the results and conclusion must be recorded Registry of respiratory symptomatic individuals in the health facility.11 In its extrapulmonary clinical form, a record must also be made in the respiratory symptomatic record book, following clinical or laboratory confirmation.
If active TB is confirmed, the health professional must include the case in Registry of people with tuberculosis and treatment follow-up; the notification/investigation form must then by duly filled out and sent to the first computerized level for input onto SINAN-Net. The Registry of people with tuberculosis and treatment follow-up remains at the health center and must be updated periodically by health professionals and used to inform the Tuberculosis Follow-up Form, which must be filled out and sent to the first computerized level monthly.11
With regard to the tuberculosis notification/investigation form, the aim of the updates made to it over the course of time has been to enable identification of individual characteristics, such as belonging to groups vulnerable to becoming ill, as well as to include new laboratory tests such as Xpert MTB/RIF test.11
TB cases are only notified after they have been confirmed, either by laboratory or clinical criteria.10 The tuberculosis notification/investigation form must be used to notify all people with active TB, i.e. new cases, relapses, readmission after loss to follow-up and transfers.1 Patients who have never been registered on SINAN and are found to have TB after death, as a result of epidemiological investigation, must be notified as ‘post-death' in the ‘type of entry‘ variable.12
The individual form is comprised of two parts: (i) notification, including identification of the notified person and data about the notifying service, standardized fields for all diseases and conditions; and (ii) investigation, including the person’s type of entry on the system, clinical form of TB, individual characteristics (vulnerable populations; and associated conditions and diseases), presence of TB-HIV co-infection and test results, among others.
The Tuberculosis Follow-up Form is used to update data related to treatment (tests in progress, number of contacts investigated, directly observed treatment carried out, follow-up bacilloscopy, use of antiretroviral therapy, transfer to another health service to continue treatment and reason for treatment outcome).1 This report, generated using SINAN data, provides a list of people in treatment at the current health center and who have been diagnosed for at least one month and one day, and whose treatment outcome status has not been informed.1,11 The report is issued by the first computerized level. And turn, health centers must return it duly filled out to the first computerized level which is responsible for inputting the data to the SINAN-Net follow-up screen.1
Case duplication and record linkage
SINAN-Net provides a report listing possible duplicated records, which must be analyzed periodically to define whether records are indeed duplicated, whether a record has been duplicated because of transfer or relapse/readmission after loss to follow-up, or whether they are homonymous cases.
True duplication occurs when the same person has been notified more than once by the same health center during the same treatment. In order to correct this situation, the first computerized level must keep the first form and complement it with data from the second form and then exclude the second form. Record exclusion must be done by the first computerized level (the level that input the form). If duplication is identified as having occurred above the first computerized level, this must be communicated to the level that input it.
A duplicated record because of transfer is the situation where the same person has been notified more than once, by different health centers, during the same treatment. Duplicated records also occur when the same person has been recorded with different entry types, because of entry due to relapse or readmission after loss to follow-up. In the first situation, the forms must be linked, while in the second situation, the ‘do not list’ option must be chosen so that these records are not listed in the duplication report but remain in the system.1
Record linkage is exclusively for transfer cases and consists of linking together the form from the service of origin with the form from the destination service. By joining the forms in this way, the oldest notification/investigation form (from the service of origin) and the most recent follow-up form (from the destination unit) remain in the system.1
Homonymous cases are records that have the same first and last name, date of birth and sex, even though they are different people. Once the suverllance technicians confirm that they are not the same person. The ‘do not list’ option must be chosen to avoid these records from appearing again on the duplication report. It is important to point out that in these cases new records input on SINAN that have characteristics similar to cases already input - i.e. name, date of birth and sex -, will result in records tagged with ‘do not list’ reappearing on the list.
Dates, deadlines and data transfer to higher levels
There are three dates on SINAN-TB deserving attention with regard to their completion on the system: notification date, diagnosis date and treatment start date. In all cases, each time a notification is made, a new notification date must be used, including in transfer situations. In the event of new treatment (relapse or readmission after loss to follow-up), a new diagnosis date and a new treatment start date are used. In the case of transfers, however, the same diagnosis date and the same treatment start date found on the oldest notification/investigation form remain.
With regard to data transfer to higher levels, this must take place for subsequent levels, it must occur weekly, from the municipal and state level, to the federal level.1
With regard to timely closure of notified cases, a period of nine months is used for cases in treatment using the basic regimen, and 15 months for meningo-encephalic TB cases, with effect from diagnosis date.11
Inclusion of new notifications on SINAN should take place within up to one year and three months after diagnosis date,13 while the duplicated record verification routine should cover the last five years.12
Information dissemination and use
Information is the main component of health action planning. It needs to be reliable, relevant and timely.2 Information deriving from SINAN-TB is used by health service managers to formulate and evaluate actions which are detailed at the municipal and state level by place of residence or place of notification.
Information feedback to health professionals, throught reports and/or other instruments, motivates recognition of the importance of filling out the notification/investigation form and the treatment follow-up form. This characteristic of the process enables health professionals to self-reflect on their actions and the quality thereof and, as a consequence, lead to indicator improvement.
The epidemiological and operational profile of TB in Brazil is publicized annually, in the month of March, throught an epidemiological bulletin. Other additional documents are also published, such as the Panorama of Tuberculosis in Brazil, which presents the data by region, state and state capital city, in a more dynamic format. All informative materials prepared by National Tuberculosis Control Program (PNCT) technical staff based on SINAN data are available at the following electronic addresses: http://portalms.saude.gov.br/saude-de-a-z/tuberculose/situacao-epidemiologica
TabNet is a tool made available by the Brazilian National Health System Information Technology Department (DATASUS)14 which enables this information to be disseminated and used. It is a public domain generic tabulator that allows anyone to generate information from the SINAN database. This online data tabulator generates tables and produces graphs and maps, by period and peace of coverage TabNet can be accessed at the following electronic address:http://www2.datasus.gov.br/DATASUS/index.php?area=0203&id=31009407&VObj=http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/tuberc
Another important channel through which TB data are publicized is the Strategic Management Support Room (SAGE), where indicators set out in Government Plans are made available and systematically updated by the PNCT. Access to SAGE is public via the following electronic address: http://sage.saude.gov.br/
Coverage
SINAN covers the whole of Brazil and provides detailed information at state and municipal level. According to the World Health Organization (WHO), Brazil detects 87% of new TB cases.15 Notification does not always take place, despite its compulsory nature. Lack of notification results in formal liabilities for all citizens and health professionals. Underdetection often occurs because of lack of knowledge and failure to recognize the importance of notification, by not sending the notification/investigation form or misplacing it between the health center and the first computerized level where it should be input, or by failure to diagnose TB in a timely manner, among other reasons.2 Strategies to raise SINAN coverage used by some Tuberculosis Control Programs include linkage of existing information systems, such as the Mortality Information System and the Laboratory Environment Manager, among others.
Main variables
The following variables are present on the notification/investigation form and the follow-up screen:
a) General data
b) Individual notification
- name;
- sex;
- date of birth;
- age;
- mother’s name;
- pregnant woman;
- race/skin color;
- schooling; and
- SUS card number.
c) Residence data
d) Complementary data
- type of entry;
- special populations (prison population;
homeless population,; health professionals; immigrants);
- beneficiary of a governmental cash transfer program;
- clinical form of TB;
- associated diseases and conditions (AIDS; alcoholism; diabetes mellitus; mental illness; illegal drug use; tobacco smoking);
- sputum bacilloscopy (diagnosis);
- chest x-ray;
- HIV test;
- antiretroviral therapy during TB treatment;
- histopathology;
- culture;
- Xpert MTB/RIF test;
- sensitivity test;
- treatment start date; and
- total contacts identified.
e) TB follow-up screen (only displayed on SINAN-Net)
Data analysis
During a stage prior to data analysis, a problem needs to be identified and a question needs to be defined that will guide the indicator generation process. Following this, the SINAN-Net database needs to be qualified and prepared: removal of duplicated records; and linkage of forms of people who have been transferred. Proceeding in this way, the SINAN-TB database will be ready for analysis. The PNCT carries out qualification/preparation of the national database using a specific algorithm developed specifically for this purpose,16 given that it is unfeasible to carry it out via SINAN-Net because of to the large number of records. This process takes place three times a year (March, May and November), this being when the national database is updated.
