Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.29 no.5 Brasília 2020 Epub 24-Nov-2020
http://dx.doi.org/10.1590/s1679-49742020000500010
Original article
Family Health Strategy and determinants of directly observed treatment for tuberculosis in Brazil: a cross-sectional study with surveillance system data, 2014-2016*
1Universidade Federal do Espírito Santo, Laboratório de Epidemiologia, Vitória, ES, Brazil.
Objective:
To assess the association between tuberculosis determinants and performance of directly observed treatment (DOT) under different levels of Family Health Strategy (FHS) coverage in Brazil.
Methods:
This was a cross-sectional study using data on tuberculosis cases notified between 2014 and 2016 on the Notifiable Health Conditions Information System, as well as data on FHS coverage in the municipality of residence. Logistic regression was used.
Results:
177,626 individuals were included; being an alcohol user (odds ratio (OR) 1.09 - 95% confidence interval % [95%CI] 1.03;1.16), being deprived of liberty (OR=1.21 - 95%CI 1.12;1.32) and positive sputum smear microscopy (OR=1.15 - 95%CI 1.10;1.21) increased the chances of DOT being performed . When stratified by FHS coverage, these associations became weak in the highest stratum of coverage.
Conclusion:
DOT being performed was associated with determinants of tuberculosis. However, association was not confirmed among residents in municipalities with higher FHS coverage.
Keywords: Tuberculosis; Social Determinants of Health; Directly Observed Therapy; Family Health Strategy; Cross-Sectional Studies
Introduction
Control of tuberculosis continues to be a challenge for health authorities. Tuberculosis is one of the leading causes of death among communicable diseases worldwide.1 Brazil is among the countries with the highest burden of the disease, with an incidence rate of 34.8 cases per 100,000 inhabitants in 2018, falling well short of the rate recommended by the World Health Organization (WHO), namely 10 cases/100,000 inhab., thus highlighting the need to make progress in order to achieve the WHO target.2
Elimination of tuberculosis has to do with its natural history, its transmission routes are hard to control and can be potentiated by social determinants of health. These determinants can be categorized into three forms of vulnerability: (i) individual; (ii) programmatic or institutional; and (iii) sociodemographic.3 These vulnerabilities, associated with long treatment time, favor scenarios of difficulties in controlling the disease and contribute to patients dropping out of treatment.1 3
Treatment of sensitive tuberculosis lasts for at least six months. However, treatment can be extended because of associated diseases or unfavorable clinical progression.4 Within this context, directly observed treatment consists of watching intake of medication for treatment and is a strategy aimed at ensuring a person’s adherence to treatment. At least 72 doses of standardized medication should be supervised: 24 doses in the first phase of treatment and 48 doses in the second phase.5 Despite directly observed treatment being recommended for all tuberculosis cases, studies describe that this approach is used above all with people with low levels of education and with determining factors related to clinical characteristics and social exclusion.6
Participation of Primary Health Care services in the control of tuberculosis is crucial.7 In particular, the Family Health Strategy (FHS) stands out as a organizational and health care process comprised of multi-professional teams which have delimited geographical territories in which to operate, with the aim of achieving comprehensive care and linkage between the FHS as an institution - in the person of the health worker - and the benefitted individual.8-9 The FHS plays an important role in increasing access to diagnosis and follow-up of people with tuberculosis, including directly observed treatment.8-9 Reduced tuberculosis mortality is associated with increased FHS coverage, and that individuals registered with the FHS are more likely to have a favorable treatment outcome.9-10
As mentioned earlier, directly observed treatment being carried out is associated with sociodemographic, contextual and clinical determinants of tuberculosis.6 A study using surveillance data for Brazil as a whole showed that people with low levels of schooling, of brown race/skin color, alcohol users and with mental disorders, having the pulmonary form of tuberculosis and positive sputum smear microscopy, are more likely to undergo directly observed treatment.6 Notwithstanding, FHS has been showing itself to be an initiative that facilitates directly observed treatment being carried out, with positive impacts on tuberculosis control, providing greater likelihood of favorable outcomes.7-10 However, there is a scarcity of studies that discuss how determinants of directly observed treatment being carried out behave under different levels of FHS coverage. We have no knowledge of evidence of FHS coverage as a factor that minimizes selection of individuals for carrying out directly observed treatment.
The objective of this study was to assess the association between determinants of tuberculosis and performance of directly observed treatment, under different levels of FHS coverage in Brazil.
Methods
This was a cross-sectional study using data from the Brazilian Notifiable Health Conditions Information System (SINAN).
Brazil’s population is estimated to be 210,147,125 inhabitants, distributed over 5,570 municipalities.11 SINAN is a fundamental source of information for the Brazilian National Health System (SUS). Health data input to SINAN is not only obligatory but also done regularly by all the country’s Federative Units, which receive the records from the municipalities containing information about individuals suspected or diagnosed as having selected diseases and health conditions of public health concern, including tuberculosis.4
Treatment of sensitive tuberculosis using standard drugs lasts for at least six months and may be extended because of associated diseases or clinical progression of the patient.4 In this context directly observed treatment is carried out as a variable analyzed at the end of the treatment process - directly observed treatment performed (no; yes; unknown) -, with the objective of confirming whether the clinical recommendation has been complied with: at least 24 supervised doses in the first phase and 48 doses in the second phase.4 This study’s researchers had access to information on individuals diagnosed as having tuberculosis between 2014 and 2016.
With the aim of providing the text with greater objectivity, we have used the term ‘observed treatment’ instead of ‘directly observed treatment’.
The study included individuals over 18 years old, with diagnosis of drug-sensitive tuberculosis, as per national recommendations, notified and monitored longitudinally on the tuberculosis module of the SINAN system, based on examination of patient follow-up bulletins.4 12
Cases that were notified after death and those with unknown or no information on observed therapy having been carried out were excluded.
Observed therapy was considered to be the dependent variable, obtained from the tuberculosis patient follow-up bulletins, at the end of treatment, with the aim of assessing whether and how it was carried out.4 The individuals were divided into two groups, classified as: with observed therapy (yes); without observed therapy (no).
The covariables were taken from the notification/investigation form containing information as at the start of treatment and include the following characteristics:
a) Sociodemographic
age (in years: under 20; 20-39; 40-59; 60 or over);
sex (female; male);
race/skin color (white; black; brown; other);
years of schooling (no schooling; 1-4; 5-8; more than 8).
b) Contextual
geographic region of Brazil (Southeast; Northeast; Midwest; South; North);
zone of residence (urban; rural; peri-urban);
population deprived of liberty (no; yes);
health worker (no; yes);
street dweller (no; yes).
c) Associated diseases/comorbidities
infection with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) (no; yes);
alcohol use (no; yes);
illicit drug use (no; yes);
tobacco smoking (no; yes);
diabetes (no; yes);
mental disorder (no; yes).
d) Current treatment of tuberculosis
type of notification (new cases; relapse; restart after treatment dropout; transfer; not known);
clinical form of tuberculosis (pulmonary; extrapulmonary; pulmonary + extrapulmonary);
sputum smear microscopy (negative; positive; not performed);
initial culture (negative; positive; in progress; not performed);
chest x-ray (not suggestive of tuberculosis; suggestive of tuberculosis).
The variable ‘FHS coverage’ in the municipality of residence of the individuals was categorized according to the distribution of the 25 and 75 percentiles of the variable, being categorized as: less than 40%; 40-70%; greater than 70%.
The data on individuals with tuberculosis held on the SINAN system were provided by the National Tuberculosis Control Program. The data on FHS coverage were taken from the Primary Health Care Management Information System, which provides information on FHS coverage for each year. FHS coverage, i.e., the ratio between the number of individuals registered by the FHS teams and the municipal population base, was calculated for the period January 1st 2014 to December 31st 2016.
Absolute and relative frequencies were used to describe the variables. Pearson’s chi-square (x2) test was used in the bivariate analysis.
A theoretical model was used for the multiple analysis which classifies determinants of tuberculosis into hierarchical levels: the first level contains the sociodemographic variables; the second contains contextual variables and dwelling variables; the third contains diseases and comorbidities; while the fourth level contains clinical information on current tuberculosis treatment.3 Based on this model,3 all the variables were included in the hierarchical logistic regression, which had ‘directly observed treatment’ as its dependent variable. The covariables were grouped into levels, and those that were statistically significant (p<0.05) were kept in the next levels. Wald’s test was used for non-dichotomous variables.
In the analytical stage, according to FHS coverage, hierarchical logistic analysis was performed for each FHS coverage stratum. Individuals with missing data were excluded from this analysis. The results of the hierarchical logistic regression analyses were expressed as odds ratios (OR) and 95% confidence intervals (95%CI).
The study project was approved by the Federal University of Espírito Santo Health Sciences Center Human Research Ethics Committee, under record number 2.088.338, dated May 29th 2017.
Results
Between 2014 and 2016, 252,675 Brazilians with tuberculosis were notified on the SINAN system. Of these, the study excluded 13,744 under 18 years old, 1,365 with post-death notification and 59,940 with unknown or no information about observed therapy. Of the final total sample of 177,626 individuals, 88,586 (49.9%) had observed therapy during tuberculosis treatment and 89,040 (50.1%) did not (Figure 1).
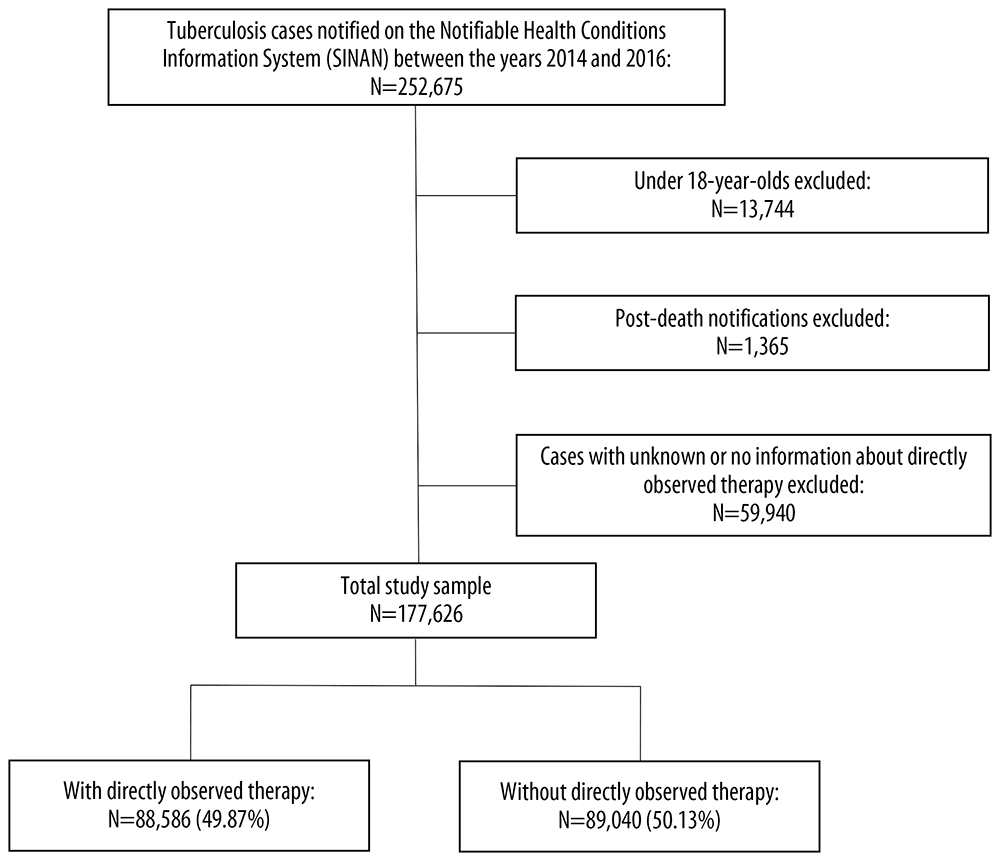
Figure 1 Selection of individuals included and excluded from the analyses of the determinants of tuberculosis and performance of directly observed treatment, Brazil, 2014-2016
Tables 1 and 2 describe the profile of the population and the distribution of the sociodemographic and contextual determinants, related to current tuberculosis treatment and associated diseases/ comorbidities, according to observed therapy having been carried out or not.
Table 1 Distribution of tuberculosis cases with and without directly observed treatment (N=177,626), by sociodemographic and contextual determinants, Brazil, 2014-2016
| Determinants | With DOTa | Without DOTa | p-valueb |
|---|---|---|---|
| N (%) | N (%) | ||
| Age, in years (n=177,594)c | |||
| <20 | 3,058 (50.48) | 3,000 (49.52) | 0.314 |
| 20-39 | 43,124 (50.04) | 43,049 (49.96) | |
| 40-59 | 29,806 (49.64) | 30,241 (50.36) | |
| ≥60 | 12,580 (49.69) | 12,736 (50.31) | |
| Sex (n=177,608)c | |||
| Female | 25,882 (47.95) | 28,097 (52.05) | <0.001 |
| Male | 62,691 (50.71) | 60,938 (49.29) | |
| Race/skin color (n=165,046)c | |||
| White | 28,474 (50.12) | 28,341 (49.88) | <0.001 |
| Black | 11,770 (49.67) | 11,927 (50.33) | |
| Brown | 40,899 (50.06) | 40,803 (49.94) | |
| Asian and indigenous | 1,833 (64.72) | 999 (35.28) | |
| Years of schooling (n=133,839)c | |||
| Illiterate | 4,827 (58.83) | 3,378 (41.17) | <0.001 |
| 1-4 | 17,773 (53.78) | 15,273 (46.22) | |
| 5-8 | 33,409 (50.36) | 32,930 (49.64) | |
| >8 | 10,957 (41.74) | 15,292 (58.26) | |
| Geographic region of Brazil (n=177,566)c | |||
| Southeast | 42,199 (52.22) | 38,607 (47.78) | <0.001 |
| Northeast | 20,984 (46.30) | 24,336 (53.70) | |
| Midwest | 5,235 (60.84) | 3,369 (39.16) | |
| South | 12,661 (49.63) | 12,852 (50.37) | |
| North | 7,499 (43.29) | 9,824 (56.71) | |
| Zone of residence (n=131,833)c | |||
| Urban | 55,692 (47.12) | 62,489 (52.88) | <0.001 |
| Rural | 7,632 (62.23) | 4,633 (37.77) | |
| Peri-urban | 630 (45.42) | 757 (54.58) | |
| Deprived of liberty (n=131,205)c | |||
| No | 56,355 (48.38) | 60,138 (51.62) | <0.001 |
| Yes | 8,814 (59.91) | 5,898 (40.09) | |
| Health worker (n=126,981)c | |||
| No | 62,251 (49.67) | 63,070 (50.33) | <0.001 |
| Yes | 675 (40.66) | 985 (59.34) | |
| Street dweller (n=130,658)c | |||
| No | 62,418 (49.65) | 63,291 (50.35) | 0.098 |
| Yes | 2,398 (48.45) | 2,551 (51.55) | |
aDOT: directly observed treatment.
bPearson’s chi-square test.
cN is different for each determinant due to notification form incompleteness
Table 2 Distribution of tuberculosis cases with and without directly observed treatment (N=177.626), by diagnosis, treatment and associated disease/comorbidity determinants, Brazil, 2014-2016
| Determinants | With DOTa | Without DOTa | p-valueb |
|---|---|---|---|
| N (%) | N (%) | ||
| HIV/AIDS (n=156,548)c | |||
| No | 72,570 (51.93) | 67,173 (48.07) | <0.001 |
| Yes | 6,603 (39.29) | 10,202 (60.71) | |
| Alcohol use (n=166,334)c | |||
| No | 66,332 (49.39) | 67,977 (50.61) | <0.001 |
| Yes | 16,861 (52.65) | 15,164 (47.35) | |
| Illicit drug use (n=131,122)c | |||
| No | 56,069 (50.09) | 55,867 (49.91) | 0.005 |
| Yes | 9,399(48.99) | 9,787 (51.01) | |
| Tobacco smoking (n=131,513)c | |||
| No | 51,365 (49.63) | 52,128 (50.37) | <0.001 |
| Yes | 14,329 (51.14) | 13,691 (48.86) | |
| Diabetes (n=165,530)c | |||
| No | 76,303 (50.06) | 76,110 (49.94) | 0.222 |
| Yes | 6,494 (49.51) | 6,623 (50.49) | |
| Mental disorder (n=165,303)c | |||
| No | 80,270 (49.84) | 80,795 (50.16) | <0.001 |
| Yes | 2,405 (56.75) | 1,833 (43.25) | |
| Type of notification (n=177,626)c | |||
| New case | 73,404 (50.51) | 71,930 (49.49) | <0.001 |
| Relapse | 6,767 (50.65) | 6,592 (49.35) | |
| Restart after treatment dropout | 6,108 (43.55) | 7,917 (56.45) | |
| Transfer | 2,149 (47.17) | 2,407 (52.83) | |
| Not known | 158 (44.89) | 194 (55.11) | |
| Clinical form (n=177,626)c | |||
| Pulmonary | 78,428 (51.22) | 74,691 (48.78) | <0.001 |
| Extrapulmonary | 8,271 (41.84) | 11,496 (58.16) | |
| Extrapulmonary + pulmonary | 1,893 (39.94) | 2,847 (60.06) | |
| Sputum smear microscopy (n=174,625)c | |||
| Negative | 18,064 (47.72) | 19,791 (52.28) | <0.001 |
| Positive | 52,966 (52.80) | 47,341 (47.20) | |
| Not performed | 16,446 (45.10) | 20,017 (54.90) | |
| Culture (n=175,970)c | |||
| Negative | 9,685 (53.88) | 8,289 (46.12) | <0.001 |
| Positive | 21,466 (54.66) | 17,804 (45.34) | |
| In progress | 2,056 (44.50) | 2,564 (55.50) | |
| Not performed | 54,657 (47.90) | 59,449 (52.10) | |
| Chest x-ray (n=140,323)c | |||
| Not suggestive of tuberculosis | 4,567 (45.92) | 5,378 (54.08) | <0.001 |
| Suspected tuberculosis | 63,955 (49.05) | 66,423 (50.95) | |
aDOT: directly observed treatment.
bPearson’s chi-square test.
cN is different for each determinant due to notification form incompleteness.
The population studied has a greater proportion of individuals between 20 and 39 years old, 48.5% (86,173), of the male sex, 69.6% (123,629), of brown race/skin color, 49.5% (81,702), with 5 to 8 years of schooling, 49.5% (66,339), and who contracted tuberculosis in its pulmonary clinical form, 86.2% (153,119).
In the case of observed therapy having been performed, statistically significant differences were found in the distribution of the following variables: sex; race/skin color; years of schooling; geographic region of Brazil; zone of residence; deprived of liberty; health worker; HIV/AIDS; alcohol use; tobacco smoking; mental disorder; type of notification; clinical form; sputum smear microscopy; culture; and chest x-ray. This was not found for age (p=0.314), street dwellers (p=0.098) and diabetes (p=0.222).
Table 3 describes the results of the hierarchical logistic regression for association between the determinants of tuberculosis and observed therapy having been carried out. This analysis only included individuals with no missing data for all the variables analyzed (44,493). Age was associated with observed therapy having been carried out: people aged 60 or over (OR=0.78 - 95%CI 0.69;0.87) were more likely to have had observed therapy. With regard to years of schooling, these were inversely associated with observed therapy, i.e. groups with 1 to 4 (OR=0.79 - 95%CI 0.73;0.86), 5 to 8 (OR=0.58 - 95%CI 0.53;0.63) and more than 8 years of schooling (OR=0.45 - 95%CI 0.41;0.49). Those who lived in the Northeast (OR=0.49 - 95%CI 0.46;0.51) and the North (OR=0.49 - 95%CI 0.46;0.53) of the country were less likely to have had observed therapy, when comparing residents of these regions with those of the Southeast. People deprived of liberty (OR=1.21 - 95%CI 1.12;1.32), with alcohol use (OR=1.09 - 95%CI 1.03;1.16) and with mental disorders (OR=1.17 - 95%CI 1.04;1.32) were more likely to have had observed therapy during tuberculosis treatment. Health workers (OR=0.77 - 95%CI 0.66;0.91) and people living with HIV/AIDS (OR=0.69 - 95%CI 0.65;0.73) were less likely to have had observed therapy.
Table 3 Association of directly observed treatment for tuberculosis and sociodemographic, contextual, diagnosis, treatment and associated disease/comorbidity determinants (N=44,493), Brazil, 2014-2016
| Determinants | ORb (IC95%c) | p-value |
|---|---|---|
| Level 1 | ||
| Age, in years | <0.001e | |
| <20 | Reference | |
| 20-39 | 0.91 (0.82;1.01) | |
| 40-59 | 0.85 (0.77;0.95) | |
| ≥60 | 0.78 (0.69;0.87) | |
| Sex | 0.064d | |
| Female | Reference | |
| Male | 1.03 (0.99;1.08) | |
| Race/skin color | <0.001e | |
| White | Reference | |
| Black | 0.85 (0.80;0.90) | |
| Brown | 0.91 (0.87;0.95) | |
| Asian and indigenous | 1.80 (1.54;2.10) | |
| Years of schooling | <0.001e | |
| No schooling | Reference | |
| 1-4 | 0.79 (0.73;0.86) | |
| 5-8 | 0.58 (0.53;0.63) | |
| >8 | 0.45 (0.41;0.49) | |
| Level 2 | ||
| Geographic region of Brazil | <0.001e | |
| Southeast | Reference | |
| Northeast | 0.49 (0.46;0.51) | |
| Midwest | 1.11 (1.01;1.21) | |
| South | 0.88 (0.83;0.92) | |
| North | 0.49 (0.46;0.53) | |
| Zone of residence | <0.001e | |
| Urban | Reference | |
| Rural | 1.65 (1.54;1.78) | |
| Peri-urban | 1.00 (0.80;1.24) | |
| Deprived of liberty | <0.001d | |
| No | Reference | |
| Yes | 1.21 (1.12;1.32) | |
| Health worker | 0.002d | |
| No | Reference | |
| Yes | 0.77 (0.66;0.91) | |
| Street dweller | 0.098d | |
| No | Reference | |
| Yes | 1.10 (0.98;1.23) | |
| Level 3 | ||
| HIV/AIDS | <0.001d | |
| No | Reference | |
| Yes | 0.69 (0.65;0.73) | |
| Alcohol use | 0.001d | |
| No | Reference | |
| Yes | 1.09 (1.03;1.16) | |
| Illicit drug use | 0.106d | |
| No | Reference | |
| Yes | 0.94 (0.88;1.01) | |
| Tobacco smoking | 0.585d | |
| No | Reference | |
| Yes | 0.98 (0.93;1.03) | |
| Diabetes | 0.386d | |
| No | Reference | |
| Yes | 1.03 (0.96;1.10) | |
| Mental disorder | 0.007d | |
| No | Reference | |
| Yes | 1.17 (1.04;1.32) | |
| Level 4 | ||
| Type of notification | <0.001e | |
| New case | Reference | |
| Relapse | 0.88 (0.81;0.95) | |
| Restart after treatment dropout | 0.74 (0.68;0.79) | |
| Transfer | 0.92 (0.82;1.02) | |
| Not known | 0.47 (0.24;0.91) | |
| Clinical form of tuberculosis | <0.001e | |
| Pulmonary | Reference | |
| Extrapulmonary | 0.80 (0.74;0.86) | |
| Extrapulmonary + pulmonary | 0.73 (0.65;0.82) | |
| Sputum smear microscopy | <0.001e | |
| Negative | Reference | |
| Positive | 1.15 (1.10;1.21) | |
| Not performed | 0.93 (0.88;0.99) | |
| Culture | <0.001e | |
| Negative | Reference | |
| Positive | 0.89 (0.83;0.96) | |
| In progress | 0.68 (0.60;0.77) | |
| Not performed | 0.68 (0.64;0.73) | |
| Chest x-ray | <0.001d | |
| Not suggestive of tuberculosis | Reference | |
| Suspected tuberculosis | 0.85 (0.78;0.93) | |
aN comprised of 44,493 individuals due to notification form incompleteness.
bOR: odds ratio.
c95%CI: 95% confidence interval.
dHierarchical logistic regression.
eWald’s test.
People with extrapulmonary tuberculosis (OR=0.80 - 95%CI 0.74;0.86) and with extrapulmonary + pulmonary tuberculosis (OR=0.73 - 95%CI 0.65;0.82) were less likely to have had observed therapy, when compared to those that only had the pulmonary form of the disease.
Table 4 shows the results of the stratified hierarchical analysis, according to FHS coverage in the municipalities of residence of the individuals, with regard to association between determinants of tuberculosis and observed therapy having been carried out. 9,167 individuals were included in the FHS coverage stratum of up to 40% of the population, 21,668 individuals in the 40 to 70% coverage stratum and 13,658 individuals in stratum in which more than 70% of the population had FHS coverage.
Table 4 Factors associated with directly observed treatment for tuberculosis and Family Health Strategy coverage rates, Brazil, 2014-2016
| Determinants | Coverage <40% (N=9,167)a | Coverage from 40 to 70% (N=21,668)a | Coverage >70% (N=13,658)a | |||
|---|---|---|---|---|---|---|
| ORb (IC95%c) | p-value | ORb (IC95%c) | p-value | ORb (IC95%c) | p-value | |
| Level 1 | ||||||
| Age, in years | 0.038d | <0.001d | 0.466d | |||
| <20 | Reference | Reference | Reference | |||
| 20-39 | 0.94 (0.72;1.22) | 0.93 (0.80;1.07) | 0.98 (0.80;1.19) | |||
| 40-59 | 0.81 (0.61;1.06) | 0.86 (0.75;1.00) | 0.96 (0.78;1.17) | |||
| ≥60 | 0.82 (0.62;1.10) | 0.75 (0.64;0.87) | 0.90 (0.73;1.11) | |||
| Sex | 0.251e | 0.468e | 0.427e | |||
| Female | Reference | Reference | Reference | |||
| Male | 1.06 (0.95;1.17) | 1.02 (0.96;1.08) | 1.03 (0.95;1.11) | |||
| Race/skin color | <0.001d | <0.001d | <0.001d | |||
| White | Reference | Reference | Reference | |||
| Black | 0.59 (0.50;0.69) | 1.04 (0.96;1.13) | 0.96 (0.84;1.08) | |||
| Brown | 0.73 (0.65;0.81) | 1.17 (1.10;1.24) | 0.84 (0.77;0.91) | |||
| Asian and indigenous | 1.02 (0.66;1.58) | 2.55 (1.97;3.31) | 1.29 (1.01;1.64) | |||
| Years of schooling | <0.001d | <0.001d | <0.001d | |||
| Illiterate | Reference | Reference | Reference | |||
| 1-4 | 0.76 (0.60;0.96) | 0.98 (0.86;1.12) | 0.89 (0.78;1.01) | |||
| 5-8 | 0.51 (0.41;0.65) | 0.73 (0.64;0.83) | 0.83 (0.72;0.94) | |||
| >8 | 0.41 (0.32;0.53) | 0.62 (0.54;0.71) | 0.60 (0.52;0.69) | |||
| Level 2 | ||||||
| Geographic region of Brazil | <0.001d | <0.001d | <0.001d | |||
| Southeast | Reference | Reference | Reference | |||
| Northeast | 0.42 (0.34;0.51) | 0.27 (0.24;0.29) | 0.66 (0.60;0.74) | |||
| Midwest | 14.74 (12.08;17.99) | 0.55 (0.47;0.64) | 0.61 (0.52;0.72) | |||
| South | 4.24 (3.58;5.01) | 0.52 (0.48;0.55) | 1.30 (1.15;1.47) | |||
| North | 2.08 (1.77;2.45) | 0.28 (0.25;0.32) | 0.78 (0.68;0.91) | |||
| Zone of residence | <0.001d | <0.001d | <0.001d | |||
| Urban | Reference | Reference | Reference | |||
| Rural | 0.91 (0.72;1.16) | 1.67 (1.42;1.97) | 1.19 (1.08;1.32) | |||
| Peri-urban | 6.25 (3.18;12.26) | 0.65 (0.46;0.92) | 0.69 (0.49;0.98) | |||
| Deprived of liberty | <0.001e | 0.154e | <0.001e | |||
| No | Reference | Reference | Reference | |||
| Yes | 3.23 (2.58;4.04) | 1.09 (0.96;1.23) | 1.45 (1.23;1.71) | |||
| Health worker | 0.186e | 0.003e | 0.005e | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.70 (0.55;0.88) | 0.65 (0.48;0.89) | 0.66 (0.50;0.88) | |||
| Street dweller | 0.033e | 0.119e | 0.862e | |||
| No | Reference | Reference | Reference | |||
| Yes | 1.41 (1.02;1.94) | 1.11 (0.97;1.29) | 1.02 (0.76;1.36) | |||
| Level 3 | ||||||
| HIV/AIDS | 0.003e | <0.001e | <0.007e | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.74 (0.61;0.90) | 0.69 (0.63;0.75) | 0.81 (0.70;0.94) | |||
| Alcohol use | 0.154e | 0.018e | 0.798e | |||
| No | Reference | Reference | Reference | |||
| Yes | 1.12 (0.95;1.33) | 1.10 (1.01;1.19) | 0.98 (0.89;1.09) | |||
| Illicit drug use | 0.001e | 0.800e | 0.121e | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.71 (0.57;0.87) | 0.98 (0.90;1.07) | 1.12 (0.97;1.29) | |||
| Tobacco smoking | 0.095e | 0.227e | 0.987e | |||
| No | Reference | Reference | Reference | |||
| Yes | 1.13 (0.97;1.31) | 0.95 (0.88;1.02) | 1.00 (0.90;1.10) | |||
| Diabetes | 0.089e | 0.802e | 0.709e | |||
| No | Reference | Reference | Reference | |||
| Yes | 1.17 (0.97;1.41) | 1.01 (0.91;1.12) | 1.02 (0.90;1.16) | |||
| Mental disorder | 0.903e | 0.074e | 0.106e | |||
| No | Reference | Reference | Reference | |||
| Yes | 1.02 (0.70;1.47) | 1.15 (0.98;1.35) | 1.22 (0.95;1.56) | |||
| Level 4 | ||||||
| Type of notification | 0.035d | <0.001d | 0.018d | |||
| New case | Reference | Reference | Reference | |||
| Relapse | 0.91 (0.73;1.13) | 0.86 (0.77;0.96) | 0.95 (0.81;1.11) | |||
| Restart after treatment dropout | 0.83 (0.67;1.04) | 0.82 (0.75;0.91) | 0.76 (0.65;0.90) | |||
| Transfer | 0.69 (0.50;0.95) | 0.93 (0.78;1.09) | 0.96 (0.79;1.16) | |||
| Not known | 0.14 (0.15;1.28) | 0.28 (0.28;1.52) | 0.31 (0.50;1.91) | |||
| Clinical form of tuberculosis | 0.032d | <0.001d | 0.018d | |||
| Pulmonary | Reference | Reference | Reference | |||
| Extrapulmonary | 0.76 (0.62;0.94) | 0.78 (0.70;0.87) | 0.86 (0.75;0.99) | |||
| Extrapulmonary + pulmonary | 1.14 (0.79;1.64) | 0.68 (0.59;0.79) | 0.74 (0.58;0.95) | |||
| Sputum smear microscopy | <0.001d | <0.001d | <0.001d | |||
| Negative | Reference | Reference | Reference | |||
| Positive | 1.35 (1.16;1.58) | 1.20 (1.11;1.29) | 1.10 (1.00;1.20) | |||
| Not performed | 1.13 (0.96;1.33) | 1.09 (1.00;1.19) | 0.86 (0.77;0.96) | |||
| Culture | <0.001d | <0.001d | <0.001d | |||
| Negative | Reference | Reference | Reference | |||
| Positive | 0.93 (0.76;1.13) | 0.77 (0.69;0.86) | 1.05 (0.90;1.21) | |||
| In progress | 0.79 (0.54;1.15) | 0.65 (0.55;0.77) | 0.71 (0.52;0.97) | |||
| Not performed | 0.62 (0.53;0.74) | 0.55 (0.50;0.61) | 0.87 (0.77;0.98) | |||
| Chest x-ray | 0.672e | 0.314e | 0.912e | |||
| Not suggestive of tuberculosis | Reference | Reference | Reference | |||
| Suspected tuberculosis | 1.05 (0.81;1.38) | 0.93 (0.82;1.06) | 0.99 (0.85;1.14) | |||
aN comprised of 44,493 individuals due to notification form incompleteness.
bOR: odds ratio.
c95%CI: 95% confidence interval.
dWald’s test.
eHierarchical logistic regression.
Individuals of Black race/skin color were less likely to have had observed therapy, in the <40% FHS coverage stratum (OR=0.59 - 95%CI 0.50;0.69), when compared to White individuals. Individuals with more than 8 years of schooling were less likely to have had observed therapy in the <40% coverage stratum (OR=0.41 - 95%CI 0.32;0.53), in the 40 to 70% coverage stratum (OR=0.62 - 95%CI 0.54;0.71) and in the >70% FHS coverage stratum (OR=0.60 - 95%CI 0.52;0.69), when compared to individuals who had no schooling.
With regard to the country’s macro-regions, individuals who lived in the Northeast were less likely to have had observed therapy in the <40% (OR=0.42 - 95%CI 0.34;0.51) and 40 to 70% FHS coverage strata (OR=0.27 - 95%CI 0.24;0.29), while those who lived in the Midwest region were less likely to have had observed therapy in the >70% FHS coverage stratum (OR=061 - 95%CI 0.52;0.72). Health workers were less likely to have had observed therapy in all three FHS coverage strata: <40% (OR=0.70 - 95%CI 0.55;0.88), 40 to 70% (OR=0.65 - 95%CI 0.48;0.89) and >70% coverage (OR=0.66 95%CI 0.50;0.88).
Individuals living with HIV/AIDS were less likely to have had observed therapy, in the <40% stratum (OR=0.74 - 95%CI 0.61;0.90), the 40 to 70% stratum (OR=0.69 - 95%CI 0.63;0.75) and in the >70% FHS coverage stratum (OR=0.81 - 95%CI 0.70;0.94).
Discussion
Directly observed treatment being carried out in Brazil was associated with social and clinical determinants of tuberculosis. Observed therapy was more likely to have been carried out for people with low levels of schooling, alcohol users, those reporting having a mental disorder, those who were part of the population deprived of liberty, living in the Midwest region of the country, having positive sputum smear microscopy and having contracted the pulmonary form of tuberculosis. However, this association lost significance when analyzing patients for whom FHS coverage was greater than 70%. This finding suggests that there is a reduction in determination to carry out observed therapy, according to the sociodemographic, contextual, associated disease and clinical characteristics presented, among individuals resident in places where FHS coverage is higher.
In particular, the relevance of this study consists of having assessed a national longitudinal database containing individual and clinical information collected routinely in health services. SINAN system information is generated by diverse health services, and, despite the recommendations for completing that information being widely disseminated by the Ministry of Health, occurrence of classification different to that which is recommended cannot be ruled out, especially with regard to observed therapy having been carried out. There are variables with incomplete or unknown information. In order to overcome data incompleteness, only forms with complete information were included in the regression models, which may also have led to biases being included in the study. Finally, there is also the possibility of an ecological effect, given that FHS coverage was only stratified for municipality of residence. However, we believe that these limitations did not interfere in the results presented. This understanding is reinforced by previous evaluations that have demonstrated the quality of SINAN, and also by the consistency of our results with findings in the cumulative literature.
In Brazil, notwithstanding the recommendation for all individuals diagnosed as having tuberculosis to undergo observed therapy, the results of this study demonstrated that just over half the individuals notified between 2014 and 2016 had observed therapy. This corroborates another Brazilian study which also used longitudinal data and described the selection of individuals for observed therapy.6 12
Individual vulnerabilities - illiteracy, alcohol use and mental disorder - are associated with greater likelihood of having treatment, according the findings of the study; and are also associated with risk of becoming ill, treatment dropout and death from tuberculosis.3 13-14 There is evidence that self-care is greater when level of education is higher, which may contribute to those with less schooling being selected for observed therapy.14-16 Alcohol use and presence of mental disorders have been associated with issues such as low quality of life, lack of hygiene, poverty, malnutrition and poor acceptance of treatment, which may also lead to the selection of these individuals for observed therapy.14-17
Living in the Southeastern, Midwestern and Southern regions of the country shows itself to be a situation associated with greater likelihood of undergoing observed therapy. This fact confirms inequalities in access to health services between the Brazilian macro-regions. An evaluative study of economic development and health service availability concluded that Northern and Northeastern region municipalities form a cluster of low economic development and low health service availability.18 When first-time health service access and number of health workers available were evaluated, there was a scarcity of Primary Health Care workers and services, thus hindering access both to diagnosis of tuberculosis and to observed therapy.19
Another study conducted in Brazil described people deprived of liberty undergoing observed therapy as having less tuberculosis treatment dropout. This corroborates the result of our study which described association between this population and observed therapy being carried out.20 The result presented in this study may be related to access to health care. The National Policy on Health in the Prison System guarantees access to health care by imprisoned people, including Primary Care and disease control actions in prison units.21-22
Regardless of FHS coverage stratum, tuberculosis patients with the extrapulmonary clinical form had less likelihood of carrying out observed therapy, compared to those who contracted the pulmonary form; however, those with positive sputum smear microscopy were more likely to do so. This may be related to the greater potential for tuberculosis transmission by individuals with the pulmonary form and positive sputum smear microscopy.23
In the analysis stratified by FHS coverage, we found that when coverage is greater than 70%, statistically significant differences are not found between determinants and observed therapy being carried out. As such, observed therapy being carried out where FHS coverage is greater does not depend on the influence of determinants of tuberculosis. Public policies have strengthened the decentralization of tuberculosis control actions to Primary Health Care, with the aim of improving access to diagnosis, case follow-up and carrying out of observed therapy.24-25 There is evidence that FHS has shown good results in terms of tuberculosis treatment, with better proportions of favorable outcomes when compared to other health services.10
FHS activities favor linkage between health services and the community. This is done according to the principle of territorialization, bringing the community closer to health services.9 Multi-professional FHS teams, familiar with the social scenarios and everyday lives of citizens, are able to devise patient embracement strategies and to gain their confidence.9-10 Community Health Agents play a fundamental role in the carrying out of observed therapy within FHS. They are responsible for making household visits, establishing and maintaining linkage between patients and FHS and, consequently, for supervising the intake of standard medication over the period of tuberculosis treatment, which can be shorter or longer depending on patient adherence and progression.5 8 24
This study highlights that regardless of FHS coverage, health workers and people living with HIV/AIDS were less likely to have carried out observed therapy. However, if health workers are at increased risk of tuberculosis infection, because of their professional practices, their lower likelihood of undergoing observed therapy may be explained by the knowledge they have of the health-illness process.
Individuals taking antiretroviral drugs also suffer the influence of social and clinical determinants with regard to adherence to treatment of HIV/AIDS: a systematic review demonstrated that social and clinical determinants were associated with reduction in adherence to using antiretroviral drugs.26 Therefore, the distancing of this population from FHS as identified here may be related to the conclusions reached by the systematic review. Historically, the clinical and treatment model adopted for people living with HIV/AIDS in Brazil was concentrated on medical specialty centers, which could also justify the fragility of their links with FHS services.27-28
There is great discussion in the literature as to the real effects of observed therapy on tuberculosis control. Systematic review studies do not demonstrate a difference in tuberculosis cure rates depending on whether observed therapy has been carried out or not.29 Worldwide, however, many tuberculosis control programs undertake observed therapy in contingency plans. In view of this issue, in its END TB strategy the World Health Organization provides for the incorporation of person-centered actions, with the aim of assessing their social, economic and clinical needs before recommending observed therapy as an action to prevent treatment dropout, more so because its imposition can overburden expenditure and increase the potential for stigmatization of tuberculosis in society.29
We conclude that in addition to clinical characteristics, the carrying out of observed therapy for tuberculosis in Brazil is associated with sociodemographic, contextual and associated disease determinants. When individuals with tuberculosis are categorized, or stratified, according to the level of FHS coverage in their municipality, the carrying out of observed therapy behaves differently in each coverage stratum; i.e., in the context of higher FHS coverage, statistical association is not found between the determinants considered and the carrying out of observed therapy. Studies which examine the characteristics of healthcare units related to the carrying out of observed therapy, and which assess the impact of FHS coverage on the carrying out of directly observed treatment, will provide batter evidence of this strategy in Brazil.
Referências
1. World Health Organization - WHO. Global tuberculosis report 2018. [Internet]. Geneva: World Health Organization; 2018 [cited 2020 Aug 28]. 265 p. Available from: https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1 [ Links ]
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Brasil livre da tuberculose: evolução dos cenários epidemiológicos e operacionais da doença. Bol Epidemiol [Internet]. 2019 [citado 2020 ago 28];50(9):1-18. Disponível em: https://portalarquivos2.saude.gov.br/images/pdf/2019/marco/22/2019-009.pdf [ Links ]
3. Maciel EL, Reis-Santos B. Determinants of tuberculosis in Brazil: from conceptual framework to practical application. Rev Panam Salud Publica [Internet]. 2015 [citado 2020 Aug 28];38(1):28-34. Available from: https://www.scielosp.org/pdf/rpsp/2015.v38n1/28-34 [ Links ]
4. Rocha MS, Bartholomay P, Cavalcante MV, Medeiros FC, Codenotti SB, Pelissari DM, et al. Sistema de Informação de Agravos de Notificação (Sinan): principais características da notificação e da análise de dados relacionada à tuberculose. Epidemiol Serv Saúde [Internet]. 2020 [citado 2020 ago 28];29(1):e2019017. Disponível em: https://doi.org/10.5123/s1679-49742020000100009 [ Links ]
5. World Health Organization - WHO. Stop TB Partnership. The global plan to stop TB 2006-2015 [Internet]. Geneva: World Health Organization; 2006 [cited 2020 Aug 28]. Available from: https://www.who.int/tb/features_archive/global_plan_to_stop_tb/en/ [ Links ]
6. Reis-Santos B, Pellacani-Posses I, Macedo LR, Golub JE, Riley LW, Maciel EL. Directly observed therapy of tuberculosis in Brazil: associated determinants and impact on treatment outcome. Int J Tuberc Lung Dis [Internet]. 2015 Oct [cited 2020 Aug 28];19(10):1188-93. Available from: https://doi.org/10.5588/ijtld.14.0776 [ Links ]
7. Wysocki AD, Ponce MAZ, Brunello MEF, Berlado AA, Vendramini SHF, Scatena LM, et al. Atenção Primária à Saúde e tuberculose: avaliação dos serviços. Rev Bras Epidemiol [Internet]. 2017 jan-mar [citado 2020 ago 28];20(1):161-75. Disponível em: https://doi.org/10.1590/1980-5497201700010014 [ Links ]
8. Bastos ML, Menzies D, Home T, Dehghani K, Trajman A. The impact of the Brazilian family health on selected primary care sensitive conditions: a systematic review. PLoS One [Internet]. 2017 Aug [cited 2020 Aug 28];12(8):e0182336. Available from: https://doi.org/10.1371/journal.pone.0182336 [ Links ]
9. Silva-Sobrinho RA, Wysocki AD, Scatena LM, Pinto ESG, Beraldo AA, Andrade RLP, et al. Assessment of primary health care in the treatment of tuberculosis in a Brazilian locality of the international triple frontier. Open Nurs J [Internet]. 2017 [cited 2020 Aug 28];11(22):124-34. Available from: https://dx.doi.org/10.2174%2F1874434601711010124 [ Links ]
10. Durovni B, Saeaceni V, Puppin MS, Tassinari W, Cruz OG, Cacalcante S, et al. The impact of the Brazilian Family Health Strategy and the conditional cash transfer on tuberculosis treatment outcomes in Rio de Janeiro: an individual-level analysis of secondary data. J Public Health (Oxf) [Internet]. 2017 Sep [cited 2020 Aug 28];40(3):e359-66. Available from: https://doi.org/10.1093/pubmed/fdx132 [ Links ]
11. Instituto Brasileiro de Geografia e Estatística - IBGE. Síntese de indicadores sociais: uma análise das condições de vida da população brasileira [Internet]. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2019 [citado 2020 ago 28]. Disponível em: https://www.ibge.gov.br/cidades-e-estados.html?view=municipio [ Links ]
12. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de recomendações para o controle da tuberculose no Brasil [Internet]. 2. ed. Brasília: Ministério da Saúde; 2019 [citado 2020 ago 28]. 364 p. Disponível em: https://sbpt.org.br/portal/wp-content/uploads/2019/06/manual_recomendacoes_tb_2ed_atualizada_8maio19.pdf [ Links ]
13. Shimeles E, Enquselassie F, Aseffa A, Tilahun M, Mekonen A, Wondimagegn G, et al. Risk factors for tuberculosis: a case-control study in Addis Ababa, Ethiopia. PLoS One [Internet]. 2019 Apr [cited 2020 Aug 28];14(4):e0214235. Available from: https://doi.org/10.1371/journal.pone.0214235 [ Links ]
14. San Pedro A, Oliveira RM. Tuberculose e indicadores socioeconômicos: revisão sistemática da literatura. Rev Panam Salud Publica [Internet]. 2013 [citado 2020 ago 28];33(2):294-301. Disponível em: https://scielosp.org/article/rpsp/2013.v33n4/294-301/ [ Links ]
15. Simou E, Britton J, Leonardi-Bee J. Alcohol consumption and risk of tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis [Internet]. 2018 Nov [cited 2020 Aug 28];22(11):1277-85. Available from: https://doi.org/10.5588/ijtld.18.0092 [ Links ]
16. Paixão LM, Gontijo ED. Profile of notified tuberculosis cases and factors associated with treatment dropout. Rev Saúde Pública [Internet]. 2007 Apr [cited 2020 Aug 28];41(2):205-3. Available from: https://doi.org/10.1590/S0034-89102007000200006 [ Links ]
17. Silva DR, Munoz-Torrico M, Duarte R, Galvao T, Bonini EH, Arbex FF, et al. Fatores de risco para tuberculose: diabetes, tabagismo, álcool e uso de outras drogas. J Bras Pneumol [Internet]. 2018 [citado 2020 ago 28];44(2):145-52. Disponível em: http://dx.doi.org/10.1590/S1806-37562017000000443 [ Links ]
18. Stopa SR, Malta DC, Monteiro CM, Szwarcwald CL, Goldbaum M, Cesar CLG. Use of and access to health services in Brazil, 2013 National Health Survey. Rev Saúde Pública [Internet]. 2017 Jun [cited 2020 Aug 28];51(Suppl):3s. Available from: https://doi.org/10.1590/s1518-8787.2017051000074 [ Links ]
19. Albuquerque MV, Viana ALA, Lima LD, Ferreira MP, Fusaro ER, Iozzi FL. Regional health inequalities: changes observed in Brasil from 2000-2016. Ciênc Saúde Coletiva [Internet]. 2017 Apr [cited 2020 Aug 28];22(4):1055-64. Available from: http://dx.doi.org/10.1590/1413-81232017224.26862016 [ Links ]
20. Macedo LR, Reis-Santos B, Riley LW, Maciel EL. Treatment outcomes of tuberculosis patients in Brazilian prisons: a polytomous regression analysis. Int J Tuberc Lung Dis [Internet]. 2013 Nov [cited 2020 Aug 28];17(11):1427-34. Available from: https://doi.org/10.5588/ijtld.12.0918 [ Links ]
21. Costa MMR, Vilaça DHV, Sousa EC, Lima Junior AA, Vieira RBR, Teotônio VLA, et al. A prevalência da tuberculose entre os privados de liberdade no Brasil: uma revisão sistemática. Braz J Hea Rev [Internet]. 2019 [citado 2020 ago 28];2(3):1719-30. Disponível em: https://www.brazilianjournals.com/index.php/BJHR/article/view/1455 [ Links ]
22. Lopes RL, Cavalcante A, Melo J, Cavalcante G, Oliveia A, Weslânnya P. Ocorrência de doenças infectocontagiosos em pessoas privadas de liberdade no sistema prisional. Interf Cientif Saúde Ambiente [Internet]. 2019 fev[citado 2020 ago 28];7(2):53-60. Disponível em: https://doi.org/10.17564/2316-3798.2019v7n2p%25p [ Links ]
23. Gomes T, Reis-Santos B, Bertolde A, Johnson JL, Riley LW, Maciel EL. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis [Internet]. 2014 Jan [cited 2020 Aug 28];14(9)1-9. Available from: https://doi.org/10.1186/1471-2334-14-9 [ Links ]
24. Cardozo-Gonzales RI, Palha PF, Harter J, Alarcon E, Lima LM, Tomberg JO. Avaliação das ações de detecção de casos de tuberculose na atenção primária. Rev Eletr Enf [Internet]. 2015 out-dez [citado 2020 ago 28];17(4). Disponível em: http://dx.doi.org/10.5216/ree.v17i4.32846 [ Links ]
25. Pelissari DM, Bartholomay P, Jacobs MG, Arakaki-Sanchez D, Anjos DSO, Costa MLS, et al. Offer of primary care services and detection of tuberculosis incidence in Brazil. Rev Saúde Pública [Internet]. 2018 May [cited 2020 Aug 28];52:53. Available from: https://doi.org/10.11606/s1518-8787.2018052000131 [ Links ]
26. Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impacto of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc [Internet]. 2013 Nov [cited 2020 Aug 28];16(3 Suppl 2):18640. Available from: https://doi.org/10.7448/ias.16.3.18640 [ Links ]
27. Ross JM, Henry NJ, Dwyer-Lindgren LA, Lobo AP, Souza FM, Biehl MH, et al. Progress toward eliminating TB and HIV deaths in Brazil, 2001-2015: a spatial assessment. BMC Med [Internet]. 2018 Sep [cited 2020 Aug 28];16(144). Available from: https://doi.org/10.1186/s12916-018-1131-6 [ Links ]
28. Melo EA, Maksud I, Agostini R. Cuidado, HIV/Aids e atenção primária no Brasil: desafio para a atenção no Sistema Único da Saúde? Rev Panam Salud Publica [Internet]. 2018 out [citado 2020 ago 28];42:e151. Disponível em: https://doi.org/10.26633/RPSP.2018.151 [ Links ]
29. Mc Laren ZM, Milliken AA, Meyer AJ, Sharp AR. Does directly observed therapy improve tuberculosis treatment? More evidence is needed to guide tuberculosis policy. BMC Infect Dis [Internet]. 2016 [cited 2020 Aug 28];16(537). Available from: https://doi.org/10.1186/s12879-016-1862-y [ Links ]
Received: May 21, 2020; Accepted: July 31, 2020











 texto em
texto em 


