Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.4 Brasília 2020 Epub 24-Jul-2020
http://dx.doi.org/10.5123/s16/79-49742020000400008
Original article
Repercussions of Zika virus emergency on the health of the population of Tocantins state, Brazil, 2015 and 2016: a descriptive study*
1Secretaria de Estado da Saúde do Tocantins, Palmas, TO, Brazil
2Universidade Federal da Bahia, Instituto de Saúde Coletiva, Salvador, BA, Brazil
3Secretaria Municipal de Saúde de Palmas, Palmas, TO, Brazil
4Centro de Integração de Dados e Conhecimentos para a Saúde, Fundação Instituto Oswaldo Cruz, Salvador, BA, Brazil
Objective
To describe the occurrence of Zika virus disease and its complications in the state of Tocantins and in its capital, the city of Palmas.
Results
Incidence of reported Zika virus disease cases in 2015 and 2016 was 295.2/100,000 inhabitants and 411.1/100,000 inhab. in the general population, and 5.9/1,000 and 27.8/1,000 live births, respectively. Higher risks occurred in women, the 20-39 year age group, municipalities in the central and northwestern regions of the state and in hotter months (February and March). Incidence of Zika-related microcephaly during pregnancy was 0.06/1,000 live births. One case of Guillain-Barré Syndrome resulting from Zika virus infection was confirmed.
Conclusion
Zika virus disease hit Tocantins intensely, although its adverse outcomes were less frequent than in other states.
Key words: Zika Virus; Zika Virus Infection; Epidemics; Microcephaly; Epidemiology, Descriptive
Introduction
The emergence and spread of Zika virus (ZIKV) in the Southern hemisphere, and particularly in the Americas, was one of the most surprising and challenging Public Health problems of current times, due to its causing a severe microcephaly epidemic and other adverse fetal outcomes. Up until the early 2000s, records of cases and sporadic outbreaks of disease caused by ZIKV were restricted to the African and Asian continents, and the disease was considered to be benign and self-limiting.1However, in 2015, the virus was identified in cities in Northeastern Brazil2 and it is estimated that by the end of 2016, more than 1,600,000 cases of this arbovirus infection had occurred in Brazil, with greatest incidence in 2016.4
In 2015, detection of the microcephaly epidemic resulting from congenital ZIKV transmission led the Brazilian Ministry of Health and the World Health Organization to declare Public Health Emergencies of National and International Concern, respectively.5 Following this, investigations enabled this causal relationship to be established, as well as identification of a range of neurological changes, making it possible to characterize a syndromic condition, which shortly afterwards received the name of Congenital Zika Syndrome (CZS).8
By the end of that year, Brazil had registered 3,174 suspected cases and 38 suspected deaths from microcephaly/CZS related to ZIKV infection.13 In the following year, 2016, 10,867 cases were notified, 7,684 (70.7%) of which were investigated. In the end, 2,366 were classified as confirmed, 49 were classified as probable and 5,269 were discarded. Among the 2,366 confirmed cases, 200 died,14 representing 8.5% lethality. On that occasion, 3,183 (29.3%) cases were being investigated which, when added to cases notified in 2017, totaled 5,739.15
In addition to severe adverse effects on fetuses, ZIKV can cause serious complications in adults, the most commonly described of which are hypertensive iridocyclitis,16 Guillain-Barré syndrome (GBS) and transverse myelitis.17
Considering the scenario set by the ZIKV emergency, the possibility of new epidemics caused by this virus occurring and variations in population and socio-environmental characteristics, capable of impacting directly on risk of the disease, it is essential to know its epidemiological profile in each territory, with the aim of producing information that informs new strategies for detection and rapid response to this emerging viral disease and/or their enhancement.
The study describes occurrence of Zika virus disease and its complications in the population of the state of Tocantins and in its capital, the city of Palmas.
Methods
A descriptive study was conducted based on Zika cases notified in Tocantins between July 2015 and December 2016. Tocantins is located in the Northern region of Brazil, has 139 municipalities and had an estimated population of 1,565,062 inhabitants in 2015; in 2014, the Gini index calculated for Tocantins was 0.468.18
The following databases were used:
Notifiable Health Conditions Information System (SINAN); and Brazilian National Health System (SUS) forms under the responsibility of sentinel units (FormSUS);
Public Health Event Registry (RESP);
Laboratory Environment Management System (GAL) of the state’s central laboratory (LACEN);
Live Birth Information System (SINASC); and
Mortality Information System (SIM).
Each case registered on one of these databases was manually searched for on the rest, with the aim of identifying duplications and omissions, as well as correcting possible errors or inconsistencies.
The RESP data used in this study were updated on August 22nd 2017. For all confirmed cases of Zika virus infection and its adverse outcomes, final diagnosis was considered to be that recorded by health services, given that they were classified according to the definition criteria established by the Ministry of Health. In short, a confirmed Zika case was considered to be a case diagnosed by laboratory test and/or clinical-epidemiological criterion, i.e. a case linked to another confirmed case and which presents pruritic maculopapular rash accompanied by two or more of the following signs: fever, conjunctival hyperemia, polyarthritis and joint edema.19 With regard to microcephaly/CZS, newborns were confirmed when they had a positive ZIKV test result and/or images compatible with those produced by this virus, regardless of whether they had microcephaly.20
Incidence and/or proportion of notified, confirmed and inconclusive cases of Zika virus disease were calculated for the total population/100,000 inhab. and for pregnant women/1,000 live births (LB), for the years 2015 and 2016. Incidence/100,000 inhab. for probable cases (notified cases after excluding discarded cases), was also calculated by sex and age range, and by municipality of residence, for the year 2016, as well as cumulative incidence – for the period 2015-2016 – of adverse effects of ZIKV infection/1,000 LB during pregnancy (miscarriage, premature birth, low birthweight and microcephaly/CNS alteration). The number of probable cases per epidemiological week (EW) was represented on a time curve. The indicators were calculated for the state of Tocantins and for its capital, the city of Palmas. In addition, for each year covererd by the study, the incidence coefficients of notified Zika virus disease, by municipality of residence, were ordered in quintiles and their spatial distribution was represented on a map of the state. The magnitude of these indicators was then compared. Microcephaly/CZS cases were described according to the trimester in which the pregnant mother had pruritic maculopapular rash.
The study project was approved by the Tocantins State Health Department Superintendency for Professional Health Management and Education, as per Ordinance No. SESAU 796, dated June 27th 2014. It was also approved by the Research Ethics Committee of the Federal University of Bahia Public Health Institute (CEP/ISC/UFBA), as per Certification of Submission for Ethical Appraisal (CAAE) No. 1.929.507/2017.
Results
Between July and December 2015 and throughout the whole of 2016, 4,472 (incidence: 295.2/100,000 inhab.) and 6,303 (incidence: 411.1/100,000 inhab.) Zika virus disease cases, respectively, were notified in the state of Tocantins. In 2015, 1.3% of cases (n=60) were confirmed and 97.3% were classified as inconclusive. In the following year, 28.0% of notified cases were confirmed (incidence: 115.1/100,000 inhab.) while 7.4% were inconclusive. In the state capital, Palmas, incidence of this arbovirus infection was 1,032.5/100,000 inhab. in 2015 and 979/100,000 inhab. in 2016. Confirmed case percentages and incidence rates were 0.7% (7.3/100,000 inhab.) in 2015 and 35.5% (347.7/100,000 inhab.) in 2016. With regard to Zika virus disease in pregnant women, 14.7% of the 149 notified cases were confirmed in 2015 and 22.5% of the 662 cases notified in 2016; confirmed case incidence in this population group was 0.9/1,000 LB (2015) and 7.5/1,000 LB (2016). In 2015, 26.2% of notified cases remained classified as inconclusive and 30.2% in 2016 (Table 1). In 2016, 63% of Zika virus disease notifications in the general population of the state of Tocantins were concentrated in Palmas, while in 2015 this proportion was 43.5%.
Table 1 – Notified (number and incidence coefficient), confirmed (number, percentage and incidence coefficient) and inconclusive (number and percentage) Zika cases by place of residence and year of occurrence, Tocantins and Palmas, July-December 2015 and January-December 2016
| Specification | Tocantins | Palmas | ||||
|---|---|---|---|---|---|---|
| 2015 | 2016 | Cumulative | 2015 | 2016 | Cumulative | |
| General population | ||||||
| Notified cases (N) | 4,472 | 6,303 | 10,775 | 2,816 | 2,740 | 5,556 |
| Incidenceª | 295.2 | 411.1 | 353.5 | 1,032.5 | 979.0 | 1,005.5 |
| Confirmed casesb (N) | 60 | 1,765 | 1,825 | 20 | 973 | 993 |
| %c | 1.3 | 28.0 | 16.9 | 0.7 | 35.5 | 17.8 |
| Incidencea | 4.0 | 115.1 | 59.9 | 7.3 | 347.7 | 178.7 |
| Inconclusive cases (N) | 4,351 | 470 | 4,821 | 2,778 | 26 | 2,804 |
| %c | 97.3 | 7.4 | 44.7 | 98.6 | 0.9 | 50.4 |
| Discarded cases (N) | 61 | 4,068 | 4,129 | 18 | 1,741 | 1,259 |
| %c | 1.3 | 64.5 | 38.8 | 0.6 | 63.5 | 31.6 |
| Pregnant women | ||||||
| Notified cases (N) | 149 | 662 | 811 | 66 | 265 | 331 |
| Incidenced | 5.9 | 27.8 | 16.5 | 12.7 | 54.6 | 33.0 |
| Confirmed casesc (N) | 22 | 149 | 171 | 12 | 77 | 89 |
| %d | 14.7 | 22.5 | 21.0 | 18.1 | 29.0 | 26.9 |
| Incidenced | 0.9 | 7.5 | 3.5 | 2.3 | 15.8 | 8.8 |
| Discarded cases (N) | 88 | 313 | 401 | 22 | 111 | 133 |
| %d | 59.1 | 47.2 | 49.4 | 33.3 | 41.8 | 40.1 |
| Inconclusive cases (N) | 39 | 200 | 239 | 32 | 77 | 109 |
| %d | 26.2 | 30.2 | 29.4 | 48.4 | 29.0 | 33.0 |
a) Cases notified between July and December 2015.
b) Incidence per 100,000 inhabitants.
c) Cases confirmed by clinical-epidemiological and/or laboratory criteria (enzyme immunoassay or molecular biology).
d) Percentage in relation to total of notified cases in the respective populations.
e) Incidence per 1,000 live births.
It can be seen in Table 2 that incidence of probable Zika virus disease cases in 2016 was higher in females, both for the state of Tocantins as a whole (205.7/100,000) and for the capital Palmas (490.7/100,000). The 20-39 year age range (201.8/100,000) and the 40-59 age range (145.3/100,000) recorded the highest levels of this indicator for the state as a whole. In the capital, considering total cases, all age ranges had rates above 200/100,000 inhab., varying from 212.5/100,000 inhab. in the 5-9 age group, to 446.9/100,000 inhab. in the 20-39 age group.
Table 2 – Number and incidence coefficienta of probable Zika casesb according to age range and sex, Tocantins and Palmas, 2016
| Age range (in years) | Tocantins | Palmas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |||||||
| N | Incidence | N | Incidence | N | Incidence | N | Incidence | N | Incidence | N | Incidence | |
| ≤4 | 73 | 107.8 | 84 | 129.3 | 157 | 118.3 | 37 | 292.4 | 37 | 308.8 | 74 | 300.4 |
| 5-9 | 47 | 65.1 | 68 | 96.6 | 115 | 80.7 | 20 | 161.3 | 33 | 263.2 | 53 | 212.5 |
| 10-19 | 122 | 82.8 | 235 | 161.6 | 357 | 121.9 | 45 | 175.1 | 106 | 394.3 | 151 | 287.2 |
| 20-39 | 281 | 104.8 | 784 | 301.9 | 1,065 | 201.8 | 137 | 246.0 | 369 | 641.4 | 506 | 446.9 |
| 40-59 | 128 | 81.8 | 316 | 211.7 | 444 | 145.3 | 55 | 215.8 | 126 | 481.9 | 181 | 350.5 |
| ≥60 | 31 | 46.8 | 66 | 101.3 | 97 | 73.8 | 10 | 159.3 | 24 | 365.4 | 34 | 264.6 |
| Total | 682 | 87.7 | 1,553 | 205.7 | 2,235 | 145.8 | 304 | 220.0 | 695 | 490.7 | 999 | 357.0 |
a) Incidence per 100,000 inhabitants.
b) Probable cases = notified, excluded or discarded cases.
In 2016, the majority of probable cases in Tocantins was concentrated between epidemiological weeks EW 7 and EW 11, with the peak being recorded in EW 8, with 367 notified cases. With effect from EW 12, the number of notifications began to fall until reaching 6 cases in week 51 (Figure 1). It can be seen that in Figure 2 that in 2015, the municipalities located in the central and northwest regions of the state had higher notified case incidence rates, in particular Colinas (1,995/100,000), Palmas (1,032.5/100,000) and Paraíso (894.5/100,000). In 2016, the highest incidence rates were found in municipalities located in the northern, northeastern and central regions of Tocantins, including Guaraí, Miracema do Tocantins, Pugmil and Silvanópolis, with rates varying between 1,022.4/100,000 and 2,035.9/100,000 inhab.
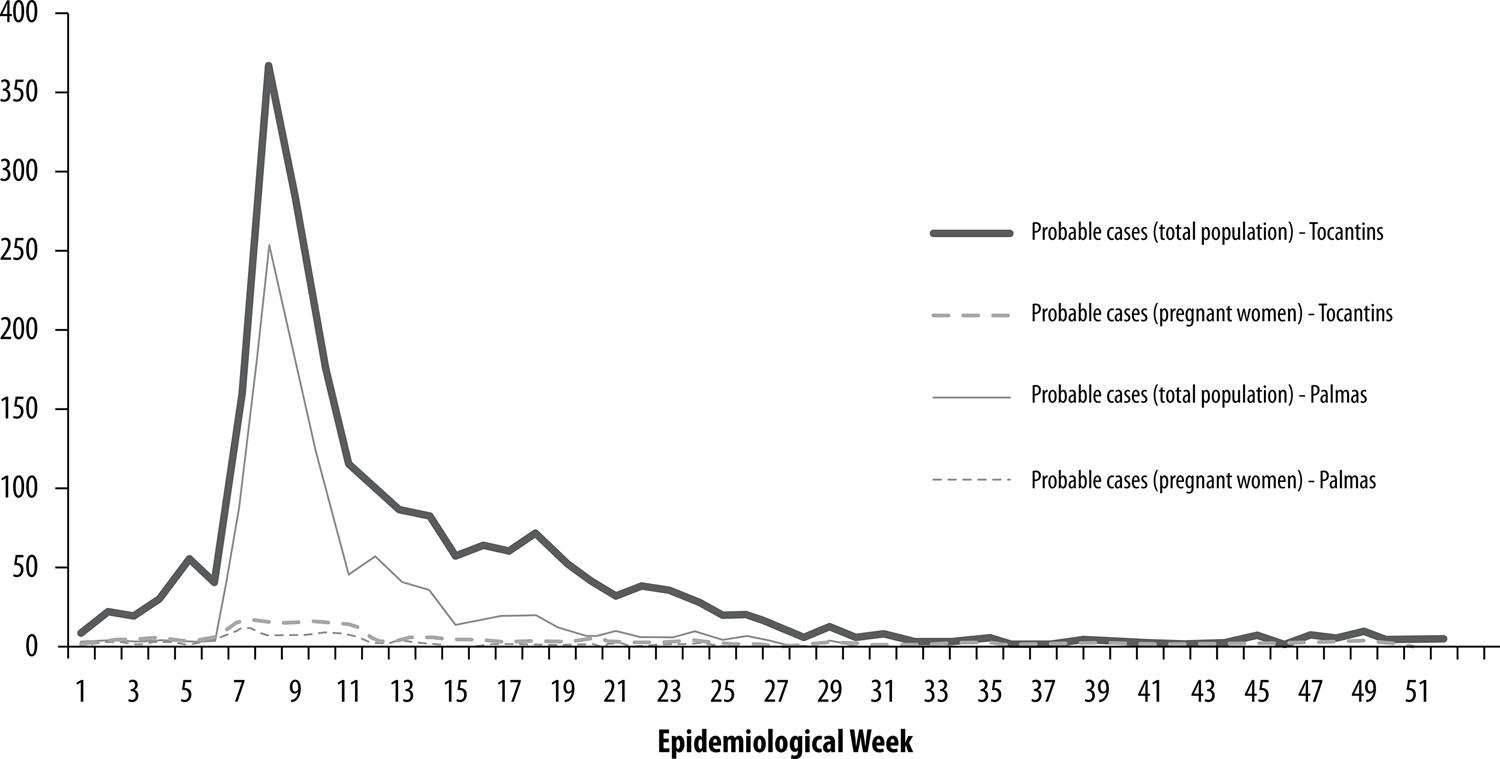
Figure 1 – Number of probable Zika casesa in the general population and in pregnant women, by epidemiological week, Tocantins and Palmas, 2016a) Probable cases = notified, excluded or discarded cases.
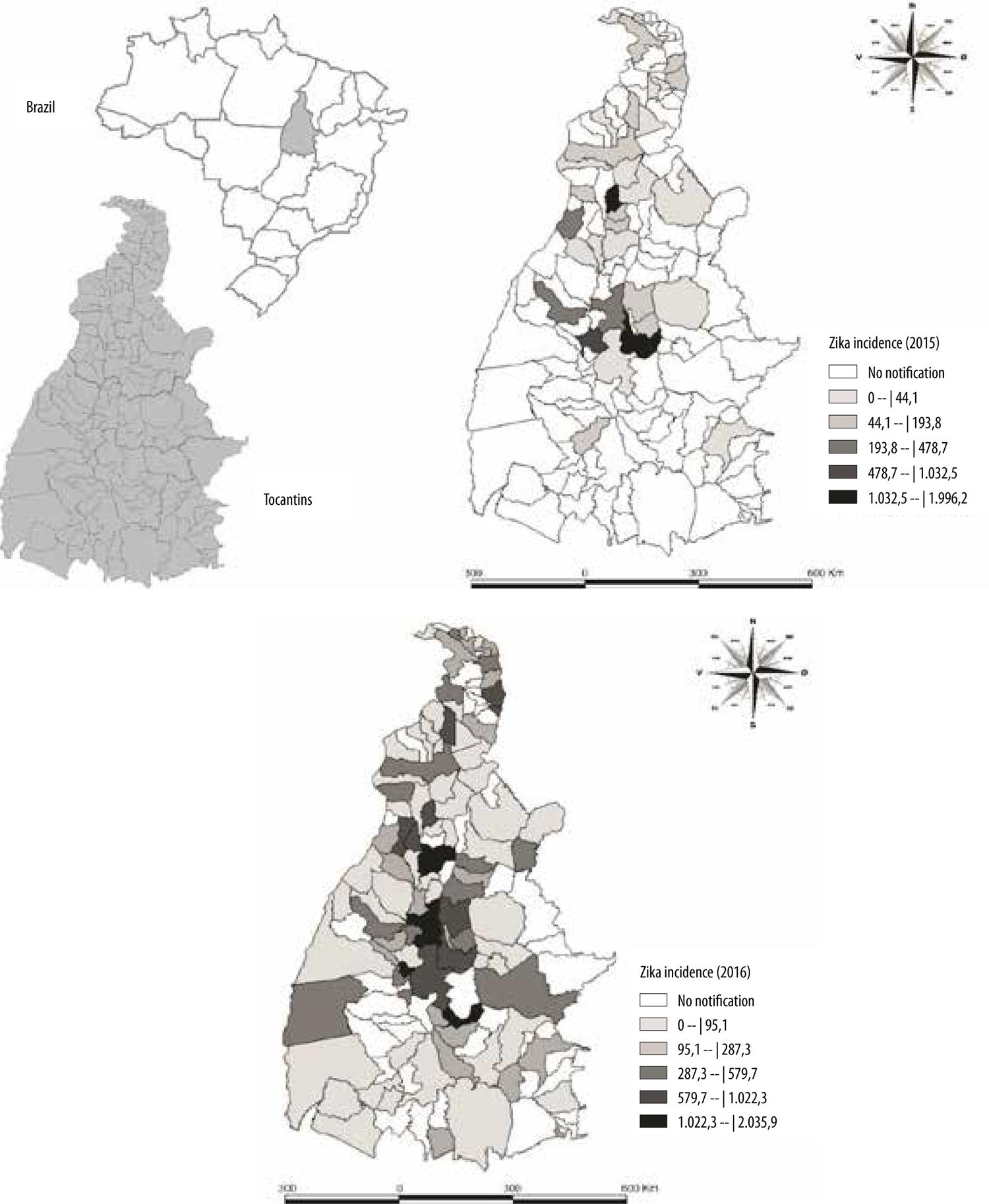
Figure 2 – Spatial distribution of the incidence coefficient of notified Zika cases (per 100,000 inhab.) by municipality of residence, Tocantins, July-December 2015 and January-December 2016
Between 2015 and 2016, out of 171 confirmed cumulative Zika cases in pregnancy in Tocantins as a whole, 35 (20.4%) resulted in adverse outcomes. Of these, 11 (31.4%) were resident in Palmas, where 89 cases of Zika in pregnant women were confirmed in the period. In 2015, no ZIKV-related cases of microcephaly and/or alterations to the CNS or alterations to other organs or systems were notified in Tocantins; while a single case of GBS due to ZIKV was confirmed in 2016. In Palmas, in particular, no cases of microcephaly related to Zika in pregnancy were recorded during the study period. In Table 3 it can be seen that during the same period the incidence rates for adverse outcomes of Zika virus infection in pregnancy in the state as a whole were: premature childbirth, 0.2/1,000 LB; low birthweight, 0.1/1,000 LB; prematurity and low birthweight, 0.2/1,000 LB; miscarriage, 0.2/1,000 LB; and microcephaly/alterations to the CNS or other system/organ, 0.1/1,000 LB. In Palmas, the incidence rates for adverse outcomes due to ZIKV in pregnancy were: premature childbirth, 0.2/1,000 LB; low birthweight, 0.3/1,000 LB; prematurity and low birthweight, 0.5/1,000 LB; and miscarriage, 0.1/1,000 LB.
Table 3 – Number, percentage and incidence coefficient (per 1,000 live births) of adverse outcomes of Zika virus infectiona during pregnancy, Tocantins and Palmas, July 2015-December 2016
| Outcomes | Tocantins | Palmas | ||||
|---|---|---|---|---|---|---|
| N | % | Incidence | N | % | Incidence | |
| Prematuridade | 8 | 22,8 | 0,2 | 2 | 18,2 | 0,2 |
| Baixo peso ao nascerb | 7 | 20,0 | 0,1 | 3 | 27,3 | 0,3 |
| Prematuridade e baixo peso | 9 | 25,7 | 0,2 | 5 | 45,4 | 0,5 |
| Aborto | 8 | 22,8 | 0,2 | 1 | 9,0 | 0,1 |
| Microcefalia/alterações do SNCc | 4 | 11,4 | 0,1 | – | – | – |
| Total | 35 | 100,0 | 0,7 | 11 | 100,0 | 1,1 |
a) Refer to products of the pregnancy of 156 women resident in Tocantins and 84 in Palmas, with Zika infection confirmed by clinical-epidemiological and/or laboratory criteria (enzyme immunoassay or molecular biology).
b) Birth weight <2,500gr.
c) CNS: central nervous system.
Notes:
i) Two pregnant women who had miscarriages and three who had live born babies with microcephaly/CNS alterations were only notified on RESP, i.e. they do not appear on SINAN.
ii) Number of live births in Tocantins between 2015 and 2016, 48,902; and in Palmas, 10,052.
Of the four newborns who had microcephaly/CZS, three had records of their mothers having had rash in the first trimester of pregnancy. Of these three cases, two were confirmed by transfontanellar ultrasound and computerized tomography, and one of these infants died shortly after birth. With regard to the fourth case, no information was recorded about Zika signs and symptoms during pregnancy.
Recording of several data items on RESP was found to be absent or incomplete. In the case of more than 80% of records of fetuses and newborns with suspected microcephaly and/or CNS alteration, there were fields with unknown information or no information about the result of the Zika laboratory test or head circumference measurement; and some cases shown as being under investigation or discarded had positive test results confirming Zika virus infection.
Discussion
The Zika virus disease epidemic in Tocantins was of great magnitude after it began in July 2015. In the same year, the state capital Palmas was the municipality with the largest proportion of notified cases of this arbovirus infection in the state, as well as having high incidence of confirmed cases. The percentage of confirmed cases was low both in the state and in the capital. Greater risk of Zika occurrence was found in the female sex and in the 20-39 year age group, while those under 10 years old and over 60 years old had lower risk. The majority of probable cases occurred between February and March. Prematurity and low birthweight were the most frequent adverse outcomes of ZIKV infection during pregnancy, and no cases of microcephaly/alterations to the central nervous system were recorded in the capital. Only one case of Guillain-Barré syndrome was notified and confirmed in Tocantins.
In the Northern region of Brazil, Tocantins was the state with the greatest risk of Zika occurring in 2016, and had Brazil’s sixth highest incidence rate, with the average national rate of this indicator in 2016 being 105.3/100,000 inhab.In Palmas, this risk was comparable to risk levels found in municipalities of Mato Grosso, the state with the highest ZIKV incidence in 2016.21 The widespread dissemination of the Aedes aegypti mosquito and its great competence as a transmitter of ZIKV, along with the inexistence of ‘herd immunity’ in the population, given that there had not been exposure to the virus up until then,11 may have contributed to the high incidence found by this study in Tocantins.
Greater risk of occurrence of the disease among females and in the 20-39 year age range is a finding consistent with the literature.22 Some authors suggest that as women tend to spend more time at home, they are more exposed to the Aedes aegypti vector, habitually found inside and around households.24Moreover, the fact of women using health services more frequently could be another hypothesis for explaining higher Zika notification in this population group.
Zika seasonality is similar to that of other arbovirus infections with more frequent notification during the hottest months of the year. It is known that environmental and climatic conditions, such as high humidity, rainfall and high temperatures (between 32º and 35ºC), favor the multiplication of vectors, especially in rainy months.25 However, information was not made available about A. aegypti infestation levels (building infestation index) in Tocantins, in order for this relationship to be verified.
The highest Zika incidence rates recorded in 2015 in Palmas, Colinas and Paraíso especially, may be explained by the fact that in that year only sentinel units in those places notified cases of Zika infection. The high number of inconclusive cases may possible have arisen from insufficiency and/or inexistence of knowledge about Zika and insufficient provision of laboratory tests by the state.
High frequency of low birthweight and prematurity is also reported as one of the adverse effects of ZIKV infection during pregnancy,26possibly due to umbilical artery flow alteration, placenta lesion and abnormal volume of amniotic fluid caused by this arbovirus.27 Although the results of this study do not enable any affirmation to be made about the existence of a relationship between the period when a pregnant woman is infected with ZIKV and the severity of neurological outcomes, this possibility cannot be ruled out, given that in the three cases of microcephaly/CZS for which this information was available, possible maternal infection (presence of rash) occurred in the first trimester of pregnancy. This finding is in agreement with those of other authors,28thus strengthening the hypothesis of the existence of this relationship.
We highlight the need for caution in interpreting the results of this study, due to the similarity between the clinical pictures of Zika, dengue and chikungunya, high frequency of inconclusive cases, and the fact that Zika virus disease only became a compulsorily notifiable condition with effect from February 2016. Inexistence of specific reliable, simple and low-cost laboratory tests for its diagnosis is yet another limiting factor, aggravated by the weakness of the state’s laboratory diagnosis network: in Tocantins, available laboratory tests were only performed in the capital. Moreover, inconsistencies in case recording between the information systems may also hinder the portrayal of an epidemiological situation closer to reality. Other problems were found during data collection in relation to records of pregnant women suspected of having the disease but only notified on the RESP and not on the SINAN, as well as several fields of the RESP form not being filled in. However, identification of some of the problems presented by the Zika surveillance information systems, principally with regard to the filling in of forms and difficulties in performing laboratory tests – among others –, beyond being an important subproduct of this study, points to the relevance and the need for them to be improved.
Despite these limitations, the conclusion is reached that the Zika epidemic in Tocantins was particularly intense. Notwithstanding, the adverse effects of ZIKV infection in the total population and among pregnant women were much less frequent than in other states in the Northeast region of Brazil in the period studied.14In particular, harm was only analyzed in the case of fetuses and live-born children shortly after birth. It is known that the live-born children of pregnant women infected with ZIKV, even if without diagnosis of microcephaly/CZS at birth, can present diverse neurological malformations, musculo-skeletal alterations, eye alterations and other health complications that will manifest themselves later.9
Nevertheless, if the effects of ZIKV infection during the period of child growth and development are not fully known, then the following are necessary: (i) special monitoring of children born in areas at risk of occurrence of Zika; and (ii) data gathering from health care records on possible cases whose clinical manifestations appeared later, such as, for example, physiotherapy records and Continuous Benefit Provision (Benefício de Prestação Continuada) records. It is important to provide special care to these children if they present alterations during their childhood. Such initiatives, of a longitudinal nature, can contribute to new hypotheses as to increased knowledge about such an important public health problem.
REFERENCES
1. Paixão ES, Barreto RF, Teixeira MG, Costa MCN, Rodrigues LC. History, epidemiology, and clinical manifestations of zika: a systematic review. Am J Public Health [Internet]. 2016 Apr [cited 2020 Jun 17];106(4):606-12. Available from: https://doi.org/10.2105/ajph.2016.303112 [ Links ]
2. Zanluca C, Melo VCA, Mosimann ALP, Santos GIV, Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz [Internet]. 2015 Jun [cited 2017 Feb 6];110(4):569-72. Available from: https://doi.org/10.1590/0074-02760150192 [ Links ]
3. Campos GS, Bandeira AC, Sardi I. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis [Internet]. 2015 Oct [cited 2017 Feb 6];21(10):1885-6. Available from: https://doi.org/10.3201/eid2110.150847 [ Links ]
4. Oliveira W K, França GVA, Carmo EH, Duncan BB, Kuchenbecker RS, Schmidt MI. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet [Internet]. 2017 Jun [cited 2018 Mar 5];390(10097):861-70. Available from: https://doi.org/10.1016/S0140-6736(17)31368-5 [ Links ]
5. Ministério da Saúde (BR). Centro de Operações de Emergências em Saúde Pública sobre Microcefalias. Informe epidemiológico nº 1/2015, semana epidemiológica 46 (15 a 21/11/2015): monitoramento dos casos de microcefalias no Brasil [internet]. Brasília: Ministério da Saúde; 2015 [citado 2018 fev 25]. (Coes – Microcefalias). Disponivel em: https://www.saude.gov.br/images/pdf/2015/novembro/24/COES-Microcefalias---Informe-Epidemiol--gico---SE-46---24nov2015.pdf [ Links ]
6. World Health Organization - WHO. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations [Internet]. Genebra: World Health Organization; 2016 [cited 2018 Oct 14]. Available from: http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/ [ Links ]
7. Teixeira MG, Costa MCN, Oliveira WK, Nunes ML, Rodrigues LC.The epidemic of zika virus–related microcephaly in Brazil: detection, control, etiology, and future scenarios. Am J Public Saúde [Internet]. 2016 Apr [cited 2020 Jun 17];106(4):601-05. Available from: https://dx.doi.org/10.2105%2FAJPH.2016.303113 [ Links ]
8. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects-reviewing the evidence for causality. N Eng J Med [Internet]. 2016 May [cited 2018 Feb 26];374(20):1981-7. Available from: https://doi.org/10.1056/nejmsr1604338 [ Links ]
9. Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, et al. Congenital zika virus infection beyond neonatal microcephaly. JAMA Neurol [Internet]. 2016 Dec [cited 2018 Jan 19];73(2):1407-16. Available from: https://doi.org/10.1001/jamaneurol.2016.3720 [ Links ]
10. Van der Linden V, Rolim Filho E, Lins OG, Van der Linden A, Aragão MF, Brainer-Lima AM, et al. Congenital zika syndrome with arthrogryposis: retrospective case series study. BMJ [Internet]. 2016 Aug [cited 2018 Sep 18];354:i3899. Available from: https://doi.org/10.1136/bmj.i3899 [ Links ]
11. Possas C, Brasil P, Marzochi MCA, Tanuri A, Martins RM, Marques ETA, et al. Zika puzzle in Brazil: peculiar conditions of viral introduction and dissemination - a review. Mem Inst Oswaldo Cruz [Internet]. 2017 May [cited 2018 Apr 10];112(5):319-27. Available from: https://doi.org/10.1590/0074-02760160510 [ Links ]
12. Araújo TVB, Rodrigues LC, Ximenes RAA, Miranda-Filho DB, Montarroyos UR, Melo APLM, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet [Internet]. 2016 Sep [cited 2018 Nov 30]. Available from: http://dx.doi.org/10.1016/ S1473-3099(16)30318-8 [ Links ]
13. Ministério da Saúde (BR). Centro de Operações de Emergências em Saúde Pública sobre Microcefalias. Informe epidemiológico nº 07, semana epidemiológica 52/2015 (27/12/2015 a 02/01/2016): monitoramento dos casos de microcefalia no Brasil [Internet]. Brasília: Ministério da Saúde; 2016 [citado 2018 out 28]. (Coes – Microcefalias). Disponível em: http://www.saude.gov.br/images/pdf/2016/janeiro/05/COES-Microcefalias---Informe-Epidemiol--gico-07---SE-52---04jan2016.pdf [ Links ]
14. Ministério da Saúde (BR). Centro de Operações de Emergências em Saúde Pública sobre Microcefalias. Informe epidemiológico nº 57, semana epidemiológica 52/2016 (25 a 31/12/2016): monitoramento dos casos de microcefalias no Brasil [Internet]. Brasília: Ministério da Saúde; 2017 [citado 2018 out 28]. Disponível em: https://www.saude.gov.br/images/pdf/2017/janeiro/12/Informe-Epidemiologico-n57-SE-52_2016-09jan2017.pdf [ Links ]
15. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Monitoramento integrado de alterações no crescimento e desenvolvimento relacionados à infecção pelo vírus Zika e outras etiologias infecciosas, até a Semana Epidemiológica 52 de 2017. Bol Epidemiol [Internet]. 2018 [citado 2019 jan 12];49(6). Disponível em: https://www.saude.gov.br/images/pdf/2018/fevereiro/20/2018-003-Final.pdf [ Links ]
16. Fontes BM. Iridociclite hipertensiva relacionada ao vírus zika. Arq Bras Oftalmol [Internet]. 2016 jan-fev [citado 2018 fev 16];79(1):63. Disponível em: https://doi.org/10.5935/0004-2749.20160020 [ Links ]
17. Malta JMAS, Vargas A, Leite PL, Percio J, Coelho GE, Ferraro AHA, et al . Síndrome de Guillain-Barré e outras manifestações neurológicas possivelmente relacionadas à infecção pelo vírus Zika em municípios da Bahia, 2015. Epidemiol Serv Saúde [Internet]. 2017 jan-mar [citado 2018 jul 2];26(1):9-18. Disponível em: https://doi.org/10.5123/s1679-49742017000100002 [ Links ]
18. Instituto Brasileiro de Geografia e Estatística - IBGE. Projeção da população do Brasil e das Unidades da Federação: nota técnica [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2016 [citado 2018 dez 10]. Disponível em: http://www.ibge.gov.br/apps/populacao/projecao/ [ Links ]
19. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de vigilância em saúde: volume único [Internet]. Brasília: Ministério da Saúde; 2017 [citado 2018 fev 25]. 705 p. Disponível em: http://portalarquivos.saude.gov.br/images/pdf/2017/outubro/06/Volume-Unico-2017.pdf [ Links ]
20. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Secretaria de Atenção em Saúde. Orientações integradas de vigilância e atenção à saúde no âmbito da Emergência de Saúde Pública de Importância Nacional [Internet]. Brasília: Ministério da Saúde; 2016 [citado 2018 out 6]. 158 p. Disponível em: http://portalarquivos.saude.gov.br/images/pdf/2016/dezembro/12/orientacoes-integradas-vigilancia-atencao.pdf [ Links ]
21. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 52, 2016. Bol Epidemiol [Internet]. 2016 [citado 2017 nov 8];47(3). Disponível em: http://portalarquivos2.saude.gov.br/images/pdf/2017/abril/06/2017-002-Monitoramento-dos-casos-de-dengue--febre-de-chikungunya-e-febre-pelo-v--rus-Zika-ate-a-Semana-Epidemiologica-52--2016.pdf [ Links ]
22. Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Camargo S, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis [Internet]. 2016 Sep [cited 2018 Feb 19];51:128-32. Available from: https://doi.org/10.1016/j.ijid.2016.08.023 [ Links ]
23. D'Ortenzio E, Matheron S, Yazdanpanah Y, Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med [Internet]. 2016 Jun [cited 2017 Feb 23];374(22):2195-8. Available from: https://doi.org/10.1056/nejmc1604449 [ Links ]
24. Donalísio MR, Glasser CM. Vigilância entomológica e controle de vetores do dengue. Rev Bras Epidemiol [Internet]. 2002 dez [citado 2014 mar 4];5(3):259-72. Disponível em: ttp://dx.doi.org/10.1590/S1415-790X2002000300005 [ Links ]
25. Viana DV, Ignotti E. A ocorrência da dengue e variações meteorológicas no Brasil: revisão sistemática. Rev Bras Epidemiol [Internet]. 2013 jun [citado 2015 out 30];16(2):240-56. Disponível em: http://dx.doi.org/10.1590/S1415-790X2013000200002 [ Links ]
26. Marrs C, Olson G, Saade G, Hankins G, Wen T, Patel J, et al. Zika virus and pregnancy: a review of the literature and clinical considerations. Am J Perinatol [Internet]. 2016 Jun [cited 2018 May 5];33(7):625-39. Available from : https://doi.org/10.1055/s-0036-1580089 [ Links ]
27. Beckham JD, Pastula DM, Massey A, Tyler KL. Zika virus as an emerging global pathogenneurological complications of zika vírus. JAMA Neurol [Internet]. 2016 Jul [cited 2018 Feb 18];73(7):875-9. Available from: https://doi.org/10.1001/jamaneurol.2016.0800 [ Links ]
28. França GVA, Schuler-Faccini L, Oliveira WK, Henriques CMP, Carmo EH, Pedi VD, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet [Internet]. 2016 Aug [citado 2019 May 10];388(10047):891-7. Available from: https://doi.org/10.1016/s0140-6736(16)30902-3 [ Links ]
29. Vianna RAO, Lovero KL, Oliveira, SA, Fernandes AR, Santos TCSD Lima LCSS et al. Children born to mothers with rash during zika virus epidemic in Brazil: first 18 months of life. J Trop Pediatr [Internet]. 2019 Dec [cited 2019 May 10];65(6):592-602. Available from: https://doi.org/10.1093/tropej/fmz019 [ Links ]
*Manuscript derived from the Masters Degree dissertation entitled ‘Repercussions of the ZIKA virus emergency on the population of Tocantins/Brazil’, defended by Meire da Silva Pereira Rodrigues at the Public Health Professional Master’s Degree Program, Institute of Public Health, Federal University of Bahia (ISC/UFBA), in 2017.
Received: March 18, 2020; Accepted: May 07, 2020











 texto em
texto em 