Another important requirement for this process is having knowledge of the specific rules, the definition of each variable and how SINAN-TB functions. This more detailed information can be found in the SINAN-TB data dictionary and in the analysis notebook (a tool describing the calculation method for a series of TB indicators), the latest versions of which are available on the Ministry of Health’s SINAN Portal page: http://portalsinan.saude.gov.br/tuberculose
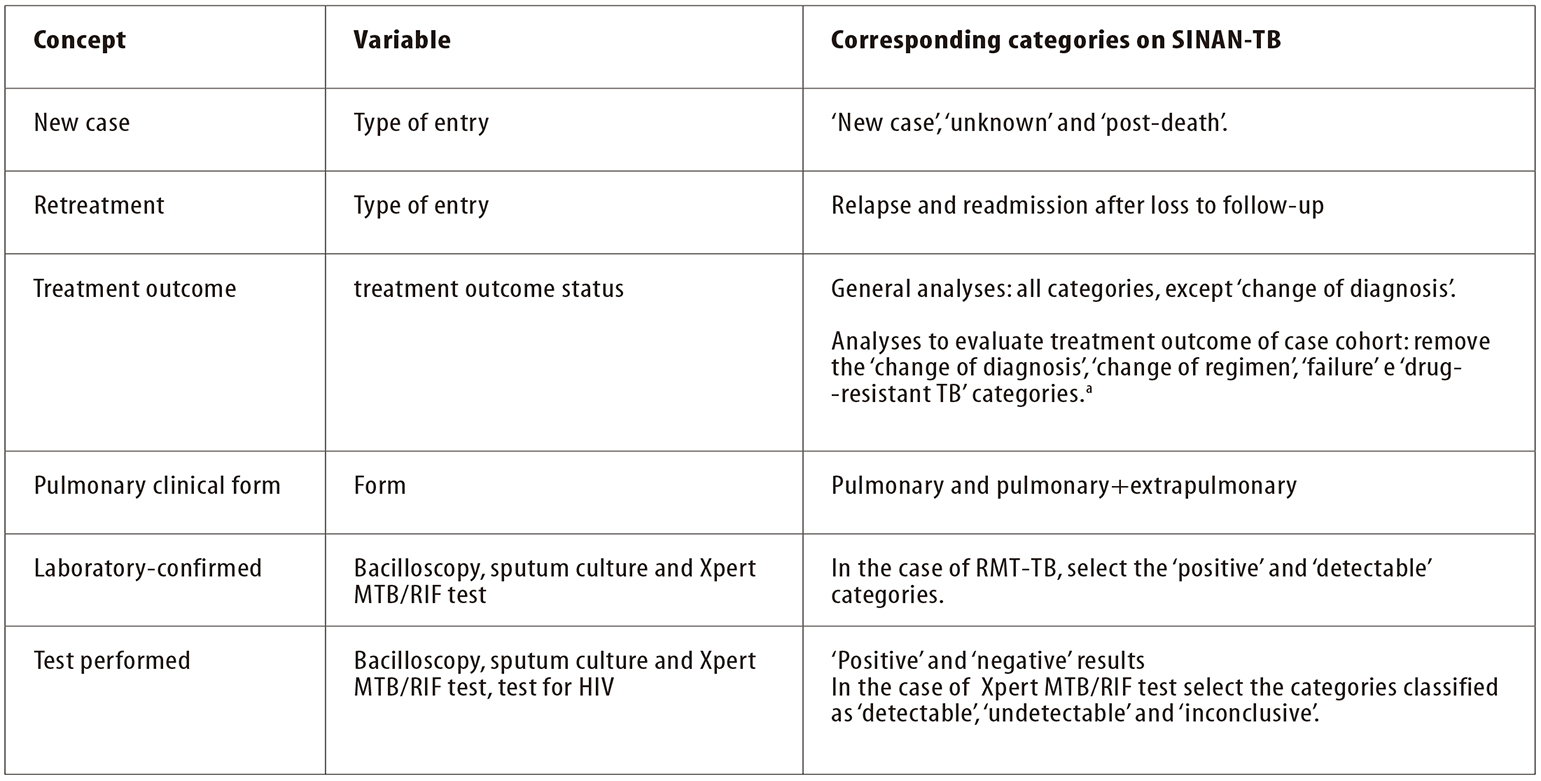
When tabulating indicators, categories of certain variables are sometimes grouped together with the aim of identifying a group or a characteristic. Figure 2 shows the concepts, the main variables and categories used when analyzing epidemiological and operational indicators. DATASUS14 provides the TabWin application17 for data analysis. This is a simple public domain tool which is quick for tabulating data from Brazil National Health System (SUS) information systems. Statistical software can also be used, such as Epi Info,18 EpiData,19 and R,20 among others.
