Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.4 Brasília 2020 Epub 14-Ago-2020
http://dx.doi.org/10.5123/s1679-49742020000400012
Original Article
Thyroid cancer in Brazil: a descriptive study of cases held on hospital-based cancer registries, 2000-2016*
1Instituto Nacional de Câncer José Alencar Gomes da Silva, Coordenação de Gestão de Pessoas, Rio de Janeiro, RJ, Brazil
2Instituto Nacional de Câncer José Alencar Gomes da Silva, Coordenação de Prevenção e Vigilância, Rio de Janeiro, RJ, Brazil
3Fundação Instituto Oswaldo Cruz, Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro, RJ, Brazil
Objective
To describe the clinical and epidemiological profile of primary thyroid cancer hospital cases in Brazil.
Methods
This is a descriptive study of cases held on hospital cancer records who had their first consultation for treatment in the period 2000-2016 and who were monitored by the hospitals providing those records.
Results
Of the 52,912 cases, 83.4% were female and 96.9% were differentiated carcinoma cases. The median time to diagnosis was shorter for anaplastic cases (11 days) and for those living in Brazil’s Southern region (5 days). Treatment was initiated within 60 days in 88.8% of cases that arrived at the hospitals without diagnosis and in 34.9% of those who arrived with diagnosis.
Conclusion
The findings are consistent with thyroid cancer epidemiology, with a predominance of female cases and differentiated carcinomas. Analysis of time-to-treatment suggests access difficulties for those who already had diagnosis when they arrived at the hospitals.
Key words: Thyroid Neoplasms; Health Information Systems; Health Profile; Epidemiology, Descriptive; Time-to-Treatment
Introduction
Thyroid cancer is the most common malign neoplasm of the endocrine system and its incidence has increased with effect from the 1990s, without considerable repercussions on the mortality and survival of populations studied worldwide.1 The difference between incidence magnitudes and mortality may be associated with earlier diagnosis and favorable prognosis of the most commonly detected histological types, i.e. differentiated carcinomas.2
According to a United States projection,8 thyroid cancer will be in fourth place among the most frequent malign neoplasms in 2030, and this pattern may possibly be found in other countries as well. Indicated as a possible hypothesis for increased incidence, overdiagnosis – attributed to the introduction of new diagnostic Technologies3 – implies detection and treatment of low risk tumors, as well as generating permanent morbidity, associated with thyroidectomy and hormone replacement.4 These issues have given relevance to the status of thyroid cancer for Public Health, even though in Brazil studies of the profile of cases cared for on the National Health System (SUS) are scarce. Hospital-based cancer registries (HBCRs) provide information that enables the situation of cases treated in SUS-accredited hospitals to be known and issues related to public cancer care in Brazil to be assessed. The objective of this study was to describe the clinical and epidemiological profile of primary thyroid cancer hospital cases in Brazil.
Methods
This is a descriptive study of thyroid cancer case data informed through HBCRs in Brazil.
The case information was retrieved from the Integrador RHC (IRHC), a web-based system developed by the José Alencar Gomes da Silva National Cancer Institute (INCA), responsible for consolidating the databases sent by SUS-accredited health establishments that provide specialized oncology care, as well as institutions that spontaneously provide oncology care information. The study used the IRHC database updated as at September 2019.
It should be emphasized that the sending of databases to the IRHC is considered to be a dynamic process, given that health establishment accreditation and disaccreditation can happen all the time. Moreover, although HBCR information is collected prospectively, databases may also be sent containing cases recorded retrospectively. For this reason, the number of establishments informing HBCRs varied over the study period.
The following variables were selected:
sex (female; male);
age (recorded in complete years, categorized into age groups: ≤19; 20-29; 30-39; 40-49; 50-59; 60-69; ≥70);
schooling (none; elementary education; high school education; higher education);
Federative Unit (UF) of residence of cases;
histological type (differentiated; medullary; anaplastic);
malignant tumor classification (TNM);
clinical stage of tumor (I; II; III; IV; IVA; IVB; IVC);
prior diagnosis and treatment (refers to case status on arrival at the institution providing HBCRs, according to the following categories: no diagnosis and no treatment; with diagnosis but no treatment; with diagnosis and with treatment; other);
treatment protocol (stratified according to the forms of treatment considered to be most relevant: surgery [alone]; surgery + radioiodinetherapy; surgery + radiotherapy; radioiodinetherapy [alone]; radiotherapy [alone]; multiple treatment protocols; no treatment);
date of first consultation (considered to be a consultation at the service responsible for diagnosis/treatment, and not screening or guidance consultations or social interviews);
date of diagnosis;
treatment start date; and
National Health Establishment Registry (CNES) (used to find the UFs where hospitals informing HBCRs were located).12
Histological type refers to tumor morphological codes according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3).13 The histological types considered in this study were those related to differentiated carcinomas (8050; 8260; 8340-8344; 8350; 8450-8460; 8290; 8330-8335), medullary carcimonas (8345; 8510-8513) and anaplastic carcinomas (8020-8035).
The ‘clinical stage of tumor’ and ‘TNM’, variables usually available in the database represent the stage of the case on their first visit to the institution informing the HBCR. With regard to cases with missing information on staging but which had information about TNM, we opted for correspondence between staging and TNM, respecting the edition in force in the year of the case’s first consultation (5th edition14 for cases between 2000 and 2004; 6th edition15 for cases between 2005 and 2012; and 7th edition16 for cases between 2013 and 2016), as done by the hospital informing the HBCR. A particularity that should be mentioned is that in the 5th TNM edition, stage IV did not have subdivisions unlike the 6th and 7th editions (IVA, IVB and IVC).
