Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.2 Brasília 2020 Epub 07-Abr-2020
http://dx.doi.org/10.5123/51679-49742020000200003
RESEARCH NOTE
Physical wastage of immunobiological products in the state of Ceará, Brazil, 2014-2016
1Secretaria da Saúde do Estado do Ceará, Coordenadoria de Promoção e Proteção à Saúde, Fortaleza, CE, Brazil
2Centro Universitário Christus, Fortaleza, CE, Brazil
3Secretaria da Saúde do Estado, Fortaleza, CE, Brazil
4Hospital Geral Waldemar de Alcântara, Fortaleza, CE, Brazil
5Universidade Estadual do Ceará, Departamento de Saúde Coletiva, Fortaleza, CE, Brazil
Objective:
to describe discarded wasted immunobiological products provided by the National Im-munization Program (PNI) to the State of Ceará between 2014 and 2016, and the costs of discarded doses.
Methods:
this was a descriptive study using data from suspect im-munobiological product evaluation forms and data from disposal approval forms.
Results:
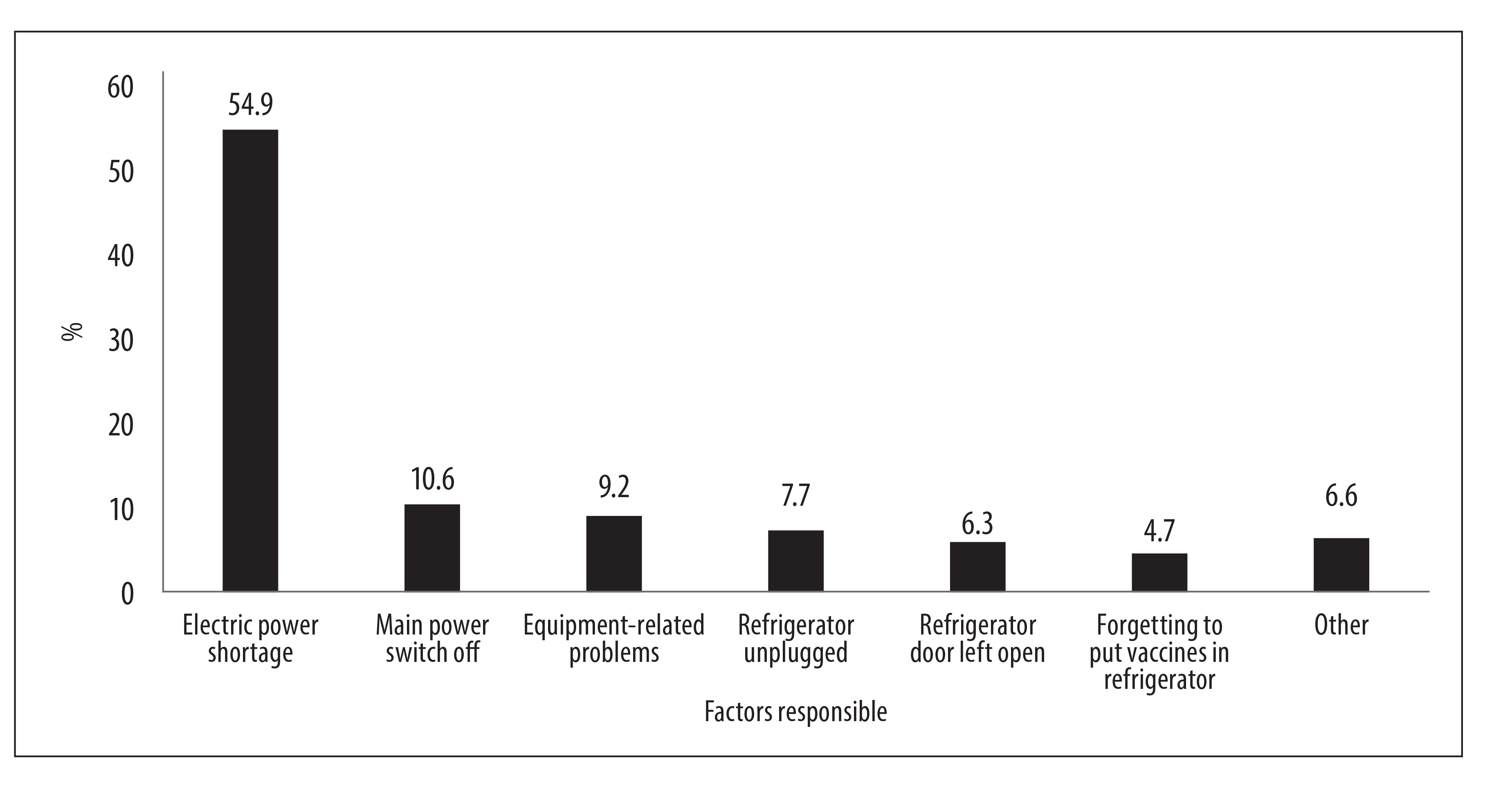
a total of 317 forms were included, 72.0% of which had a disposal approval form, and 160,767 discarded doses were identified, at a total cost of BRL 1,834,604.75; wastage accounted for 0.45%, 0.93% and 0.53% of the total cost of vaccines in 2014, 2015 and 2016, respectively; the main reason for the wastage identified was electric power shortage (54.9%).
Conclusion:
we identified a large number of discarded wasted doses, with high absolute cost; tighter control is necessary, as failures in conservation dynamics may interfere with the supply of immunobiologicals.
Keywords: Immunization; Vaccines; Refrigeration
Introduction
Public immunization policies are acknowledged as providing the best cost-benefit ratio and the best epidemiological and socially far-reaching impact, strengthening both health promotion and disease prevention.1
Vaccines have been used in Brazil as disease control measures since the 19th century. Brazil’s National Immunization Program (PNI) was launched in 1973 with the purpose of coordinating actions intended to immunize the population and control and eradicate vaccine-preventable diseases. Brazil’s PNI is cited by the Pan American Health Organization (PAHO) as a global Public Health reference in the area of immunization.2
Immunobiologicals are thermolabile pharmacological products, sensitive to heat, cold and light. In order to maintain their strength, they must be stored, transported, organized, monitored, distributed and managed adequately,1 and in order to ensure that they keep their immunogenicity, vaccines must be kept at adequate temperatures, right from production through to their use.3
It is important to highlight that in the period 2010-2015, PNI’s budget increased more than 140%, from BRL 1.2 billion in 2010, to BRL 2.9 billion in 2015.4 PNI also has several Information Systems providing data that support cold chain monitoring, analysis and evaluation throughout the country, right from stock and distribution, through to vaccine wastage, whether this be technical or physical, thus ensuring the diagnosis necessary for organizing and planning future distributions.5) The cold chain is defined as a technical and administrative system controlled by PNI, by means of norms, planning, evaluation and funding, with the purpose of ensuring the efficacy and efficiency of the process.
Technical wastage is considered to be justifiable wastage because it results from multidose vaccine vials being opened and expiring as soon as they are opened.6) In turn, physical wastage is wastage that is considered to be avoidable such as, for example, broken vials, electric power shortage, equipment failure, product expiry, inadequate procedures and transport shortcomings, among others.7
The objective of this study was to describe physical wastage of immunobiologicals provided by the National Immunization Program (PNI) to the State of Ceará between 2014 and 2016, and the costs of unused doses.
Methods
A descriptive study was conducted based on secondary data documented on forms used to evaluate suspect immunobiological products exposed to inadequate temperature, as well as data from physical wastage disposal approval forms, for the period from January 2014 to December 2016 in Ceará state. The evaluation forms, together with the technical disposal or reuse approval forms, were retrieved from the state’s cold chain database.
In this study physical wastage was considered to be that arising from inadequate storage, packaging and conservation, handling and transport problems, such as broken or cracked vials, expired immunobiologicals, temperature deviation due to equipment failure and labeling problems.
Suspect immunobiological product evaluation forms are filled in by health professionals responsible for municipal vaccine rooms at the time of occurrence and are forwarded to the respective Regional Health Coordination Service (CRES) within the state. The CRES, in turn, send the printed forms to the state’s cold chain management body which is responsible for providing a technical report approving disposal or reuse, taking into consideration (i) data on the last temperature reading before and after the occurrence, (ii) length of time during which immunobiologicals were exposed to temperature alteration from the beginning until the end of the occurrence, (iii) maximum, minimum and momentary temperatures recorded during the exposure period, and vaccine conservation practices adopted, and (iv) written records of the products, demonstrating whether or not they had suffered previous temperature alterations. Data was collected from the evaluation forms in April and May 2017.
Data analysis was performed by means of a descriptive statistical study, showing the types of immunobiologicals wasted, the amount of wasted doses in absolute numbers, and the percentage of the main factors that led to the occurrence of physical wastage.
Taking the supply notes for products provided to the Ceará cold chain by the Ministry of Health, via the Strategic Supplies Information System (SIES), we calculated the cost, at current prices, of all vaccines, sera and immunoglobulins provide to Ceará state per annum in the period selected. The calculation was performed taking into consideration price adjustments during the period as updated on each supply note issued by the Ministry. Based on these price adjustments, we calculated the cost of physical wastage of vaccines each year, compared to the total cost of the vaccines.V
Results
The study assessed 317 forms from the 22 CRES and their respective municipalities. Two hundred and fifty seven (72.0%) of the forms had corresponding disposal approval forms issued following cold chain management analysis and evaluation; the remainder had been authorized for reuse.
Table 1 shows the description of physical immunobiological wastage during the study period and the cost (in BRL) of wasted doses. In 2014-2016, 160,767 doses of vaccines, sera and immunoglobulin forming part of the products provided by PNI/Ministry of Health to the state of Ceará were wasted, at a total cost of BRL 1,834,604.75. Physical wastage recorded on the disposal approval forms accounted for 0.45%, 0.93% and 0.53% of the total cost of vaccines in 2014, 2015 and 2016, respectively.
Table 1 - Imunobiologicals discarded owing to physical wastage and respective costs, Ceará, 2014-2016
| Immuno/serum | Number of doses | Average cost per dose (BRL) | Cost (in BRL) | ||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2014 | 2015 | 2016 | ||
| Anti-bothropic serum | 30 | 50 | - | 76,8 | 1.870.8 | 5.298,8 | - |
| Anti bothropic/laquetic serum | 20 | 10 | - | 101,3 | 1.634,8 | 1.210,3 | - |
| Anti-crotalic serum | 67 | 52 | - | 76,3 | 3.620,6 | 5.132,9 | - |
| Anti-elapidic serum | - | 10 | - | 66,0 | - | 660,0 | - |
| Anti-bothropic/crotalic serum | 29 | - | - | 122,4 | 3.551,3 | - | - |
| Anti-scorpion serum | 14 | 26 | - | 37,4 | 420,9 | 1.163,5 | - |
| Anti-rabies serum | 48 | 12 | 13 | 45,2 | 1.500,9 | 637,2 | 690,3 |
| Anti-tetanus serum | 33 | 11 | - | 62,1 | 1.584,0 | 840,1 | - |
| Anti-human rabies serum | 2.224 | 2.751 | 466 | 33,7 | 65.140,9 | 86.216,3 | 18.882,3 |
| BCGa vaccine | 1.772 | 4.770 | 2.027 | 1,3 | 2.419,4 | 6.630,3 | 2.776,9 |
| Yellow fever vaccine | 47 | 660 | 300 | 1,7 | 1,3 | 1.702,8 | 774,0 |
| Hepatitis A vaccine | 99 | 1.115 | 1.231 | 22,0 | 1.965,1 | 22.132,7 | 32.449,1 |
| Hepatitis B vaccine | 2.712 | 9.727 | 4.434 | 1,5 | 3.932,4 | 15.757,7 | 7.316,1 |
| HPV vaccine | 1.128 | 4.510 | 2.805 | 38,5 | 34.990,5 | 190.682,8 | 118.651,5 |
| Influenza vaccine | 2.740 | 2.989 | 2.141 | 10,6 | 23.125,6 | 26.990,6 | 30.873,2 |
| Varicella vaccine | - | 99 | 583 | 39,1 | - | 4.752,9 | 17.664,9 |
| dTb vaccine | 1.895 | 3.780 | 3.955 | 0,2 | 480,3 | 1.096,2 | 1.344,7 |
| DTPc vaccine | 3.542 | 6.584 | 1.816 | 0,6 | 1.948,1 | 3.752,8 | 1.743,3 |
| DTPad vaccine | 88 | 1.452 | 993 | 25,9 | 1.657,7 | 31.537,4 | 36.949,5 |
| Rubella and measles vaccine | - | 1.714 | 1.626 | 1,3 | - | 2.262,4 | 2.130,0 |
| Meningococcal virus | 2.267 | 3.490 | 2.228 | 30,8 | 48.423,1 | 95.486,4 | 97.519,5 |
| Oral rotavirus vaccine | 1.648 | 2.437 | 1.714 | 23,7 | 35.959,3 | 55.246,7 | 46.038,0 |
| 5-in-1 vaccine | 3.450 | 4.853 | 1.944 | 6,4 | 22.942,5 | 30.962,1 | 12.461,0 |
| Pneumococcal vaccine 10v | 1.146 | 3.987 | 2.528 | 41,0 | 37.829,4 | 163.785,9 | 124.023,6 |
| Pneumococcal vaccine 23v | 3 | 254 | 52 | 19,2 | 46,1 | 4.373,8 | 1.312,4 |
| Quadrivalent vaccine | 610 | 468 | 88 | 31,0 | 17.690,0 | 14.985,3 | 2.817,7 |
| Measles, mumps and rubella vaccine | 3.754 | 8.612 | 3.836 | 6,8 | 25.527,2 | 58.561,6 | 26.084,8 |
| IPVe | 3.166 | 4.062 | 2.678 | 6,6 | 17.729,6 | 28.149,6 | 19.977,8 |
| OPVf | 3.463 | 12.769 | 3.104 | 0,7 | 2.468,5 | 9.576,7 | 2.669,4 |
| HIBg | 1 | - | - | 2,2 | 2,2 | - | - |
| Total | 38.943 | 81.262 | 40.562 | - | 359.867,0 | 869.586,9 | 605.150,8 |
a) BCG: bacillus Calmette-Guérin.
b) HPV.
c) dT: diphtheria and tetanus.
d) DTP: diphtheria, tetanus and pertussis.
e) DTPa: diphtheria, tetanus and pertussis (acellular).
f) IPV: inactivated poliovirus vaccine.
g) OPV: oral poliovirus vaccine.
h) HIB: Haemophilus Influenza B.
The immunobiological products with the highest number of wasted doses were hepatitis B vaccine, diphtheria and tetanus adsorbed vaccine/adult (dT), diphtheria, tetanus and pertussis adsorbed vaccine (DTP), oral poliovirus vaccine (OPV) and measles, mumps and rubella vaccine (MMR), probably because these vaccines are provided in multidose vials.
The mean reason for physical waste was electric power shortage (54.9%), followed by the main power switch being turned off (10.6%), equipment-related problems (9.2%), refrigerator being unplugged (7.7%), refrigerator door left open (6.3%) and forgetting to put vaccines in the refrigerator (4.7%), among others (Figure 1).
Discussion
This study revealed that in a three-year period (2014-2016) in the state of Ceará, more than 160,000 doses of vaccine were discarded as physical wastage, corresponding to a cost of almost BRL 2 million. Percentage wastage accounted for under 1% of PNI’s annual investment in immunobiologicals in Ceará in the same period.
In financial terms, wastage cost least in 2014, totaling BRL 359,867.02. 2014 was also the year in which the lowest number of wasted doses and occurrences were recorded, in comparison with each of the two following years. In particular, 2015 was the year in which the greatest number of doses was wasted and at the highest cost, totaling BRL 869,586.91 - or 47.4% of the entire cost of wastage for the three-year period studied. Notwithstanding, in the same year there were only 77 occurrences of discarded products, i.e. an amount lower than expected, when compared to the wastage/cost ratio found in 2014 and 2016. The high financial amount generated by this wastage can therefore be explained by the greater quantity of suspect immunobiologicals included on the same form, as well as by the variations in the prices of these products which occur every year when the supply contracts between the Ministry of Health and the manufacturing laboratories are renewed.
In this study, electric power shortage was the main reason for wastage of stored vaccines. The highest proportion of physical wastage in the state of Santa Catarina and Amazonas was also caused by electric power shortage, i.e. 35.7% and 41.2% respectively. On the other hand, in the states of Mato Grosso do Sul (38.6%) and Rio Grande do Norte (40.4%), the highest proportion of identified wastage was caused by recording errors and therefore did not correspond to physical wastage.8
Analysis of physical wastage in the state of Paraná between 2009 and 2012, found that wastage of immunobiologicals due to avoidable causes accounted for 3,437,552 doses, with the highest levels (38%) occurring in 2011. The highest percentage recorded was for wastage owing to equipment failure (76.5%), followed by electric power shortage (7%).9
The greater part of physical wastage could be avoided through permanent training of health professionals working in cold chains, as well as preventive and corrective maintenance of refrigeration equipment, control of vaccine batches and expiry dates, among other equally simple actions.10 In order to solve the problem of electric power shortage, mostly responsible for the physical wastage found by this study, prevention measures are urgently required, such as installing generators and correctly carrying out PNI recommended contingency protocols.
In financial terms, despite physical wastage accounting for a small proportion of the total amount invested by the Ministry of Health in immunization actions, even so wasted doses cost almost BRL 2 million. These are resources which, if they were not wasted, could be used in the most varied ways to benefit Ceará’s population. Furthermore, while this wastage continues to occur, imunobiologicals may be in shortage in other parts of the country, placing responsibility on PNI to manage the cold chain and distribution of immunobiologicals better.
It can therefore be considered that any shortcomings in cold chain can place a burden on the public budget, lead to waste, restrict the population’s access to immunization and have reduced vaccine coverage as their outcome.
Referências
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Manual de rede de frio: do programa nacional de imunizações [Internet]. 4. ed. Brasília: Ministério da Saúde; 2013 [citado 2019 dez 10]. 144 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_rede_frio4ed.pdf [ Links ]
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças Transmissíveis. Programa nacional de imunizações - 40 anos [Internet]. Brasília: Ministério da Saúde; 2013 [citado 2019 dez 10]. 236 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/programa_nacional_imunizacoes_pni40.pdf [ Links ]
3. Raglione D, Bezerra GAM, Lopes MH, Nerger MLB, Guimarães TC, Sartori MAC. Avaliação da rede de frio para conservação de vacinas em unidades básicas de saúde das Regiões Sul e Centro-Oeste do Município de São Paulo em 2011-2012. Epidemiol Serv Saúde [Internet]. 2016 jan-mar [citado 2019 dez 10];25(1):65-74. Disponível em: Disponível em: http://www.scielo.br/pdf/ress/v25n1/2237-9622-ress-25-01-00065.pdf . doi: 10.5123/s1679-49742016000100007 [ Links ]
4. Fundação Oswaldo Cruz. Ministério da Saúde inicia campanha nacional de multivacinação [Internet]. Brasília: Fundação Oswaldo Cruz; 2016 [citado 2017 jul 15]. Disponível em: Disponível em: https://portal.fiocruz.br/pt-br/content/ministerio-da-saude-inicia-campanha-nacional-de-multivacinacao [ Links ]
5. Ministério da Saúde (BR). Sistema de Informação do Programa Nacional de Imunizações SI-PNI [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2017 jul 10]. Disponível em: Disponível em: http://pni.datasus.gov.br/apresentacao.asp [ Links ]
6. Pereira DDS, Neves EB, Gemelli M, Ulbricht L. Análise da taxa de utilização e perda de vacinas no programa nacional de imunização. Cad Saúde Colet [Internet]. 2013 [citado 2019 dez 10];21(4):420-4. Disponível em: Disponível em: http://www.scielo.br/pdf/cadsc/v21n4/v21n4a10.pdf . doi: 10.1590/S1414-462X2013000400010 [ Links ]
7. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças Transmissíveis. Manual de normas e procedimentos para vacinação [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2019 dez 10]. 176 p. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_procedimentos_vacinacao.pdf [ Links ]
8. Samad S. Perdas de vacinas: razões e prevalência em quatro unidades federadas do Brasil. Dissertação [Mestrado]. São Paulo (SP): Universidade Federal de São Paulo; 2011. Disponível em: http://repositorio.unifesp.br/handle/11600/9923 [ Links ]
9. Oliveira VC, Caveião C, Crosewski F. Gerenciamento de Enfermagem no controle de perdas evitáveis de imunobiológicos. Cogitare Enferm [Internet]. 2014 out-dez [citado 2019 dez 10];19(4):679-86. Disponível em: Disponível em: https://revistas.ufpr.br/cogitare/article/view/36358 . doi: 10.5380/ce.v19i4.36358 [ Links ]
10. Crosewski F, Larocca LM, Chaves MMN. Perdas evitáveis de imunobiológicos na instância local: reflexões acerca do processo de trabalho da enfermagem. Saúde Debate [Internet]. 2018 jan-mar [citado 2019 dez 10];42(116):203-13. Disponível em: Disponível em: http://www.scielo.br/pdf/sdeb/v42n116/0103-1104-sdeb-42-116-0203.pdf . doi: 10.1590/0103-1104201811616 [ Links ]
Received: February 19, 2019; Accepted: November 01, 2019











 texto en
texto en