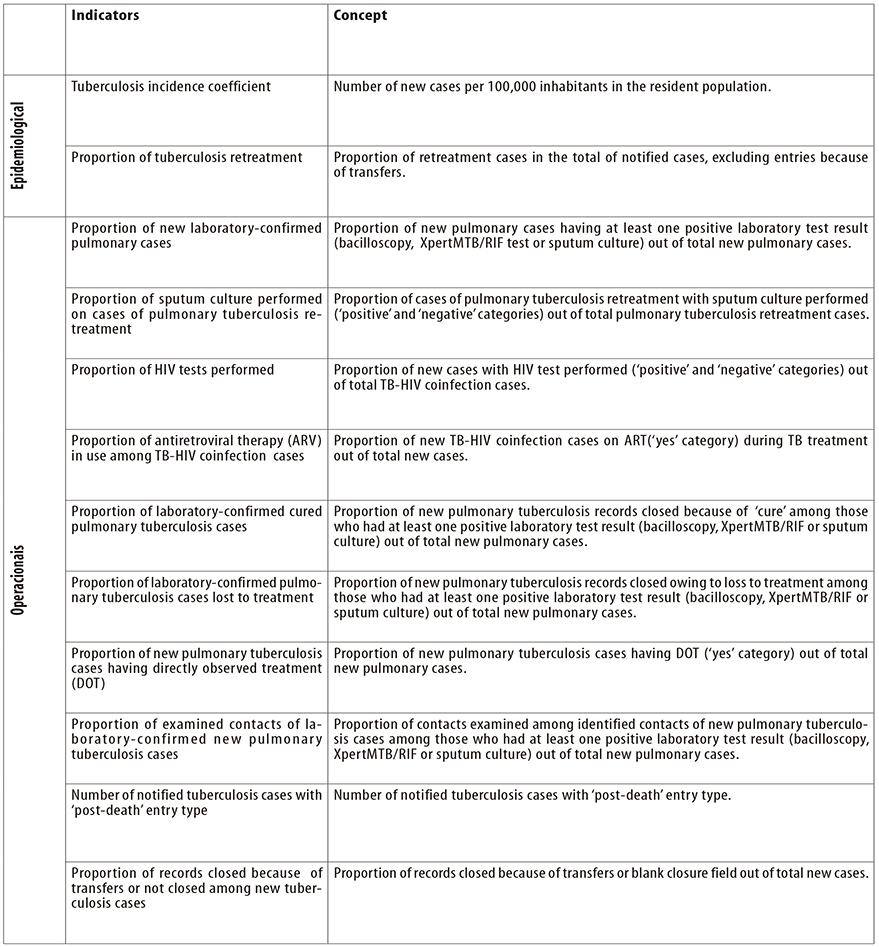
a) Record closures because of ‘change of regimen’, ‘failure’ and ‘drug-resistant TB’ (TB-DR) are excluded from analysis, as the patient will continue treatment on another system, namely the Special Treatment of Tuberculosis Information System (SITE-TB).
Figure 2 - Main variables and categories used in analyses of tuberculosis epidemiological and operational indicators
Uses
Data derived from SINAN-TB enable tuberculosis actions and incidence to be monitored, via indicators that evaluate both the epidemiological and the operational profile in relation to TB. The indicators can be monitored every three months and publicized annually, providing details for the national, state, regional and municipal levels, and even by health centers, according to place of residence or notification. Figure 3 shows the main indicators.
Access to data
The national data without patient identification generated by SINAN-Net can be requested via the Electronic System of the Citizens’ Information Service (e-SIC). In order to request them, interested parties must follow the guidance available at the following electronic address: http://www.acessoainformacao.gov.br/sistema/site/primeiro_acesso.html
To request national data with patient identification by name, interested parties must follow the procedure defined on the website of the Health Ministry’s Health Surveillance Secretariat(SVS/MS): http://portalms.saude.gov.br/vigilancia-em-saude
State and municipal data must be requested from the respective health departments.
Limitations and challenges
The system’s limitations include (i) low completeness of variable fields which are essential for SINAN-TB, such as schooling and race/skin color, special populations (prision population; homeless population; health professionals; immigrants), beneficiaries of government income transfer programs, associated diseases and conditions (AIDS; alcoholism; diabetes mellitus; mental illness; illegal drug use; tobacco smoking) and antiretroviral therapy during TB treatment; and likewise, (ii) low completeness of fields relating to treatment follow-up (treatment follow-up bacilloscopy; DOT carried out; contacts examined).
Another factor relates to unclosed records or records closed because of transfers remaining in the system, as well as failure to update variables related to laboratory tests which remain in course, such as in the ‘culture’ field, HIV test and sensitivity test (Figure 4). As a consequence, the analyses generated based on the existing data do not faithfully represent the actions carried out, thus compromising evaluation of measures taken and also management of surveillance activities. This is evidence of the need to recognize the importance of systematic evaluation of the quality of the information collected and input to SINAN, above all at the first computerized level, before transferring data to higher levels.2
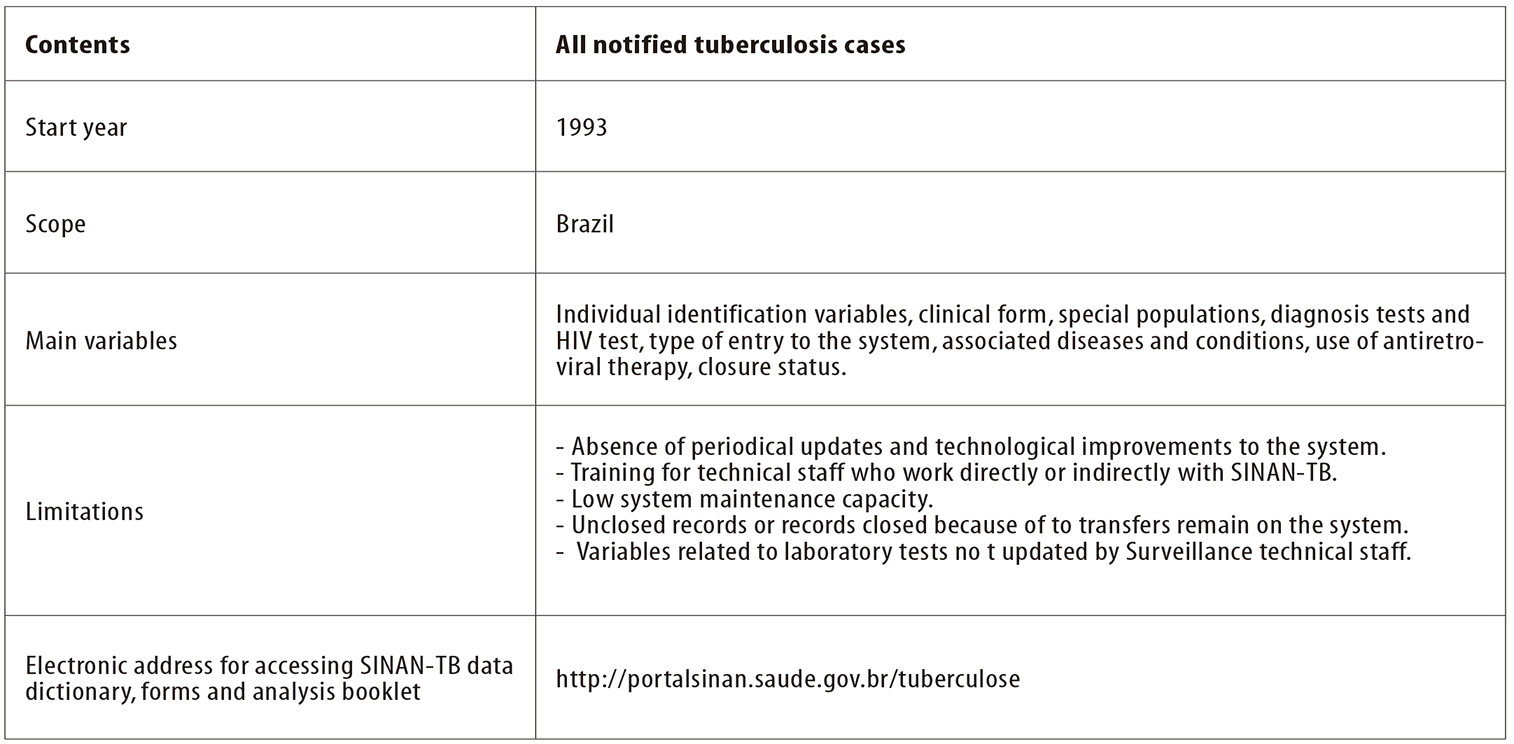
Figure 4 - Summary of the profile of the Notifiable Diseases Information System for Tuberculosis (SINAN-TB)