The median time interval between first consultation and diagnosis was measured for cases who arrived at the HBCR institution without diagnosis and without treatment. We also calculated the median time interval between diagnosis and start of treatment for (i) cases who arrived without diagnosis and without treatment and for (ii) cases who arrived with diagnosis but without treatment, excluding those with unknown information about treatment and those who received no treatment whatsoever. Time between diagnosis and treatment, measured in absolute values, was stratified into ≤60 days and >60 days, in accordance with the provisions of Law No. 12732/2012.17
A descriptive analysis of the data was made by distributing the study population variables. Measures of central tendency and dispersion were calculated for the continuous variables. With the aim of not being affected by the influence of outliers, we opted to use the median and respective interquartile range (IQR). With regard to the categorical variables, proportions were calculated and comparisons between groups were performed using Pearson’s chi-squared test with a 5% significance level. A matrix containing absolute number of cases per UF of residence versus UF of hospital providing HBCR was built, whereby the proportion of cases provided with care in their own UF of residence was calculated. The female/male (F/M) sex ratio was calculated and was also analyzed according to histological type and age group. All the analyses were performed using R, version 3.6.1.
The study project was approved by the Sergio Arouca National Public Health School/Oswaldo Cruz Foundation Research Ethics Committee Cep/ENSP/Fiocruz): Certification of Submission for Ethical Appraisal (CAAE) No. 62062116.8.0000.5240, on January 31st 2017.
Results
Of the 83,151 Brazilian primary thyroid cancer cases informed via HBCRs, 80,463 had their first consultation for tumor treatment between 2000 and 2016, and 56.394 had follow-up at their HBCR institution. Of these, 51,291 were differentiated carcinoma cases, 1,042 were medullary carcinoma cases and 579 were anaplastic carcinoma cases, totaling 52,912 cases selected for the study.
Proportional distribution of cases informed via HBCRs increased over the years, from 1.1% in 2000 to 11.0% in 2014. There was a reduction in case proportion in 2015 (8.5%) and 2016 (7.3%), although there was also a reduction in institutions providing HBCRs with effect from 2014 (Figure 1).
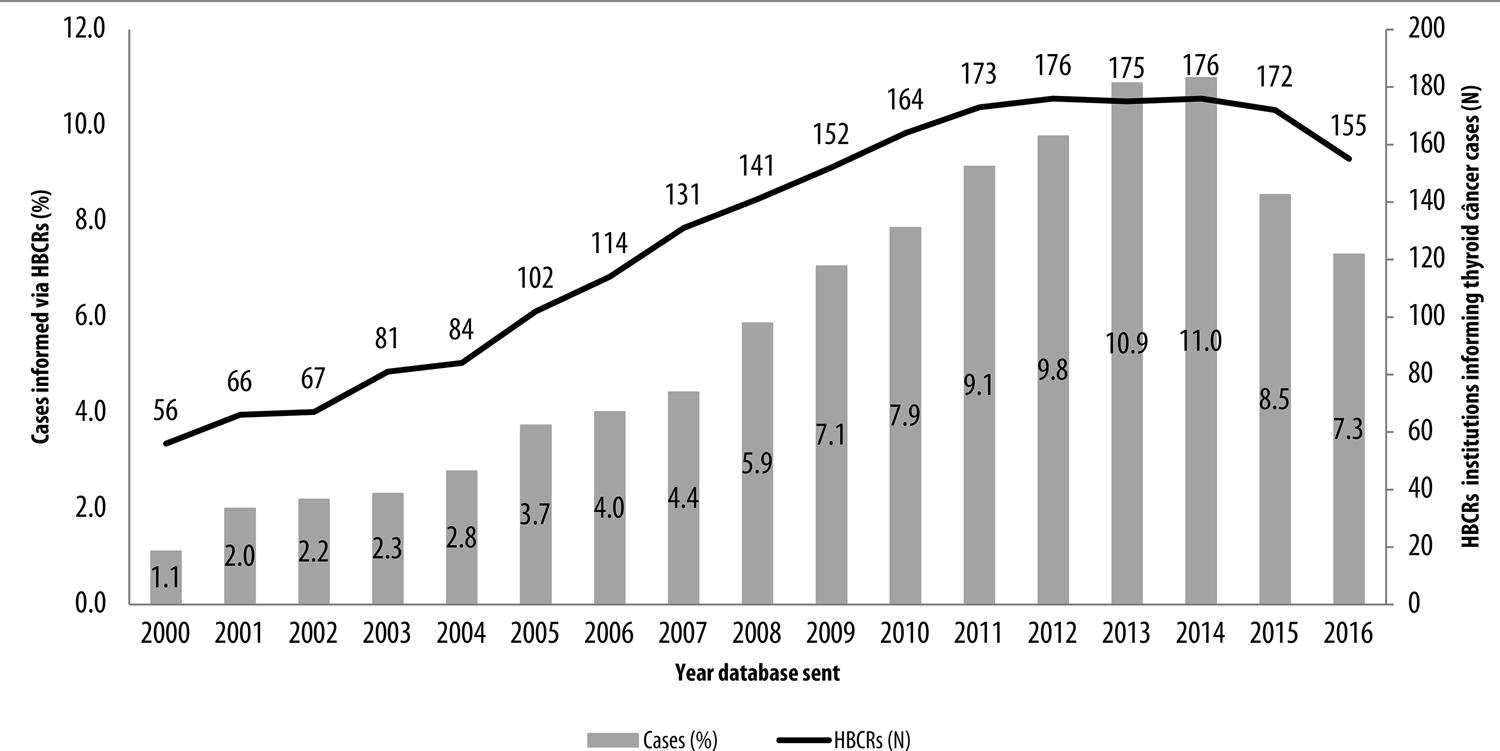
Figure 1 – Proportion of thyroid cancer cases and number of hospital-based cancer registries (HBCRs) informed per annum, Brazil, 2000-2016
Of the 52,912 cases, 83.4% were female: the sex ratio (F/M) was 5:1, and median age was 46 years (IQR = 35-56). The F/M ratio by histological type was 5.3 for differentiated carcinoma, 1.5 for medullary carcinoma and 2.2 for anaplastic carcinoma. The F/M ratio by age group for differentiated carcinoma was: 3.7 for the ≤19 year group; 5.7 for the 20-29 group; 5.5 for the 30-39 group; 6.1 for the 40-49 group; 5.3 for the 50-59 group; 4.4 for the 60-69 group; and 3.7 for the ≥70 group.
With regard to histological types, 96.9% of cases were diagnosed as having differentiated carcinomas (includes papillary and follicular carcinomas), 2.0% as having medullary carcinomas and 1.1% as having anaplastic carcinomas, whose respective median ages were 45 (IQR = 35-56), 49 (IQR = 36-60) and 66 (IQR = 56-76) years.
An increase in the proportion of differentiated carcinoma cases was found up to the 40-49 age group, as well as for medullary cases up to the 50-59 age group. Anaplastic carcinoma increase was progressive over the age groups (Table 1).
Table 1 – Sociodemographic and tumor characteristics by histological type of thyroid cancer cases informed by hospital-based cancer registries (HBCRs), Brazil, 2000-2016
| Variablesa | Differentiatedb | Medullary | Anaplastic | Total | p-valuec | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Sex | ||||||||
| Female | 43,081 | 84.0 | 623 | 59.8 | 400 | 69.1 | 44,104 | < 0.001 |
| Male | 8,205 | 16.0 | 419 | 40.2 | 179 | 30.9 | 8,803 | |
| Total | 51,286 | 100.0 | 1.042 | 100.0 | 579 | 100.0 | 52,907 | |
| Age group (in years) | ||||||||
| ≤19 | 1,377 | 2.7 | 61 | 5.9 | 6 | 1.0 | 1,444 | < 0.001 |
| 20-29 | 5,837 | 11.4 | 93 | 8.9 | 10 | 1.7 | 5,940 | |
| 30-39 | 11,014 | 21.5 | 161 | 15.5 | 19 | 3.3 | 11,194 | |
| 40-49 | 12,728 | 24.8 | 212 | 20.3 | 45 | 7.8 | 12,985 | |
| 50-59 | 11,040 | 21.5 | 235 | 22.6 | 102 | 17.6 | 11,377 | |
| 60-69 | 6,272 | 12.2 | 183 | 17.6 | 155 | 26.8 | 6,610 | |
| ≥70 | 3,019 | 5.9 | 97 | 9.3 | 242 | 41.8 | 3,358 | |
| Total | 51,287 | 100.0 | 1.042 | 100.0 | 579 | 100.0 | 52,908 | |
| Schooling | ||||||||
| None | 1,819 | 5.0 | 57 | 7.2 | 71 | 18.6 | 1,947 | < 0.001 |
| Elementary education | 16,277 | 44.4 | 464 | 58.8 | 230 | 60.4 | 16,971 | |
| High school education | 10,930 | 29.8 | 171 | 21.7 | 51 | 13.4 | 11,152 | |
| Higher education | 7,645 | 20.8 | 97 | 12.3 | 29 | 7.6 | 7,771 | |
| Total | 36,671 | 100.0 | 789 | 100.0 | 381 | 100.0 | 37,841 | |
| Case region of residence | ||||||||
| North | 1,762 | 3.4 | 29 | 2.8 | 12 | 2.1 | 1,803 | < 0.001 |
| Northeast | 21,027 | 41.1 | 247 | 23.8 | 152 | 26.3 | 21,426 | |
| Midwest | 1,184 | 2.3 | 40 | 3.9 | 16 | 2.8 | 1,240 | |
| Southeast | 20,249 | 39.6 | 536 | 51.7 | 267 | 46.2 | 21,052 | |
| South | 6,892 | 13.5 | 185 | 17.8 | 131 | 22.7 | 7,208 | |
| Total | 51,114 | 100.0 | 1.037 | 100.0 | 578 | 100.0 | 52,729 | |
| Prior diagnosis and treatment | ||||||||
| No diagnosis and no treatment | 31,979 | 62.9 | 674 | 65.9 | 366 | 64.1 | 33,019 | < 0.001 |
| With diagnosis but no treatment | 14,277 | 28.1 | 275 | 26.9 | 167 | 29.2 | 14,719 | |
| With diagnosis and with treatment | 3,996 | 7.9 | 68 | 6.6 | 35 | 6.1 | 4,099 | |
| Other | 584 | 1.1 | 6 | 0.6 | 3 | 0.5 | 593 | |
| Total | 50,836 | 100.0 | 1.023 | 100.0 | 571 | 100.0 | 52,430 | |
| Staging | ||||||||
| I | 23,521 | 73.6 | 159 | 25.0 | – | – | 23,680 | < 0.001 |
| II | 2,729 | 8.5 | 120 | 18.8 | – | – | 2,849 | |
| III | 3,969 | 12.4 | 141 | 22.1 | – | – | 4,110 | |
| IV | 130 | 0.4 | 19 | 3.0 | 70 | 21.7 | 219 | |
| IVA | 1,025 | 3.2 | 107 | 16.8 | 134 | 41.6 | 1,266 | |
| IVB | 160 | 0.5 | 17 | 2.7 | 55 | 17.1 | 232 | |
| IVC | 418 | 1.3 | 74 | 11.6 | 63 | 19.6 | 555 | |
| Total | 31,952 | 100.0 | 637 | 100.0 | 322 | 100.0 | 32,911 | |
| Treatment protocol | ||||||||
| Surgery (alone) | 27,424 | 53.5 | 654 | 62.9 | 177 | 30.6 | 28,255 | < 0.001 |
| Surgery + radioiodinetherapy | 5,879 | 11.5 | 31 | 3.0 | 9 | 1.6 | 5,919 | |
| Surgery + radiotherapy | 6,068 | 11.8 | 79 | 7.6 | 47 | 8.1 | 6,194 | |
| Radioiodinetherapy (alone) | 3,688 | 7.2 | 11 | 1.1 | 12 | 2.1 | 3,711 | |
| Radiotherapy (alone) | 1,216 | 2.4 | 31 | 3.0 | 89 | 15.4 | 1,336 | |
| Multiple treatment protocols | 5,906 | 11.5 | 187 | 18.0 | 177 | 30.6 | 6,270 | |
| No treatment | 1,037 | 2.0 | 46 | 4.4 | 67 | 11.6 | 1,150 | |
| Total | 51,218 | 100.0 | 1.039 | 100.0 | 578 | 100.0 | 52,835 | |
a) Missings: sex (N=5; 0.01%); age group (N=4; 0.01%); schooling (N=15,071; 28.5%); case region of residence (N=183; 0.4%); staging (N=20,001; 37.8%); prior diagnosis and treatment (N=482; 0.9%); treatment protocol (N=63; 0.2%).
b) Includes papillary and follicular carcinomas.
c) Pearson’s chi-squared test.
The majority of cases had studied as far as the elementary education level, regardless of histological type. However, 18.6% of anaplastic carcinoma cases had no schooling, followed by 7.2% of medullary carcinoma cases and 5.0% of differentiated carcinoma cases. Among the differentiated carcinoma cases, 50.6% had completed high school and/or higher education. Notwithstanding, missing information on this variable reached 28.5% (Table 1).
The vast majority of cases were resident in the Southeastern, Northeastern and Southern regions of the country. Southeastern region residents accounted for 51.7% of medullary carcinoma cases and 46.2% of anaplastic carcinoma cases. Together the Northeastern and Southeastern regions concentrated approximately 80.0% of differentiated carcinoma cases (Table 1).
Over 60.0% of cases arrived at the institutions without diagnosis and without treatment, and more than 20.0% were found to be in the ‘with diagnosis but without treatment’ category, for all histological types (Table 1).
With regard to differentiated carcinomas, 73.6% of cases were classified as stage I. In relation to medullary carcinomas, 25.0% of cases were at stage I and 22.1% were at stage III, while 41.6% of anaplastic carcinoma cases were found to be at stage IVA and 19.6% at stage IVC. Anaplastic carcinoma cases whose first consultation took place between 2000 and 2004 were recorded as stage IV (21.7%), with no subdivisions. However, 37.8% of the information on this variable was missing (Table 1).
With regard to treatment, ‘surgery alone’ was the treatment protocol received in the majority of cases and was performed on 62.9% of medullary carcinoma cases and 53.5% of differentiated carcinoma cases. In relation to anaplastic carcinomas, 30.6% of cases were submitted to ‘surgery alone’ and 15.4% to ‘radiotherapy alone’, whereby this latter form of treatment accounted for a higher proportion when compared to ‘radiotherapy alone’ for the other histological types: 2.4% of differentiated cases and 3.0% of medullary cases. The same behavior was found for ‘no treatment’, accounting for 11.6% of anaplastic carcinoma cases, 2.0% of differentiated carcinoma cases and 4.4% of medullary carcinoma cases (Table 1).
Analysis of time intervals by histological type (Table 2) indicated that among cases who arrived at the institution without diagnosis and without treatment, median time between first consultation and diagnosis was shortest (11 days) for anaplastic carcinomas. Similarly, anaplastic carcinoma cases who arrived at the institution with diagnosis but without treatment had the shortest median time (38 days) between diagnosis and first treatment. As such, 74.7% of anaplastic carcinoma cases received treatment in up to 60 days following diagnosis, compared to 34.5% of differentiated carcinoma cases and 32.1% of medullary carcinoma cases.
Table 2 – Time (in days) between first consultation and diagnosis and time between diagnosis and start of treatment according to status upon arrival at hospital-based cancer registries (HBCRs) institutions and histological types, Brazil, 2000-2016
| Time interval (days) | Histological types | Without diagnosis and without treatment | With diagnosis but without treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQRª) | ≤60 days (%) | >60 days (%) | N | Median (IQRª) | ≤60 days (%) | >60 days (%) | ||
| Between first consultation and diagnosis | Differentiated | 31,751 | 28 (6 - 84) | − | − | − | − | − | − |
| Medullary | 671 | 35 (9 - 88) | − | − | − | − | − | − | |
| Anaplastic | 363 | 11 (3 - 33) | − | − | − | − | − | − | |
| All | 32,785 | 28 (6 - 84) | − | − | − | − | − | − | |
| Between diagnosis and treatment | Differentiated | 26,020 | 0 (0 - 1) | 27,535 (88.8) | 3,467 (11.2) | 12,707 | 91 (48 - 165) | 4,442 (34.5) | 8,447 (65.5) |
| Medullary | 580 | 0 (0 - 14) | 563 (86.7) | 86 (13.3) | 539 | 92 (47 - 182) | 77 (32.1) | 163 (67.9) | |
| Anaplastic | 287 | 0 (0 - 16) | 290 (92.4) | 24 (7.6) | 146 | 38 (20 - 62) | 109 (74.7) | 37 (25.3) | |
| All | 26,887 | 0 (0 - 2) | 28,388 (88.8) | 3,577 (11.2) | 13,092 | 90 (47 - 164) | 4,628 (34.9) | 8,647 (65.1) | |
a) Interquartile range.
Distribution of time intervals by Brazilian regions (Table 3) showed that median time between first consultation and diagnosis for cases that arrived at the institution without diagnosis and without treatment was 28 days. This median time varied between the Brazilian regions, with the shortest interval being found in the South (5 days) and the longest interval in the North (73 days). Median time between diagnosis and treatment was zero for all regions except the Northern region (1 day), meaning that diagnosis and treatment were concomitant. In relation to cases who arrived at the institution with diagnosis but without treatment, median time was 90 days between diagnosis and treatment, with the shortest interval in the Southern region (72 days) and the longest interval in the Northern region (142 days). As such, a higher proportion of cases was found whose treatment started after more than 60 days, varying from 55.2% in the Southern region to 73.3% in the Northeast. We found that the state of São Paulo provided care to 66.9% of cases resident in the state of Goiás, 33.0% of cases resident in the Federal District and 18.5% of cases resident in Acre (Table 4).
Table 3 – Time (in days) between first consultation and diagnosis and time between diagnosis and start of treatment according to status upon arrival at hospital-based cancer registries (HBCRs) institutions and region of residence, Brazil, 2000-2016
| Time interval (days) | Regions | Without diagnosis and without treatment | With diagnosis but without treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQRª) | ≤60 days (%) | >60 days (%) | N | Median (IQRª) | ≤60 days (%) | >60 days (%) | ||
| Between first consultation and diagnosis | North | 570 | 73 (18 - 164) | − | − | − | − | − | − |
| Northeast | 12,495 | 36 (10 - 100) | − | − | − | − | − | − | |
| Midwest | 552 | 22 (5 - 80) | − | − | − | − | − | − | |
| Southeast | 14,505 | 28 (4 - 76) | − | − | − | − | − | − | |
| South | 4,663 | 5 (0 - 55) | − | − | − | − | − | − | |
| Brazil | 32,785 | 28 (6 - 84) | − | − | − | − | − | − | |
| Between diagnosis and treatment | North | 341 | 1 (0 - 74) | 471 (83.5) | 93 (16.5) | 579 | 142 (70 - 270) | 124 (38.6) | 457 (61.4) |
| Northeast | 8,803 | 0 (0 - 7) | 10,616 (88.7) | 1,350 (11.3) | 4,389 | 115 (61 - 205) | 1,206 (26.7) | 3,309 (73.3) | |
| Midwest | 520 | 0 (0 - 0) | 486 (86.7) | 72 (13.3) | 251 | 95 (37 - 194) | 97 (38.6) | 154 (61.4) | |
| Southeast | 13,022 | 0 (0 - 9) | 12,478 (87.4) | 1,794 (12.6) | 6,490 | 77 (42 - 141) | 2,564 (39.4) | 3,941 (60.6) | |
| South | 4,201 | 0 (0 - 0) | 4,355 (94.2) | 268 (5.8) | 1,383 | 72 (30 - 125) | 637 (44.8) | 786 (55.2) | |
| Brazil | 26,887 | 0 (0 - 2) | 28,388 (88.8) | 3,577 (11.2) | 13,092 | 90 (47 - 164) | 4,628 (34.9) | 8,647 (65.1) | |
a) Interquartile range.
Table 4 – Matrix of absolute number of thyroid cancer cases per Federative Unit (UF) of residence and UF of hospital-based cancer registries (HBCRs) informing institution and proportion of cases receiving care in their respective UFs of residence, Brazil, 2000-2016
| Case UF of residencea | UF of HBCR informing institution | % of cases cared for in UF of residence | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | AL | AM | AP | BA | CE | DF | ES | GO | MA | MG | MS | MT | PA | PB | PE | PI | PR | RJ | RN | RO | RR | RS | SC | SE | SP | TO | Total | ||
| AC | 42 | − | − | − | − | − | − | − | − | − | 1 | − | − | − | − | − | − | − | − | 1 | 22 | − | − | − | − | 15 | − | 81 | 51,9 |
| AL | − | 650 | − | − | 6 | − | − | − | − | − | − | − | − | − | 1 | 9 | − | 1 | − | − | − | − | − | − | 5 | 9 | − | 681 | 95,4 |
| AM | − | − | 440 | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | − | 1 | − | − | 50 | − | 494 | 89,1 |
| AP | − | − | − | 26 | − | 1 | − | − | − | − | − | − | − | 26 | − | − | − | − | − | − | 3 | − | − | − | − | 4 | − | 60 | 43,3 |
| BA | − | 1 | − | − | 7.727 | 1 | 8 | 9 | − | − | 6 | 1 | 1 | 0 | − | 7 | − | 1 | 2 | − | − | − | 1 | 1 | 25 | 44 | − | 7.835 | 98,6 |
| CE | − | − | − | − | 8 | 2.578 | − | − | − | 1 | − | − | − | − | − | − | 1 | − | 3 | 1 | − | − | − | 1 | 1 | 6 | − | 2.600 | 99,2 |
| DF | − | − | − | − | 3 | − | 164 | − | − | 1 | 3 | − | − | − | − | − | − | − | 1 | 1 | − | − | − | − | − | 85 | − | 258 | 63,6 |
| ES | − | − | − | − | 1 | − | − | 573 | − | − | 3 | − | 1 | − | − | − | − | − | 10 | − | − | − | − | − | − | 13 | − | 601 | 95,3 |
| GO | − | − | − | − | − | 2 | 22 | − | 15 | − | 3 | − | − | − | − | − | 1 | − | − | − | − | − | 1 | − | − | 89 | − | 133 | 11,3 |
| MA | − | − | − | − | 1 | − | − | 1 | − | 1.594 | 2 | − | − | 2 | 1 | 2 | 128 | − | 1 | − | − | − | − | 3 | − | 13 | 4 | 1.752 | 91,0 |
| MG | − | − | − | − | 7 | 1 | 5 | 2 | − | − | 3.487 | 1 | 1 | − | − | − | − | 6 | 8 | − | − | − | − | 1 | 2 | 383 | − | 3.904 | 89,3 |
| MS | − | − | − | − | − | − | − | − | − | − | 1 | 363 | 1 | − | − | − | 1 | 8 | 1 | − | − | − | − | − | − | 137 | − | 512 | 70,9 |
| MT | − | − | − | − | 1 | − | − | − | − | − | 1 | − | 273 | − | − | − | − | − | − | − | − | − | − | − | − | 60 | 2 | 337 | 81,0 |
| PA | − | − | 30 | − | − | 1 | − | − | − | 2 | − | − | 2 | 704 | − | − | 16 | − | 1 | − | − | − | − | − | − | 29 | 6 | 791 | 89,0 |
| PB | − | − | − | − | 8 | 2 | − | − | − | − | − | 1 | − | 3 | 1.084 | 19 | 2 | 1 | 2 | 10 | − | − | 2 | 2 | 1 | 4 | − | 1.141 | 95,0 |
| PE | − | 1 | − | − | 15 | 22 | − | − | − | − | 2 | − | − | − | 7 | 2.672 | − | 2 | 2 | 1 | − | − | 1 | − | 1 | 27 | − | 2.753 | 97,1 |
| PI | − | − | − | − | 1 | 2 | − | − | − | − | 1 | − | − | − | − | 1 | 714 | − | − | − | − | − | − | − | − | 10 | − | 729 | 97,9 |
| PR | − | − | − | − | 2 | 1 | − | − | − | − | 1 | 2 | 1 | − | − | − | − | 2.059 | − | − | − | − | 2 | 17 | − | 33 | − | 2.118 | 97,2 |
| RJ | − | − | 1 | − | 2 | − | − | − | − | − | 19 | − | − | − | − | − | − | − | 1.769 | − | − | − | 1 | 1 | 3 | 23 | − | 1.819 | 97,3 |
| RN | − | − | − | − | 4 | 6 | − | − | − | − | − | − | − | − | 1 | − | − | 1 | − | 3.480 | − | − | − | − | − | − | − | 3.492 | 99,7 |
| RO | − | − | − | − | 1 | − | − | − | − | − | − | − | 4 | − | − | − | 1 | 2 | − | − | 164 | − | − | 1 | − | 34 | − | 207 | 79,2 |
| RR | − | − | 8 | − | − | − | − | − | − | − | 1 | − | − | − | − | − | 1 | − | 1 | − | 1 | 10 | − | − | − | 2 | − | 24 | 41,7 |
| RS | − | − | − | − | − | − | − | − | − | − | 1 | − | 1 | − | − | − | − | 3 | − | − | − | − | 2.099 | 3 | 1 | 3 | − | 2.111 | 99,4 |
| SC | − | − | − | − | − | − | 1 | − | − | − | − | − | − | − | 1 | − | − | 34 | − | − | − | − | 27 | 2.894 | − | 22 | − | 2.979 | 97,1 |
| SE | − | − | − | − | 27 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 406 | 10 | − | 443 | 91,6 |
| SP | − | − | − | − | 12 | − | 1 | − | − | − | 20 | 1 | − | − | − | − | − | 7 | 2 | − | − | − | 2 | 2 | 1 | 14.680 | − | 14.728 | 99,7 |
| TO | − | − | − | − | − | − | 1 | − | − | − | 1 | − | − | − | − | − | 1 | 1 | − | − | − | − | − | − | − | 12 | 130 | 146 | 89,0 |
| Total | 42 | 652 | 479 | 26 | 7.827 | 2.617 | 202 | 585 | 15 | 1.598 | 3.553 | 369 | 285 | 735 | 1.095 | 2.710 | 866 | 2.126 | 1.803 | 3.494 | 192 | 10 | 2.137 | 2.926 | 446 | 15.797 | 142 | 52.729 | − |
a) Missing: UF of residence (N=183; 0.4%).
Discussion
The results of this study showed an increase over the years in the number of thyroid cancer cases in Brazil informed via HBCRs. Whereas the number of institutions providing HBCR information increased more than threefold between 2000 and 2014, the number of cases reported in the same period increased approximately tenfold. The reduction in case numbers seen in the years following this period may be related to delays in the institutions submitting their databases.18
Although this study did not deal with population morbidity, it should be noted that a substantial increase in thyroid cancer incidence has been described with effect from the 1990s in a variety of countries including Brazil.2 Some authors suggest that the pattern of incidence growth is real, considering that: this increase is occurring in all tumor sizes and stages, although it is more noticeable in small nodules; it almost exclusively affects the papillary histological type; it is greater among females than among males; and it reveals a birth cohort effect which is possibly a reflection of changes in risk factors related to forms of environmental exposure.4 However, other authors defend the hypothesis that this increase reflects overdiagnosis, due to improved ability to detect malignancy in minute thyroid nodules, made possible by increased availability of ultrasound examinations and feasibility of cytology using material obtained through fine-needle aspiration puncture.3
As this is a hospital-based study, the ratio found between the female and male sexes, i.e. 5:1, is greater than the ratio of around 3:1 described in population-based studies, principally for differentiated carcinomas.11 However, the ratio found in this study is quite consistent with the fact of thyroid cancer being one of the few cancers that are predominant in females.24 Moreover, when this sex ratio was analyzed by age groups and histological types, specifically in the case of differentiated carcinomas, it increased during child-bearing age and decreased after the menopause.11 This finding suggests the possibility of female sex hormones playing an important role in the pathogenesis of the disease, i.e.: the potential of endogenous hormones and endocrine disruptors, such as polyhalogenated aromatic hydrocarbons, in particular polybrominated diphenyl ethers, in the development of thyroid cancer.26
Analysis of age according to histological types is in agreement with the literature, which describes mean age at diagnosis of between 45 to 55 years for differentiated carcinomas, 50 years for medullary carcinomas and 60 years or over for anaplastic carcinomas.25
Inadequate filling in of medical records by health workers hinders the accuracy of HBCRs and has a direct impact on information quality. In this study in particular, information was partially missing for the ‘schooling’ and ‘clinical stage of tumor’ variables. Level of schooling is not a risk factor that is consolidated in the literature,2 although some studies do describe higher thyroid cancer incidence among people with high school or higher education,30 suggesting a detection bias caused by greater medical surveillance and access to diagnostic technologies.25On the other hand, clinical stage of tumor is essential, and the existence of so many cases without this information made it impossible to make more in-depth assumptions and analyses about this variable.
The first treatment received at the institutions is closely related to the stage of the disease, this being the reason why tumors diagnosed at initial stages tend to be submitted to surgery. With the exception of anaplastic carcinomas, over 70.0% of the other histological types received surgical treatment as part of the treatment protocol. In the case of differentiated carcinomas, the main treatment approach consists of thyroidectomy, followed by thyroid tissue ablation using radioactive iodine (I131), whereby the former has excellent prognosis even when ablation is not successful.25 As such, the implications of treating a considerable number of malign neoplasms that would neither affect the health nor the survival of the individuals concerned need to be taken into consideration: apart from providing little benefit, these treatments generate permanent morbidity related to thyroidectomy and thyroid hormone replacement. However, in view of the uncertainty about thyroid cancer evolution, regardless of clinical and epidemiological profile, the treatment chosen has been immediate surgery rather than watchful waiting.4
The time intervals that elapsed between first consultation, diagnosis and start of treatment are a relevant aspect which points to probable difficulties in accessing oncology care. It should be noted that Law No. 12732, dated November 22nd 2012, determines the right to receive first treatment on the SUS within 60 days with effect from diagnosis.17 In this sense, with regard to thyroid cancer cases who arrive at HBCR institutions without diagnosis and without treatment, divergences were identified in the median time between first consultation and diagnostic confirmation, both in terms of histological types and Brazilian regions. Median time was shortest for anaplastic carcinomas and this was also found for Southern Brazil in comparison to the country’s other regions.
Still in relation to cases who arrived without diagnosis and without treatment at HBCR institutions, the length of time between diagnosis and start of treatment was short, and was similar between histological types and regions of the country. It is noteworthy that the greater proportion of cases received first treatment within 60 days with effect from diagnosis. On the other hand, for cases who arrived at HBCR institutions with diagnosis but without treatment, median time to start of treatment was longer and was more than 60 days in the majority of cases. This may possibly be a reflection of access difficulties faced by those who received diagnosis in services not accredited to provide cancer treatment. In addition, the fact of anaplastic carcinoma cases receiving diagnosis and treatment in a shorter time may be associated with the more lethal characteristics of this tumor.
Case distribution according to UFs of residence and HBCR institution revealed a migratory flow, probable not taken into account by the cancer care plans of the respective states; this is the case of people resident in the states of Acre, Goiás and the Federal District, who sought cancer care in São Paulo. It is possible that problems in accessing health services or shortcomings in the cancer care network can contribute to migration in search of care, hindering the start of treatment within 60 following diagnosis.
There are some limitations related to HBCR information, including missing information for some variables and possible case under-recording. Errors in the morphological coding of malign thyroid tumors could also account for some cases not being included in the study population. The shortage of publications based on HBCR information makes it difficult to compare results. Notwithstanding these limitations, the findings of this investigation are consistent thyroid cancer epidemiology described in the literature.25
Analysis of the situation of cancer morbidity in hospitals can enable health planning that facilitates care flow in the health service network, providing timely diagnosis and treatment, as well as reducing the need to travel in order to get cancer care. We hope this study will contribute to Public Health in the sense of raising the awareness of health service managers to use the information generated though Hospital-based cancer registries, in order to inform the process of building and evaluating cancer care plans, identifying possible obstacles in the care network and evaluating the quality of medical record information, as well as the quality of care provided within SUS.
REFERENCES
1. Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R and Ferlay J, editors. Cancer incidence in five Continents, Vol. XI [Internet]. Lyon: International Agency for Research on Cancer; 2017 [cited 2019 Jul 22]. Available from: https://ci5.iarc.fr/CI5-XI/Default.aspx [ Links ]
2. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer [Internet]. 2015 May [cited 2019 Oct 22];136(9):2187-95. Available from: https://doi.org/10.1002/ijc.29251 [ Links ]
3. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid [Internet]. 2015 Oct [cited 2020 Jun 22];25(10):1127-36. Available from: https://doi.org/10.1089/thy.2015.0116 [ Links ]
4. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol [Internet]. 2013 [cited 2019 Aug 22]:965212. Available from: https://doi.org/10.1155/2013/965212 [ Links ]
5. Davies L, Morris L, Hankey B. Increases in thyroid cancer incidence and mortality. JAMA [Internet]. 2017 Jul [cited 2019 Sep 25];318(4):389-90. Available from: https://doi.org/10.1001/jama.2017.7906 [ Links ]
6. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA [Internet]. 2017 Apr [cited 2019 Sep 25];317(13):1338-48. Available from: https://doi.org/10.1001/jama.2017.2719 [ Links ]
7. Davies L. Overdiagnosis of thyroid cancer. BMJ [Internet]. 2016 Nov [cited 2019 Aug 24];355:i6312. Available from: https://doi.org/10.1136/bmj.i6312 [ Links ]
8. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res [Internet]. 2014 Jun [cited 2019 Jul 29];74(11):2913-21. Available from: https://doi.org/10.1158/0008-5472.can-14-0155 [ Links ]
9. Borges AKM, Miranda-Filho A, Koifman S, Koifman RJ. Thyroid cancer incidences from selected South America population-based cancer registries: an age-period-cohort study. J Glob Oncol [Internet]. 2017 Sep [cited 2019 Jul 29];4:1-11. Available from: https://doi.org/10.1200/jgo.17.00024 [ Links ]
10. Vaccarella S, Franceschi S, Bray F, Wild C, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med [Internet]. 2016 Aug [cited 2019 Aug 15];375:614-17. Available from: https://doi.org/10.1056/NEJMp1604412 [ Links ]
11. Dal Maso L, Lise M, Zambon P, Falcini F, Crocetti E, Serraino D, AIRTUM Working Group, at al. Incidence of thyroid cancer in Italy, 1991-2005: time trends and age-period-cohort effects. Ann Oncol [Internet]. 2011 Apr [cited 2019 Aug 15];22(4):957-63. Available from: https://doi.org/10.1093/annonc/mdq467 [ Links ]
12. Instituto Nacional de Câncer José Alencar Gomes da Silva - INCA. Registros hospitalares de câncer: planejamento e gestão [Internet]. 2. ed. Rio de Janeiro: INCA; 2010 [citado 2019 maio 23]. 536 p. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//registros-hospitalares-de-cancer-2010.pdf [ Links ]
13. Organização Mundial de Saúde - OMS. CID-O: classificação internacional de doenças para oncologia. São Paulo: Editora da Universidade de São Paulo; Fundação Oncocentro de São Paulo; 2013. [ Links ]
14. Sobin LH, Gospodarowicz M, Wittekind C. TNM: classificação de tumores malignos. 5. ed. Rio de Janeiro: INCA; 1998. Capítulo: Glândula tireoide (CID-O C73). [ Links ]
15. Sobin LH, Gospodarowicz M, Wittekind C. TNM: classificação de tumores malignos. 6. ed. Rio de Janeiro: INCA; 2004. Capítulo: Glândula tireoide (CID-O C73). [ Links ]
16. Sobin LH, Gospodarowicz M, Wittekind C. TNM: classificação de tumores malignos. 7. ed. Rio de Janeiro: INCA; 2012. Capítulo: Glândula tireoide (CID-O C73). [ Links ]
17. Brasil. Presidência da República. Casa Civil. Lei nº 12.732, de 22 de novembro de 2012. Dispõe sobre o primeiro tratamento de paciente com neoplasia maligna comprovada e estabelece prazo para seu início [Internet]. Diário Oficial da União, Brasília (DF), 2012 nov 23 [citado 2020 jun 22];Seção 1:1. Disponível em: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm [ Links ]
18. Organização Pan-Americana da Saúde. Instituto Nacional de Câncer (BR). Ministério da Saúde (BR). Perfil da assistência oncológica no Brasil, de 2007 a 2011. Inf Vigil Câncer [Internet]. 2015 jan-jul [citado 2020 abr 5];6:1-12. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//informativo-vigilancia-do-cancer-n6-2015.pdf [ Links ]
19. Instituto Nacional de Câncer (BR). Ministério da Saúde (BR). Perfil da assistência oncológica no Brasil entre 2012 e 2016. Inf Vigil Câncer [Internet]. 2020 jan-jul [citado 2020 abr 20];7:1-16. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//informativo-vigilancia-do-cancer-n7-2020.pdf [ Links ]
20. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control [Internet]. 2009 Jul [citado 2019 Sep 25];20(5):525-31. Available from: https://doi.org/10.1007/s10552-008-9260-4 [ Links ]
21. Sierra MS, Soerjomataram I, Forman D. Thyroid cancer burden in Central and South America. Cancer Epidemiol [Internet]. 2016 Sep [cited 2019 Aug 15];44 Suppl 1:S150-7. Available from: https://doi.org/10.1016/j.canep.2016.07.017 [ Links ]
22. Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev [Internet]. 2009 Mar [cited 2019 Jul 29];18(3):784-91. Available from: https://doi.org/10.1158/1055-9965.epi-08-0960 [ Links ]
23. Zheng T, Holford TR, Chen Y, Ma JZ, Flannery J, Liu W, Russi M, Boyle P. Time trend and age-period-cohort effect on incidence of thyroid cancer in Connecticut, 1935–1992. Int J Cancer [Internet]. 1996 Aug [cited 2019 Jul 29];67(4):504-9. Available from: https://doi.org/10.1002/(sici)1097-0215(19960807)67:4%3C504::aid-ijc7%3E3.0.co;2-w [ Links ]
24. Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid [Internet]. 2009 Oct [cited 2020 Jun 22];19(10):1061-6. Available from: https://doi.org/10.1089/thy.2008.0342 [ Links ]
25. Ron E, Schneider AB. Thyroid cancer. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, editors. Cancer epidemiology and prevention. 4th ed. New York: Oxford University Press; 2017. [ Links ]
26. Krassas GE. Thyroid disease and female reproduction. Fertil Steril [Internet]. 2000 Dec [cited 2019 Aug 15];74(6):1063-70. Available from: https://doi.org/10.1016/s0015-0282(00)01589-2 [ Links ]
27. Yane K, Kitahori Y, Konishi N, Okaichi K, Ohnishi T, Miyahara H, et al. Expression of the estrogen receptor in human thyroid neoplasms. Cancer Lett [Internet]. 1994 Aug [cited 2019 Oct 15];84(1):59-66. Available from: https://doi.org/10.1016/0304-3835(94)90358-1 [ Links ]
28. Franceschi S, Dal Maso L. Hormonal imbalances and thyroid cancers in humans. IARC Sci Publ. 1999;(147):33-43. [ Links ]
29. Zhang Y, Guo GL, Han X, Zhu C, Kilfoy BA, Zhu Y, et al. Do polybrominated diphenyl ethers (PBDE) increase the risk of thyroid cancer? Biosci Hypotheses [Internet]. 2008 [cited 2019 Jul 8];1(4):195-9. Available from: https://doi.org/10.1016/j.bihy.2008.06.003 [ Links ]
30. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer [Internet]. 2001 Sep [cited 2019 Jul 8];93(5):745-50. Available from: https://doi.org/10.1002/ijc.1377 [ Links ]
*Manuscript based on the Ph.D. thesis entitled ‘Thyroid cancer: a study of the age-period-cohort effect on incidence, analysis of the oncology care profile in the Brazilian National Health System and survival of a hospital cohort in Rio de Janeiro’, submitted by Anne Karin da Mota Borges to the Public Health and Environment Postgraduate Program, Sergio Arouca National Public Health School/Oswaldo Cruz Foundation (ENSP/Fiocruz), in 2017.
Received: December 26, 2019; Accepted: May 23, 2020











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI

