Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.2 Brasília 2020 Epub 24-Abr-2020
http://dx.doi.org/10.5123/s1679-49742020000200015
ORIGINAL ARTICLE
Epidemiological situation and control of schistosomiasis in Pernambuco, Brazil: a descriptive study, 2010-2016*
1Fundação Instituto Oswaldo Cruz, Instituto Aggeu Magalhães, Recife, PE, Brazil
2 Universidade de Pernambuco, Faculdade de Enfermagem Nossa Senhora das Graças, Recife, PE, Brazil
Objective:
to describe schistosomiasis control actions and its epidemiological situation in Pernambuco, Brazil, 2010-2016.
Methods:
this was a descriptive study using data from the Schistosomiasis Surveillance and Control Program Information System for 116 municipalities, including indicators related to control actions (population surveyed, tests performed, treatment coverage) and epidemiological actions (positivity, parasite load, other helminthiases).
Results:
Health Regions II, III, IV, V and XII, which are traditionally endemic, registered higher average percentages for control actions (population surveyed [6.5%, 6.0%, 2.0%, 12.0%, and 13.0%], tests performed [75.0%, 75.5%, 74.0%, 74.0%, and 68.5%], and treatment coverage [71.0%, 82.5%, 82.0%, 91.0%, and 73.0%], respectively), and higher average percentages for epidemiological variables (positivity [3.5%, 8.0%, 1.0%, 2.0%, and 6.5%], high parasite load [0.1%, 0.7%, 0.02%, 0.03%, and 0.5%], and other helminthiases [4.0%, 11.0%, 4.0%, 6.0%, and 8.0%], respectively).
Keywords: Schistosomiasis; Information Systems; Endemic Diseases; Neglected Diseases; Public Health Surveillance; Epidemiology Descriptive
Introduction
Schistosomiasis control has made progress in Brazil, being linked to the process of epidemiology and disease control decentralization actions and reinforced by the 2006 Basic Operational Norms.1 Within this new context, municipal health departments have been encouraged to gain knowledge of the local reality of schistosomiasis and to overcome the diagnosis-treatment dyad which was characteristic of classic control actions, in order for these actions to become part of Primary Health Care.2-4 Schistosomiasis is still a public health problem, as in 2017 it affected approximately 1.5 million people in Brazil, 80% of whom lived in the country’s Northeast region.1 Moreover, the severe clinical forms in which schistosomiasis manifests itself contribute to its magnitude and transcendency.5,6
Pernambuco is one of the Northeastern states with high schistosomiasis prevalence.2 As a classically chronic and rural disease, associated with poverty and occurring above all in the Zona da Mata (an area where the soil is fertile, rivers are perennial and not subject to periodic droughts), schistosomiasis took on new facets to its epidemiological profile when it began to occur in the acute form, in outbreaks located along the state’s coast.3,4 In 2015, mean positivity was 3% in the areas examined in Pernambuco, accounting for approximately 180 deaths a year between 2005 and 2014. This mortality rate is five times greater than the national rate.7 These particularities contribute to the Brazilian epidemiological transition pattern, with old and new problems existing alongside each other, making it a hard-to-control nosological entity.2,3
The contribution of government bodies, considering the large number of factors associated with the disease and its different forms of expression, is an essential condition for supporting schistosomiasis control actions. The Schistosomiasis Control Program (PCE), formerly called the Special Schistosomiasis Control Program (PECE), implemented in 1976, was launched by the Ministry of Health with the aim of providing guidance to municipal health departments on control of the disease, concentrating its actions on diagnosis and treatment of infected cases in endemic areas.1 In order to make control feasible at the local level, PCE advocates the use of the Schistosomiasis Surveillance and Control Program Information System (SISPCE), managed by the Health Ministry’s Health Surveillance Secretariat. The system is responsible for compiling, along with other information, data on coproscopic surveys and treatment of cases among the population of these areas.1,7
In 2011, Pernambuco launched its Program to Address Neglected Diseases (SANAR),7 an advanced initiative in its approach to schistosomiasis, aligned with World Health Organization (WHO) recommendations for responding to neglected diseases, performing mass treatment in areas with high positivity and integrating surveillance activities with those of Primary Care. SANAR places emphasis on the importance of health education and controlling host mollusks, in addition to routine activities already carried out: diagnosis, treatment and data input to SISPCE by surveillance sectors.8 Initially, SANAR’s objective was to reduce the burden of these diseases in 108 priority municipalities by the end of the 2011-2014 four-year period. Following this, in the 2015-2018 four-year period, the program increased the geographic area of its actions to cover 144 municipalities.9
The objective of this study was to describe schistosomiasis control actions and epidemiological situation in the state of Pernambuco, Brazil, between 2010 and 2016.
Methods
This was a descriptive study using SISPCE secondary data on endemic schistosomiasis areas in Pernambuco, comprising 116 endemic municipalities distributed over seven Health Regions: I, II, III, IV, V, VIII and XII. The researchers chose the period 2010-2016 because the regionalization process was reorganized in Pernambuco in 2010.10
Pernambuco is comprised of 184 municipalities and the island of Fernando de Noronha, with an estimated population in 2019 of 9,557,071 inhabitants occupying an area of 98,068.021km2, representing demographic density of 89.62 inhab./km2. It is a state marked by social inequalities.11
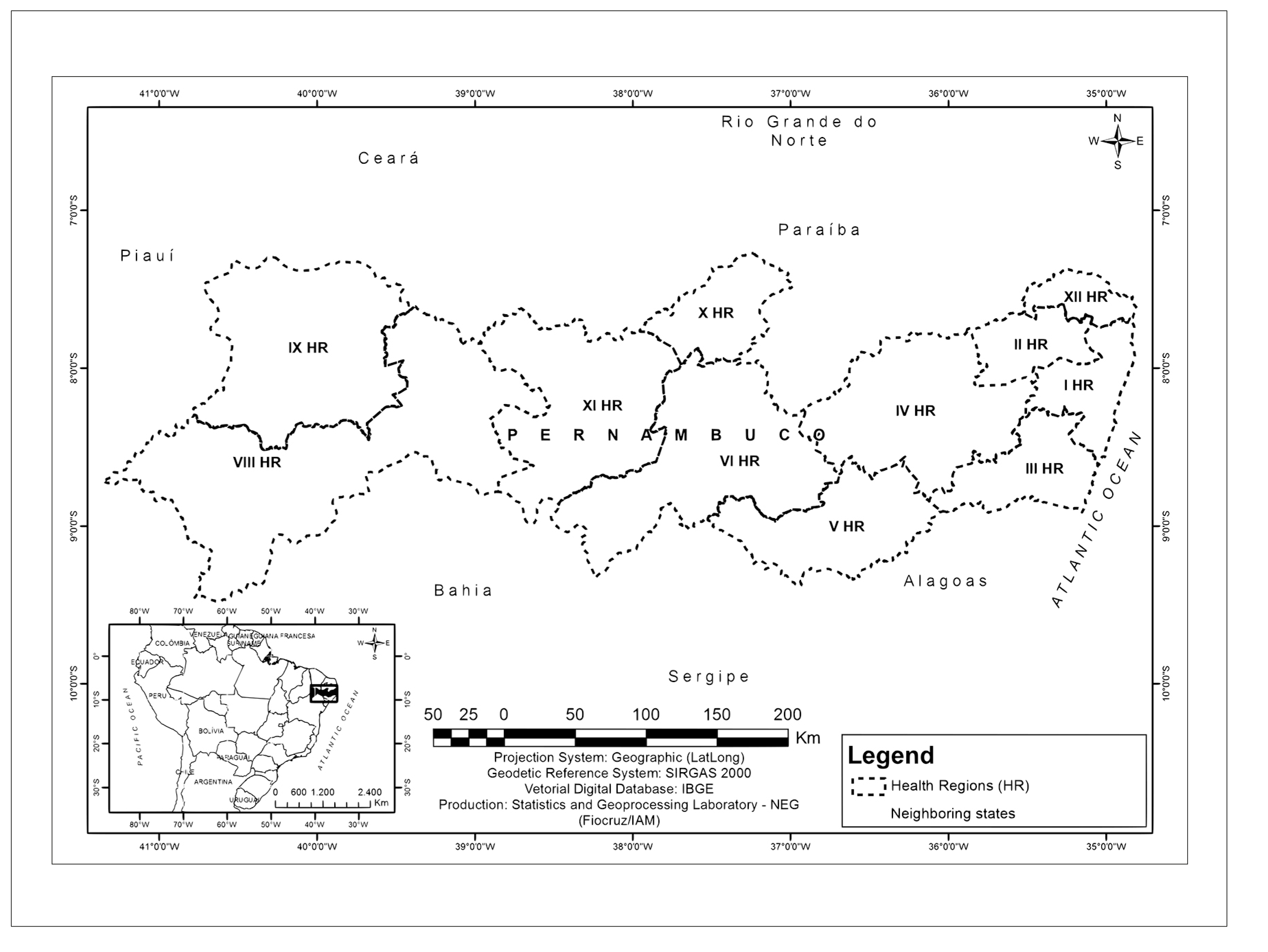
In order to assist health service organization, the state has been divided into 12 regions, distributed over four macro-regions based on natural geographic characteristics: (i) the Metropolitan macro-region, covering the coast and the Zona da Mata, comprised of Health Regions I, II, III and XII; (ii) the Agreste macro-region, comprised of Health Regions IV and V; (iii) the Sertão macro-region, comprised of Health Regions VI, X and XI; and (iv) the River São Francisco Valley and Araripe macro-region, comprised of Health Regions VII, VIII and IX.7,10-12 (Figures 1 and 2).
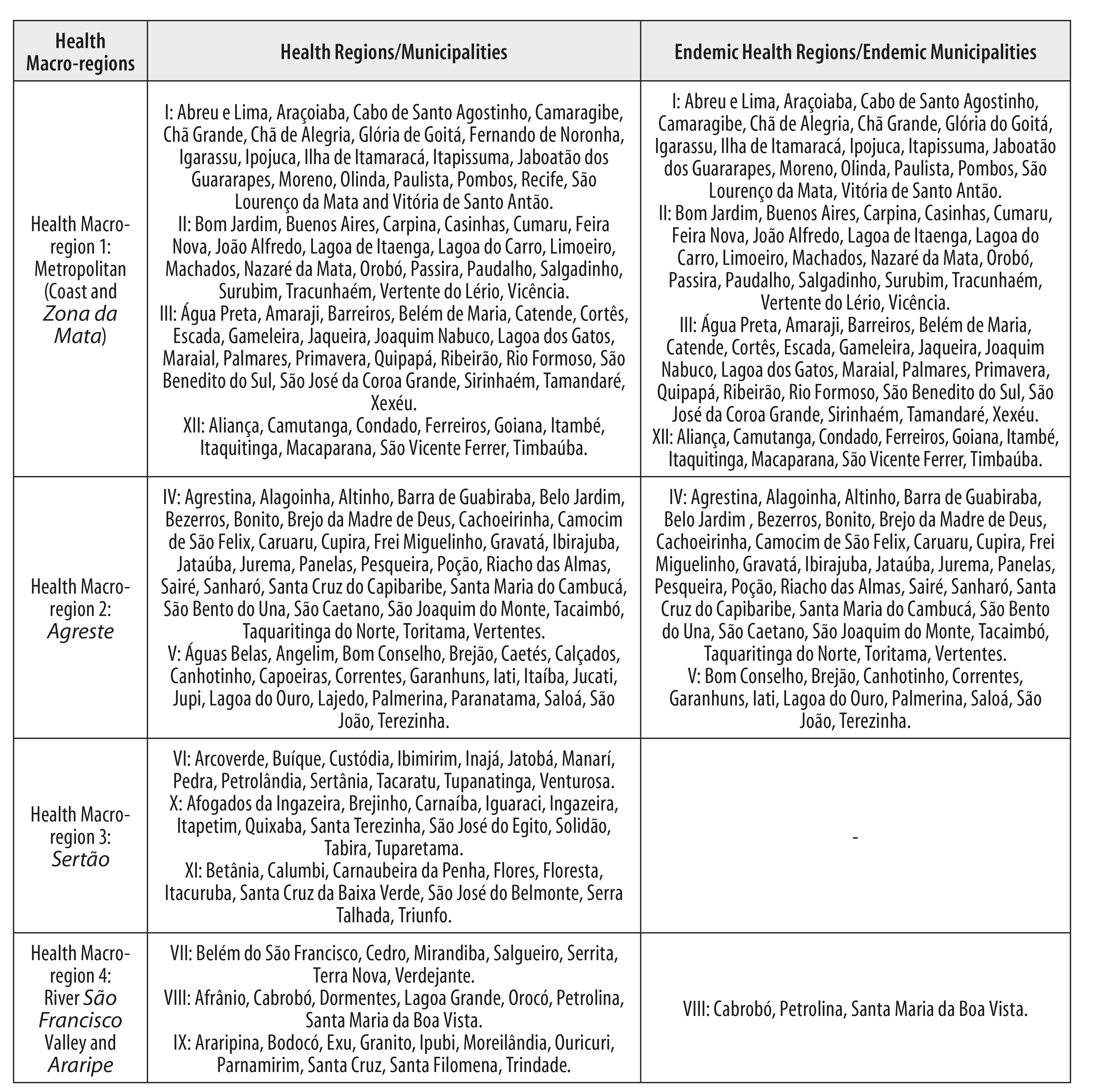
Figure 2 - Municipality distribution by health macro-region and health region, Pernambuco, 2010-2016
This division is important, given that, historically, schistosomiasis occurred in the Zona da Mata area, which is naturally humid, with abundant freshwater rivers and rainfall, as well as occurring in the Agreste area. Issues related to migratory flow have lead to the disease spreading to the coast and leaving the Sertão area at risk of active transmission becoming established.2,4,13 These areas were only included as a priority when changes were found in the profile of schistosomiasis occurrence.2-4,7,12 In 2000, an outbreak of acute schistosomiasis occurred in Porto de Galinhas.14 Interventions took place and the disease was believed to have been controlled at that coastal resort. However, a new survey was conducted in 2011 and new cases with characteristics of the chronic form of the disease were found.14,15 In addition, areas vulnerable to the establishment of the transmission cycle, with presence of bodies of water and snails, have been reported in the Sertão macro-region since 2011.13
This study was based on data from PCE Form 101, a standard SISPCE form intended to gather information about field activities carried out by health departments in endemic municipalities. The study covered the period from October to December 2018.12
The indicators to be calculated, regarding epidemiological control actions, were classified according to Ministry of Health defined standards,12 as follows:
a) control actions
- population surveyed (individuals targets of coproscopic surveys [%]);
- tests performed (individuals undergoing coproscopic tests [%]);
- treatment coverage (treated individuals [%]);
b) epidemiological actions
- positivity (individuals with positive schistosomiasis test results [%]);
- low and medium parasite load (individuals with up to 16 eggs per gram of feces [%]);
- high parasite load (individuals with more than 17 eggs per gram of feces [%]); and
- positivity for other helminthiases (individuals with positive test results for other geo-helminthiases [%]).
The means used to calculate the relative frequencies of the variables is explained in Figure 3, which also shows the parameters defined by the Ministry of Health12 to guide endemic municipalities on the local epidemiological situation of schistosomiasis and control actions.
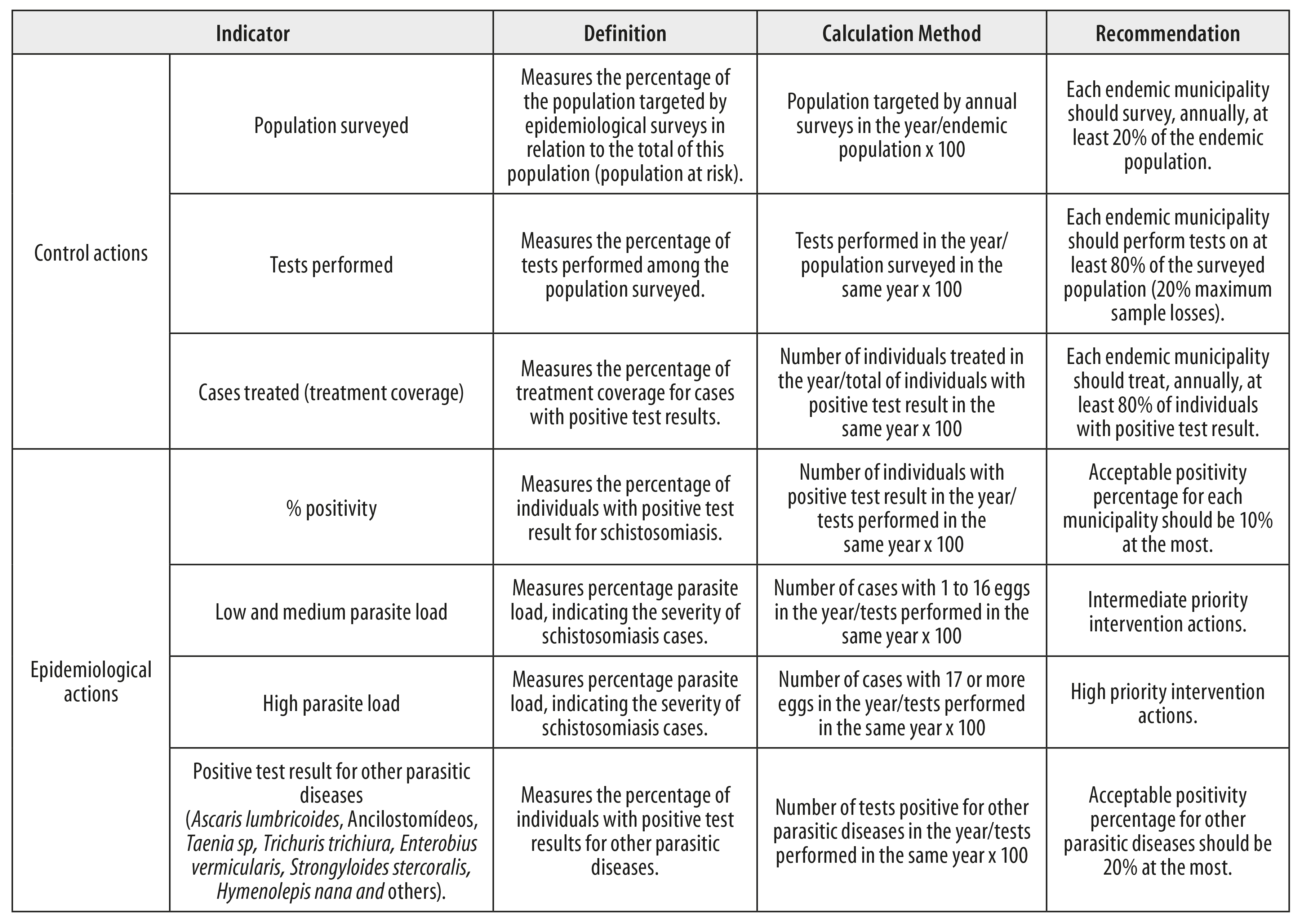
Figure 3 - Indicators for schistosomiasis control actions and epidemiological actions selected by the Schistosomiasis Surveillance and Control Program Information System
The data were tabulated using Excel Microsoft Office 2016. The non-population-weighted mean was calculated for each variable, by Health Region. This calculation enables municipal parameters to be applied for these Regions.
The study project was approved by the Aggeu Magalhães Institute Research Ethics Committee, Oswaldo Cruz Institute Foundation/Pernambuco: Opinion No. 3.098.896, issued on December 20th 2018, and as per Certification of Submission for Ethical Appraisal (CAAE) No. 03980918.3.0000.5190.
Results
The population surveyed in the 116 endemic municipalities of Pernambuco, in the period studied, totaled 1,496,463 individuals.
Considering the years for which records existed, Health Region XII, belonging to the Zona da Mata macro-region, had the highest surveyed population percentage, namely 19.0% in 2014, while Health Region IV, located in the Agreste, had the lowest percentage: 0.2% from 2011 to 2013. 1,071,982 coproscopic tests were performed. The majority of the Regions examined approximately 70.0% of the target population, whereby Health Region IV had the highest rate (86.0%), in 2012, and Health Region VIII had the lowest rate (50.5%), in 2015. A total of 35,973 individuals received treatment, with the highest percentage (100.0%) being recorded in Health Region III in 2014, while the lowest percentage (47.05) was recorded in Health Region I in 2016 (Table 1).
Table 1 - Percentages of population surveyed, tests performed and treatment coverage, by Pernambuco health regions, 2010-2016
| Health Region | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | |||||||||||||||
| PS | TP | TC | PS | TP | TC | PS | TP | TC | PS | TP | TC | PS | TP | TC | PS | TP | TC | PS | TP | TC | |
| I | 3.0 | 68.0 | 71.0 | 2.5 | 72.0 | 72.0 | 3.0 | 68.0 | 73.0 | 3.0 | 67.0 | 78.0 | 4.0 | 67.0 | 73.5 | 3.0 | 67.0 | 58.0 | 2.0 | 73.0 | 47.0 |
| II | 3.0 | 78.0 | 81.0 | 3.0 | 82.0 | 87.5 | 3.0 | 76.0 | 83.0 | 3.0 | 77.0 | 76.0 | 11.0 | 77.0 | 66.0 | 12.0 | 72.0 | 59.0 | 9.0 | 73.0 | 57.0 |
| III | 5.0 | 77.0 | 86.0 | 5.5 | 75.0 | 86.0 | 3.0 | 77.0 | 91.0 | 7.0 | 71.0 | 82.5 | 9.0 | 74.0 | 100.0 | 8.5 | 79.0 | 59.0 | 5.0 | 76.0 | 67.0 |
| IV | - | - | - | 0.2 | 78.0 | 87.5 | 0.2 | 86.0 | 79.0 | 0.2 | 81.5 | 95.0 | 4.0 | 73.0 | 91.0 | 5.0 | 75.0 | 81.5 | 2.0 | 73.0 | 63.5 |
| V | - | - | - | - | - | - | - | - | - | - | - | - | 13.0 | 71.5 | 93.0 | 11.0 | 75.0 | 90.0 | 10.0 | 75.0 | 87.0 |
| VIII | - | - | - | - | - | - | - | - | - | - | - | - | 0.1 | 84.0 | - | 0.4 | 50.5 | - | - | - | - |
| XII | 9.0 | 72.0 | 81.0 | 10.0 | 72.0 | 70.0 | 11.0 | 68.0 | 80.0 | 12.0 | 68.0 | 79.0 | 19.0 | 69.0 | 72.5 | 18.0 | 67.0 | 59.0 | 10.0 | 65.5 | 59.0 |
a) PS: population surveyed.
b) TP: tests performed.
c) TC: treatment coverage.
Positive schistosomiasis results totaled 47,467 cases. Health Region XII had highest positivity, 15.2% in 2010, while it was lowest in Health Region IV, 0.5% in 2016. Health Regions III and XII had the highest percentages, followed by Health Region II, principally in 2010 (7.3%) and 2011 (7.9%). With effect from 2012, these percentages began to reduce in all the Health Regions studied (Table 2).
Table 2 - Percentages of positivity, parasite load and positivity for other helminthiases, by Pernambuco health regions, 2010-2016
| Health Region | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | ||||||||||||||||||||||
| P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | P | PL low and medium | PL high | POH | |
| I | 5.2 | 4.7 | 0.5 | 12.0 | 4.4 | 4.0 | 0.4 | 10.0 | 4.5 | 4.2 | 0.3 | 9.0 | 4.1 | 3.8 | 0.3 | 7.0 | 2.9 | 2.8 | 0.1 | 5.0 | 2.3 | 2.1 | 0.2 | 4.0 | 1.9 | 1.8 | 0.1 | 3.0 |
| II | 7.3 | 7.0 | 0.3 | 2.0 | 7.9 | 7.5 | 0.4 | 4.0 | 4.2 | 4.1 | 0.1 | 12.0 | 3.8 | 3.7 | 0.1 | 4.5 | 3.1 | 2.9 | 0.2 | 3.0 | 2.5 | 2.4 | 0.1 | 3.0 | 2.1 | 2.0 | 0.1 | 3.0 |
| III | 9.6 | 9.0 | 0.6 | 17.0 | 12.3 | 11.3 | 1.0 | 16.0 | 10.6 | 10.0 | 0.6 | 14.0 | 8.6 | 8.1 | 0.5 | 13.0 | 6.4 | 6.0 | 0.4 | 6.0 | 6.5 | 5.8 | 0.7 | 9.0 | 5.2 | 4.7 | 0.5 | 6.5 |
| IV | - | - | - | - | 1.5 | 1.5 | - | - | 2.0 | 1.9 | 0.1 | 3.5 | 1.0 | 1.0 | - | 0.5 | 0.6 | 0.5 | 0.1 | 3.0 | 0.7 | 0.7 | - | 5.0 | 0.5 | 0.5 | - | 4.0 |
| V | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.3 | 2.2 | 0.1 | 4.0 | 1.6 | 1.6 | - | 5.0 | 1.4 | 1.4 | - | 5.0 |
| XII | 15.2 | 13.6 | 1.6 | 10.5 | 8.0 | 7.5 | 0.5 | 9.0 | 6.2 | 5.7 | 0.5 | 10.5 | 5.8 | 5.4 | 0.4 | 9.0 | 5.1 | 4.7 | 0.4 | 7.0 | 5.0 | 4.7 | 0.3 | 6.0 | 3.7 | 3.5 | 0.2 | 5.5 |
P: positivity.
PL: parasite load.
POH: positivity for other helminthiases.
Note:
Health Region VIII had no positivity for schistosomiasis, nor for other helminthiases, in any of the years in the time series covered by the study.
Among individuals with a positive coproscopic test result, 44,245 had low and medium parasite load (1-16 eggs per gram of feces). Health Region XII had the highest percentage of low and medium parasite load (13.6% in 2010), while Health Region IV had the lowest (0.5% in 2014 and 2016). In general, in all regions the loads recorded tended to reduce (Table 2).
High parasite load was found in 3,222 individuals. Health Region XII had the highest rate (1.6% in 2010); in all the other regions the rate was below 1.0%.
Regarding other helminthiases, 2,240 individuals were found to be positive. Analysis by year shows that Health Region III had the highest rate (17.0% in 2010), while Health Region IV had the lowest rate (0.5% in 2013) (Table 2).
Discussion
Up until 2014, the control action indicators either remained mathematically stable or improved in all Health Regions, even though in the majority of them they were below the recommended ideal, i.e. more than 20% of the population surveyed and tests performed and treatment coverage in excess of 80%.1,7 After 2014, indicator percentages can be seen to fall, and this occurred up until the end of the time series in all Health Regions: those belonging to the Zona da Mata (Health Regions II, III and XII) and to the Agreste (Health Regions IV and V), which are traditionally endemic and targets of actions, continued to have the highest percentages. The epidemiological indicators were found to have reduced between 2010 and 2016 in the majority of Health Regions; while the Regions in the Zona da Mata area had the highest positivity rates and the highest parasite loads at the end of that period. It is noteworthy that percentage control actions and epidemiological actions in the majority of the Regions were not within the percentage indicated by the national government (under 10%).12
Inconsistencies related to typing errors and/or losses in the process between data collection and data input on the system are limitations of this study. The information provided here refers only to the percentage of the endemic population submitted to coproscopic surveys, so that no population inference can be made based on the analysis performed here. Another limitation, relating specifically to use of the SISPCE system, lies in the unavailability of information about malacology, educational activities and sanitation activities, thus making more complete characterization of control actions impossible. This system, brought into force in 1995, is the only public domain source providing information on routine PCE activities in all endemic municipalities,1 thus advocating in favor of its high coverage and data availability. The information available, for the purposes of descriptive analysis of control actions and the epidemiological situation, is robust, given the existence of operational standardization since the decentralization period, as put forward in Ministry of Health12 and SANAR7 publications.
The increase in control action percentages up until 2014 can be explained by the integration of routine actions carried out by surveillance sectors (case diagnosis and treatment), and Family Health Strategy (ESF) team actions. This form of integration was proposed by SANAR in 2011.7 A critical review of the literate supports this hypothesis as it infers that the carrying out of traditional actions, together with actions proposed for Primary Care, has contributed to improved levels of knowledge and empowerment of both health professionals and the community, resulting in improved schistosomiasis care and control actions.8
The better control action results for traditionally endemic Health Regions can be explained by the state’s tradition of carrying out all-round disease surveillance and control actions. A study of surveillance evolution in Brazil supports these conclusions when it affirms that communicable disease control in Brazil is well structured, has existed since before the National Epidemiological Surveillance System was organized in 1975, as well as being based on a vertical logic under the responsibility of the Campaign Superintendency.15 This action model is therefore ingrained in the way schistosomiasis control is approached in Pernambuco.
The reduction in control action percentages after 2014 may have arisen from changes resulting from the launch of a second SANAR phase. The first phase received more financial investment, which may have encouraged active participation on the part of the stakeholders. Contingency policies lead to a review of priorities as contained in a second plan for the period 2015-2018, indicating lower adherence on the part of implementing stakeholders.2,9,16 A literature review study of factors related to policy implementation in low and middle-income countries corroborates these findings by showing that workers tend to resist to change because it disorganizes established power structures.17
The increase in the survey target population and in tests performed, although the latter are below the level recommended by the Ministry of Health, and the reclassification of new areas as being endemic (Health Regions IV and VIII) are related to sample-based coproscopic surveys and malacological surveys proposed by SANAR. Studies highlight the need for these actions to be carried out in order to identify areas with the potential for transmission.4,13 One of these studies, conducted in a municipality in Pernambuco’s Zona da Mata, proves the existence of new transmission areas based on the results of malacological surveys.4 Another study, conducted in 2012 with the aim of verifying the occurrence of snails of the Biomphalaria genus in two dams of importance for the River São Francisco, found that snails of this genus did exist there.13 Although they were not contaminated with Schistosoma mansoni, the presence of these snails, along with favorable environmental conditions and the influx of tourists, is a risk for the establishment of the active schistosomiasis transmission cycle.13
Growth of the tourism sector, along with accelerated real estate development, has interfered with environments where ecological equilibrium is relatively fragile. This also adds to the challenge of the increase in the population to be surveyed and performance of coproscopic tests in all of Pernambuco’s Health Regions. Studies conducted in 2015 and 2016 about the risk of tourism in areas vulnerable to S. mansoni, pointed to the exodus of people with schistosomiasis to urban and rural touristic areas in search of work, thus facilitating the schistosomiasis transmission cycle with effect from when the host has contact with human beings.14,18 This situation can be understood as part of the negative aspects of the process of globalization: when contagious people and agents are taken from their original territory, the occurrence of diseases of considerable impact is facilitated in other regions and places where health systems are not prepared to deal with situations that are complex and unprecedented for them.19
The treatment coverage percentages identified in this study suffered oscillations, and in most years did not meet the level recommended by the Health Ministry. This is a finding of concern, to the extent that low treatment coverage levels imply that the transmission cycle persists. A studying assessing this indicator in 11 municipalities in the Metropolitan Region of Recife between 2003 and 2005, found evidence that seven of them had low treatment coverage percentages.20 A second study, with the same objective, regarding municipalities in Pernambuco’s Zona da Mata region, found that in half of them coverage treatment was below the 80% Health Ministry recommended level,21 thus reinforcing that coverage levels below 80% are a recurring fact in the state. This result may possibly be related to shortage of health professionals, absence of positive case referral to health centers, as well as weaknesses in mobilizing the population to get treated with praziquantel.22-24
Reduction in schistosomiasis positivity and parasite load and reduction in positivity for other helminthiases did not occur in a uniform manner between the Health Regions: those belonging to the Zona da Mata region had the worst results. Overall reduction may be associated with mass treatment being implemented in hyperendemic places.7,24,25 This strategy was proposed in order to control/eliminate neglected tropical diseases in places with positivity equal to or greater than 10%, considering the large number of false-negative results found in parasitology tests.7 Mass treatment takes place by administrating broad-spectrum drugs (praziquantel, albendazole, mebendazole), which given their simplicity - increase their scope when they are combined, in addition to reducing health system costs7,9,24,26,27 A study of schistosomiasis hyperendemic sites in Jaboatão dos Guararapes between 2011 and 2013, confirmed that mass treatment was useful for reducing both positivity and parasite load.24
Individuals with positive test results for schistosomiasis and other helminthiases continuing to be found in traditionally endemic regions is related both to issues concerning the physiopathology of severe forms of the disease, in places where socio-economic conditions are precarious, and also to the inefficacy of praziquantel as an isolated strategy for eliminating schistosomiasis in hyperendemic regions. A study of the use/efficacy of this medication on its own when administered for four years (2010-2014) in 67 Kenyan children found that the parasite load in 15 of them did not reduce, highlighting the parasite’s ability to persist in endemic areas despite systematic treatment.28 Another study evaluating the effectiveness of mass treatment of schistosomiasis worldwide, makes provisos with regard to this strategy when it highlights that praziquantel efficacy is only 50% and that this component, on its own, is not sufficient to eliminate schistosomiasis as a Public Health problem. For this reason its authors defend the efficiency of integrated control.29 Socio-economically precarious communities where low schooling levels together with environmental problems prevail, are characterized by these additional factors which result in these problems continuing, within a context in which reinfection is facilitated.1-7,9,10,12,14,16,18,23-29
The reduction in the percentage of positivity for other helminthiases may be associated with treatment with low toxicity medication (albendazole, mebendazole), which has taken place for decades in Brazil and has been reinforced by SANAR.7,25 Data from the national survey on the prevalence of Manson’s schistosomiasis and geo-helminthiases showed that periodic use of these low cost and easy to administer forms of medication is responsible for the reduction in approximately 80% of cases of other helminthiases.30
The fact that other helminthiases and schistosomiasis persist, above all in their severe form in traditionally endemic regions, suggests the need for these actions to be reinforced.
REFERENCES
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços. Guia de Vigilância em Saúde: volume 3 [Internet]. Brasília: Ministério da Saúde; 2017 [citado 2020 mar 6]. Disponível em: Disponível em: https://www.hc.ufu.br/sites/default/files/tmp//volume_3_guia_de_vigilancia_em_saude_2017.pdf [ Links ]
2. Costa CS, Rocha AM, Silva GS, Jesus RPFS, Albuquerque AC. Programa de controle da esquistossomose: avaliação da implantação em três municípios da Zona da Mata de Pernambuco, Brasil. Saúde Debate [Internet]. 2017 mar [citado 2020 mar 6];41(spe):229-41. Disponível em: Disponível em: https://doi.org/10.1590/0103-11042017s17 [ Links ]
3. Oliveira ECA, Pimentel TJF, Araujo JPM, Oliveira LCS, Fernando VCN, Loyo RM, et al. Investigação sobre os casos e óbitos por esquistossomose na cidade do Recife, Pernambuco, Brasil, 2005-2013. Epidemiol Serv Saúde [Internet]. 2018 [citado 2020 mar 6];27(4):e2017190. Disponível em: Disponível em: https://doi.org/10.5123/s1679-49742018000400010 [ Links ]
4. Gomes ECS, Mesquita MCS, Rehn VNC, Nascimento WRC, Loyo R, Barbosa CS. Transmissão urbana da esquistossomose: novo cenário epidemiológico na Zona da Mata de Pernambuco. Rev Bras Epidemiol [Internet]. 2016 out-dez [citado 2020 mar 6];19(4):822-34. Disponível em: Disponível em: https://doi.org/10.1590/1980-5497201600040012 [ Links ]
5. Rocha TJM, Santos MCS, Lima MVM, Calheiros CML, Wanderley FS. Aspectos epidemiológicos e distribuição dos casos de infecção pelo Schistosoma mansoni em municípios do Estado de Alagoas, Brasil. Rev Pan-Amaz Saúde [Internet]. 2016 jun [citado 2020 mar 6];7(2):27-32. Disponível em: Disponível em: http://dx.doi.org/10.5123/S2176-62232016000200003 [ Links ]
6. Palasio RGS, Bortoleto NA, Rosa-Xavier IG, Andrighetti MTM, Tuan R, Chiaravalloti-Neto F. Schistosomiasis in the Middle Paranapanema river region, state of São Paulo, Brazil: does it matter today for public health? Rev Soc Bras Med Trop [Internet]. 2019 Jun [cited 2020 Mar 6];52:e20180447. Available from: Available from: https://doi.org/10.1590/0037-8682-0447-2018 [ Links ]
7. Secretaria Estadual de Saúde (PE). Secretaria Executiva de Vigilância em Saúde. SANAR: programa de enfrentamento das doenças negligenciadas no Estado de Pernambuco, 2011-2014 [Internet]. 2. ed. Recife: Secretaria Estadual de Saúde de Pernambuco; 2014 [citado 2020 mar 6]. 39 p. (Série A. Normas e Manuais Técnicos). Disponível em: Disponível em: http://portal.saude.pe.gov.br/sites/portal.saude.pe.gov.br/files/plano_sanar_2011-2014.pdf [ Links ]
8. Bizimana P, Giuseppina O, Geertruyden J-PV, Nsabiyumva F, Nkeshimana A, Muhimpundu E, et al. Integration of schistosomiasis control activities withion the primary health care system: a critical review. Parasites Vectors [Internet]. 2019 Aug [cited 2020 Mar 6];12(393):1-11. Available from: Available from: https://doi.org/10.1186/s13071-019-3652-z [ Links ]
9. Secretaria Estadual de Saúde (PE). Secretaria Executiva de Vigilância em Saúde. Plano integrado de ações para o enfretamento às doenças negligenciadas no Estado de Pernambuco / SANAR 2015 - 2018 [Internet]. 2. ed. Recife: Secretaria Estadual de Saúde; 2015 [citado 2020 mar 6]. 46 p. (Série A. Normas e Manuais Técnicos). Disponível em: Disponível em: http://portal.saude.pe.gov.br/sites/portal.saude.pe.gov.br/files/plano_sanar_2_edicao_29.08.17.pdf [ Links ]
10. Secretaria Estadual de Saúde (PE). Secretaria Executiva de Regulação em Saúde. Plano diretor de regionalização [Internet]. Recife: Secretaria Estadual de Saúde ; 2011 [citado 2020 mar 6]. 20 p. Disponível em: Disponível em: http://ead.saude.pe.gov.br/mod/resource/view.php?id=707 [ Links ]
11. Instituto Brasileiro de Geografia e Estatística. Cidades: Recife [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2017 [citado 2019 ago 06]. Disponível em: Disponível em: https://cidades.ibge.gov.br/brasil/pe/panorama [ Links ]
12. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Vigilância da esquistossomose mansoni: diretrizes técnicas [Internet]. 4. ed. Brasília: Ministério da Saúde ; 2014 [citado 2020 mar 6]. 144 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/vigilancia_esquistossome_mansoni_diretrizes_tecnicas.pdf [ Links ]
13. Souza MG. Investigação da presença e contaminação de Biomphalaria spp. por Schistosoma mansoni em dois açudes sob influência indireta do Projeto São Francisco [monografia]. Petrolina (PE): Universidade Federal do Vale do São Francisco; 2012. Disponível em: http://www.cemafauna.univasf.edu.br/arquivos/files/0000026B.pdf [ Links ]
14. Barbosa CS, Souza ATOF, Leal Neto OB, Gomes ECS, Araujo KCGM, Guimarães RJPS. Turismo de risco para esquistossomose mansônica em Porto de Galinhas, Estado de Pernambuco, Brasil. Rev Pan-Amaz Saúde [Internet]. 2015 set [citado 2020 mar 6];6(3):51-8. Disponível em: Disponível em: http://dx.doi.org/10.5123/S2176-62232015000300007 [ Links ]
15. Teixeira MG, Costa MC, Carmo EH, Oliveira WK, Penna GO. Vigilância em Saúde no SUS - construção, efeitos e perspectivas. Ciênc Saúde Coletiva [Internet]. 2018 jun [citado 2020 mar 6];23(6):1811-18. Disponível em: Disponível em: https://doi.org/10.1590/1413-81232018236.09032018 [ Links ]
16. Dubeux LS, Jesus RPFS, Samico I, Mendes MFM, Wanderley FSO, Tomasi E, et al. Avaliação do programa de enfrentamento às doenças negligenciadas para o controle da esquistossomose mansônica em três municípios hiperendêmicos, Pernambuco, Brasil, 2014. Epidemiol Serv Saúde [Internet]. 2019 jul [citado 2020 mar 6];28(2):e2018085. Disponível em: Disponível em: https://doi.org/10.5123/s1679-49742019000200008 [ Links ]
17. Campos P, Reich M. Political analysis for health policy implementation. Health Syst Reform [Internet]. 2019 Aug [cited 2020 Mar 6];5(3):224-35. Available from: Available from: https://doi.org/10.1080/23288604.2019.1625251 [ Links ]
18. Barreto MS, Gomes ECS, Barbosa CS. Turismo de risco em áreas vulneráveis para a transmissão da esquistossomose mansônica no Brasil. Cad Saúde Pública [Internet]. 2016 abr [citado 2020 mar 6];32(3):e00190815. Disponível em: Disponível em: https://doi.org/10.1590/0102-311X00190815 [ Links ]
19. Piot P, Soka MJ, Spencer J. Emergent threats: lessons learnt from Ebola. Int Health [Internet]. 2019 Aug [cited 2020 Mar 6];11(5):334-7. Available from: Available from: https://doi.org/10.1093/inthealth/ihz062 [ Links ]
20. Quinino LRM, Costa JMBS, Aguiar LR, Wanderley TNG, Barbosa CS. Avaliação das atividades de rotina do Programa de Controle da Esquistossomose em municípios da Região Metropolitana do Recife, Pernambuco, entre 2003 e 2005. Epidemiol Serv Saúde [Internet]. 2009 dez [citado 2020 mar 6];18(4):335-43. Disponível em: Disponível em: http://dx.doi.org/10.5123/S1679-49742009000400003 [ Links ]
21. Quinino LRM, Barbosa CS, Samico I. O programa de controle da esquistossomose em dois municípios da zona da mata de Pernambuco: uma análise de implantação. Rev Bras Saúde Mater Infant [Internet]. 2010 nov [citado 2020 mar 6];10(1):119-29. Disponível em: Disponível em: https://doi.org/10.1590/S1519-38292010000500011 [ Links ]
22. Barreto AVMS, Melo ND, Ventura JVT, Santiago RT, Silva MBA. Análise da positividade da esquistossomose mansoni em Regionais de Saúde endêmicas em Pernambuco, 2005 a 2010. Epidemiol Serv Saúde [Internet]. 2015 jan-mar [citado 2020 mar 6];24(1):87-96. Disponível em: Disponível em: https://doi.org/10.5123/S1679-49742015000100010 [ Links ]
23. Tuhebwe D, Bagonza J, Kiracho EE, Yeka A, Elliott AM, Nuwaha F. Uptake of mass drug administration programme for schistosomiasis control in Koome Islands, Central Uganda. PLoS One [Internet] 2015 Apr [cited 2020 Mar 6];10(4):e0123673. Available from: Available from: https://doi.org/10.1371/journal.pone.0123673 [ Links ]
24. Gomes ACL, Galindo JM, Lima NN, Silva EVG. Prevalência e carga parasitária da esquistossomose mansônica antes e depois do tratamento coletivo em Jaboatão dos Guararapes, Pernambuco. Epidemiol Serv Saúde [Internet]. 2016 abr-jun [citado 2020 mar 6];25(2):243-50. Disponível em: Disponível em: https://doi.org/10.5123/s1679-49742016000200003 [ Links ]
25. Saucha CVV, Silva JAM, Amorim LB. Condições de saneamento básico em áreas hiperendêmicas para esquistossomose no estado de Pernambuco em 2012. Epidemiol Serv Saúde [Internet]. 2015 jul-set [citado 2020 mar 6];24(3):497-506. Disponível em: Disponível em: https://doi.org/10.5123/S1679-49742015000300015 [ Links ]
26. Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AGP. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med [Internet]. 2014 Oct [cited 2020 Mar 6];5:65-75. Available from: Available from: https://doi.org/10.2147/RRTM.S44274 [ Links ]
27. Smits HL. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti Infect Ther [Internet]. 2009 Feb [cited 2020 Mar 6];7(1):37-56. Available from: Available from: https://doi.org/10.1586/14787210.7.1.37 [ Links ]
28. Lelo AE, Mburu DN, Magoma GN, Mungai BN, Kihara JH, Mwangi IN, et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in Mwea, central Kenya, a heavy transmission area. PLoS Negl Trop Dis [Internet]. 2014 Oct [cited 2020 Mar 6];8(10):e3221. Available from: Available from: https://doi.org/10.1371/journal.pntd.0003221 [ Links ]
29. Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. A new global strategy for the elimination of schistosomiasis. Int J Infect Dis [Internet]. 2017 Jan [cited 2020 Mar 6];54:130-7. Available from: Available from: https://doi.org/10.1016/j.ijid.2016.09.023 [ Links ]
30. Katz N. Inquérito nacional de prevalência da esquistossomose mansoni e geo-helmintoses [Internet]. Belo Horizonte: CPqRR; 2018 [citado 2020 mar 6]. 76 p. Disponível em: Disponível em: https://www.arca.fiocruz.br/handle/icict/25662 [ Links ]
*Article derived from the final course assignment entitled ‘Characterization of the epidemiological situation of schistosomiasis in the State of Pernambuco, 2010-2016’, submitted by Maria Isabelle Barbosa da Silva Brito to the Public Health Multiprofessional Residency Postgraduate Program, Aggeu Magalhães Institute, Oswaldo Cruz Institute Foundation/Pernambuco, on April 30th 2019.
ADDENDUM This document has an addendum: https://doi.org/10.1590/S1679-49742022000100033
Received: September 19, 2019; Accepted: February 11, 2020











 texto em
texto em