Operational errors also stand out which are attribuited to lack of knowledge by technical staff of case definitions, criteria for performing duplication and linkage routines, as well as the specific rules for SINAN-TB variables. Another issue to be addressed is the strengthening of integration between SINAN interlocutors at the state level and the Tuberculosis Control Program teams, so as to facilitate training and identification and solving of local problems.
Another notable point is low maintenance capacity, reflected in system technical failures and functionality failures and consequent risk of SINAN-TB being transformed from a database fit for analyzing, monitoring and evaluating actions carried out, into a merely bureaucratic system (Figure 4).
Other factors include obsolete language, interoperability with other health information systems and lack of SINAN versatility, with regard to its ability to adapt to changes. The pattern of disease occurrence, in addition to incorporation of new tools and strategies for their control, often requires updating of the data collected via the notification/investigation forms. Given that the system is not versatile, it is not always able to accompany such updating in a timely manner.
SINAN has undergone a variety of improvements and updates since it was first deployed and its most recent version was released in 2015. During the course of time, the TB notification/investigation form has also been modified with the aim of accompanying new TB surveillance concepts, in addition to enhancing information about the carrying out of diagnosis tests and about special populations. However, as shown here, the system has limitations that have direct impact on true and timely analysis of the epidemiological and operational situation of TB (Figure 4).
Considering that the Notifiable Diseases Information System for Tuberculosis is the main source used by health surveillance services to inform data analysis as well as the planning and monitoring of actions aimed at TB control at the three government levels, its current challenge lies in implementing a version of SINAN-TB that has a unique identifier per person, integrated with other information systems and built based on new technologies, so that tuberculosis data transfer and analysis is more streamlined in Brazil.
Referências
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Sistema de informação de agravos de notificação - Sinan: normas e rotinas [Internet]. 2. ed. Brasília: Ministério da Saúde; 2007 [citado 2019 ago 23]. 68 p. (Série A. Normas e Manuais Técnicos). Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/07_0098_M.pdf [ Links ]
2. Ministério da Saúde (BR). Organização Pan-Americana da Saúde. Fundação Oswaldo Cruz. A experiência brasileira em sistemas de informação em saúde: falando sobre os sistemas de informação em saúde no Brasil [Internet]. Brasília: Ministério da Saúde ; 2009 [citado 2019 ago 23]. 2 v. (Série B. Textos Básicos de Saúde). Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/experiencia_brasileira_sistemas_saude_volume2.pdf [ Links ]
3. Brasil. Ministério da Saúde. Portaria de Consolidação MS/GM nº 4, de 28 de setembro de 2017. Consolidação das normas sobre os sistemas e os subsistemas do Sistema Único de Saúde [Internet]. Diário Oficial da União, Brasília (DF), 2017 out 03 [citado 2019 ago 23];Seção 1:suplemento, página 288. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2017/prc0004_03_10_2017.html [ Links ]
4. Brasil. Presidência da República. Lei no 6.259, de 30 de outubro de 1975. Dispõe sobre as organizações de Vigilância Epidemiológica, sobre o Programa Nacional de Imunizações, estabelece normas relativas à notificação compulsória de doenças, e dá outras providências [Internet]. Diário Oficial da União, Brasília (DF), 1975 out 31 [citado 2019 ago 23]. Disponível em: Disponível em: http://www.planalto.gov.br/ccivil_03/leis/L6259.htm [ Links ]
5. Brasil. Presidência da República. Decreto no 78.231, de 12 de agosto de 1976. Regulamenta a Lei nº 6.259, de 30 de outubro de 1975, que dispõe sobre a organização das ações de Vigilância Epidemiológica, sobre o Programa Nacional de Imunizações, estabelece normas relativas à notificação compulsória de doenças, e dá outras providências [Internet]. Diário Oficial da União, Brasília (DF), 1976 out 12 [citado 2019 ago 23]. Disponível em: Disponível em: http://www.planalto.gov.br/ccivil_03/decreto/1970-1979/D78231.htm [ Links ]
6. Brasil. Ministério da Saúde. Instrução Normativa MS/SVS no 2, de 22 de novembro de 2005. Regulamenta as atividades de vigilância epidemiológica com relação à coleta, fluxo, periodicidade de envio de dados da notificação compulsória de doenças por meio do Sistema de Informação de Agravos de Notificação - Sinan [Internet]. Diário Oficial da União, Brasília (DF), 2005 nov 23 [citado 2019 ago 23]; Seção 1:46. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/svs/2005/int0002_22_11_2005.html [ Links ]
7. Laguardia J, Domingues CMA, Carvalho C, Lauerman CR, Macário E, Glatt R. Sistema de Informação de Agravos de Notificação (Sinan): desafios no desenvolvimento de um sistema de informação em saúde. Epidemiol Serv Saúde [Internet]. 2004 set [citado 2019 ago 23];13(3):135-47. Disponível em: Disponível em: http://scielo.iec.gov.br/pdf/ess/v13n3/v13n3a02.pdf . doi: 10.5123/S1679-49742004000300002 [ Links ]
8. Brasil. Ministério da Saúde. Portaria MS/GM no 1882, de 18 de dezembro de 1997. Estabelece o Piso da Atenção Básica - PAB - e sua composição [Internet]. Diário Oficial União, Brasília (DF), 1997 dez 22 [citado 2019 ago 23]; Seção 1:10. Disponível em: Disponível em: http://www.saude.pr.gov.br/arquivos/File/CIB/LEGIS/PortGM1882_18Dezembro_1997.pdf [ Links ]
9. Ministério da Saúde (BR). Organização Pan-Americana da Saúde. Fundação Oswaldo Cruz. A experiência brasileira em sistemas de informação em saúde: produção e disseminação de informações sobre saúde no Brasil. Brasília: Ministério da Saúde ; 2009 [citado 2019 ago 23]. 2 v. (Série B. Textos Básicos de Saúde). Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/experiencia_brasileira_sistemas_saude_volume1.pdf [ Links ]
10. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Manual do Sistema de Agravos de Notificação - Sinan-Net, versão 5.0. Brasília: Ministério da Saúde ; 2014 [citado 2019 ago 23]. 248 p. Disponível em: Disponível em: http://vigilancia.saude.mg.gov.br/index.php/download/manual-do-sinannet-5-0/ [ Links ]
11. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de recomendações para o controle da tuberculose no Brasil [Internet]. 2. ed. Brasília: Ministério da Saúde ; 2018 [citado 2019 ago 23]. 364 p. Disponível em: Disponível em: http://portalarquivos2.saude.gov.br/images/pdf/2019/marco/28/manual-recomendacoes.pdf [ Links ]
12. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Nota informativa CGPNCT/DEVIT/SVS nº 14, de 17 de novembro de 2017. Recomendações relacionadas à notificação de tuberculose e rotinas de duplicidade e vinculação de registros no Sinan [Internet]. Brasília: Ministério da Saúde ; 2017 [citado 2019 ago 23]. Disponível em: Disponível em: http://www.dive.sc.gov.br/conteudos/agravos/notas_tecnicas/NotaInformativa-n14SINAN-TB.pdf [ Links ]
13. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços. Guia de vigilância em saúde: volume 2 [Internet]. Brasília: Ministério da Saúde ; 2017 [citado 2019 ago 23]. 3 v. Disponível em: Disponível em: http://portalarquivos2.saude.gov.br/images/pdf/2017/setembro/05/Guia-de-Vigilancia-em-Saude-2017-Volume-2.pdf [ Links ]
14. Ministério da Saúde (BR). Departamento de Informática do Sistema Único de Saúde [Internet]. Brasília: Ministério da Saúde ; 2018 [citado 2019 ago 23]. Disponível em: Disponível em: http://www.datasus.gov.br [ Links ]
15. World Health Organization. Global tuberculosis report 2017: leave no one behind - unite to end TB [Internet]. Geneva: World Health Organization; 2017 [cited 2019 Aug 23]. Available from: Available from: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf?u%20a=1 [ Links ]
16. Oliveira GP, Bierrenbach ALS, Camargo Júnior KR, Coeli CM, Pinheiro RS. Acurácia das técnicas de relacionamento probabilístico e determinístico: o caso da tuberculose. Rev Saúde Pública [Internet]. 2016 ago [citado 2019 ago 23];50:49. Disponível em: Disponível em: http://www.scielo.br/pdf/rsp/v50/pt_0034-8910-rsp-S1518-87872016050006327.pdf . doi: 10.1590/S1518-8787.2016050006327 [ Links ]
17. Ministério da Saúde (BR). TabWin: tabulador para Windows versão 3.6b [Internet]. Brasília: Ministério da Saúde ; 2019 [citado 2019 ago 23]. Disponível em: Disponível em: http://www.datasus.gov.br/tabwin [ Links ]
18. Centers for Disease Control and Prevention. Epi Info [computer program]. EPI Info: a database and statistics program for public health professionals Version 7.2 [Internet]. Atlanta: Centers for Disease Control and Prevention; 2017 [cited 2019 Aug 23]. Available from: Available from: https://www.cdc.gov/epiinfo/support/downloads.html [ Links ]
19. Lauritsen JM, Bruus M, Myatt MA. An extended tool for validated data entry and documentation of data (v2.1) [Internet]. Odense (DK): EpiData Association; 2002 [cited 2019 Aug 23]. (Portuguese version by João Paulo Amaral Haddad - Brazil dialect). Available from: Available from: http://www.epidata.dk/downloads/epdintro_pt.pdf [ Links ]
20. R Core Team (2017). R: A language and environment for statistical computing. R Vienna (AT): Foundation for Statistical Computing; 2017 [cited 2019 Aug 23]. Available from: Available from: https://www.R-project.org/ [ Links ]
21. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Perspectivas brasileiras para o fim da tuberculose como problema de Saúde Pública. Bol Epidemiol [Internet]. 2016 [citado 2019 ago 23];47(13);1-15. Disponível em: Disponível em: http://portalarquivos2.saude.gov.br/images/pdf/2016/marco/24/2016-009-Tuberculose-001.pdf [ Links ]
22. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Indicadores prioritários para o monitoramento do Plano Nacional pelo Fim da Tuberculose como Problema de Saúde Pública no Brasil. Bol Epidemiol [Internet]. 2017 [citado 2019 ago 23];48(8):1-11. Disponível em: Disponível em: http://portalarquivos2.saude.gov.br/images/pdf/2017/marco/23/2017-V-48-N-8-Indicadores-priorit--rios-para-o-monitoramento-do-Plano-Nacional-pelo-Fim-da-Tuberculose-como-Problema-de-Sa--de-P--blica-no-Brasil.pdf [ Links ]
Received: February 21, 2019; Accepted: July 16, 2019











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI