Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.30 no.3 Brasília set. 2021 Epub 19-Jul-2021
http://dx.doi.org/10.1590/s1679-49742021000300011
ORIGINAL ARTICLE
Epidemiological profile of a cohort of symptomatic pregnant women with suspected Zika virus infection in the State of São Paulo, Brazil, 2015-2018*
1Universidade de São Paulo, Faculdade de Saúde Pública, São Paulo, SP, Brazil
2Secretaria de Estado da Saúde, Centro de Vigilância Epidemiológica ‘Prof. Alexandre Vranjac’, São Paulo, SP, Brazil
Objective:
To describe the epidemiological profile of pregnant women with suspected Zika virus infection, reported on the Center for Strategic Information for Health Surveillance System, in the state of São Paulo, Brazil, its range of abnormalities and/or pregnancy outcomes.
Methods:
Descriptive epidemiological study of a cohort of symptomatic pregnant women with suspected Zika virus infection and their pregnancy outcomes, living in the state of São Paulo, reported between 2015-2018.
Results:
Of the 2,329 pregnant women studied, 29.3% were confirmed to have the infection, almost half of them were single (44.8%), the majority of them were white woman (74.2%), with complete high school education (53.6%), and concentrated in the northeast region of the state. The proportion of newborns with central nervous system abnormalities was approximately 4.0%.
Conclusion:
The results found characterize Zika virus transmission in the state of São Paulo and may support public health actions in places with higher risk of disease transmission.
Keywords: Zika Virus; Pregnant Women; Congenital Abnormalities; Epidemiology, Descriptive
Introduction
Among the emerging diseases of the 21st century, Zika virus (ZIKV) infection has been one of the major concerns and challenges for public health worldwide, because of its magnitude, given its rapid global spread and its serious impact, due to the microcephaly epidemic and other fetal alterations associated with it. As of July 2019, 87 countries and territories had evidence of autochthonous transmission of the ZIKV. Incidence of ZIKV infection in the Americas peaked in 2016, and declined substantially throughout 2017 and 2018. However, all areas with prior reports of ZIKV transmission have the potential for re-emergency or re-introduction of the disease.1
Since the identification of Zika virus in Brazil, there has been nine times increase in microcephaly cases, compared to the average of the previous five years.2-4 The highest prevalence in 2015 and 2016 was observed in the Northeast region, with a reduction between 2015 and 2016 (from 12.64 to 7.13 cases per 10,000 live births), which influenced the decrease in the prevalence of this malformation nationwide (from 3.85 to 3.07 per 10,000 live births). However, in the Midwest, Southeast and North regions, the prevalence was higher in 2016, compared to that observed in 2015.5.6
Many studies have been conducted to evaluate the association between ZIKV infection during pregnancy and the occurrence of congenital malformations.7-10 Such studies have shown that ZIKV infection during pregnancy can cause numerous fetal alterations, including microcephaly, craniofacial disproportion, spasticity, seizures, irritability, brainstem dysfunction, dysphagia, limbs contractures, hearing and eye abnormalities, and brain abnormalities detected by neuroimaging. There is consensus that the ZIKV is a cause of microcephaly and other neurological complications, which together constitute congenital Zika virus syndrome.11.12
This study aimed to describe the epidemiological profile of pregnant women with suspected ZIKV infection reported on the Center for Strategic Information for Health Surveillance System (CeVeSP), in the state of São Paulo, its range of abnormalities and pregnancy outcomes.
Methods
This was a descriptive epidemiological study on a cohort of symptomatic pregnant women with suspected exposure to ZIKV and their conceptuses.
This cohort of pregnant women was extracted from the Center for Strategic Information for Health Surveillance System of the São Paulo State Health Department, which records all pregnant women with suspected ZIKV infection and, through this same registration form, detects pregnancy outcome (a healthy or impaired child).
This study took place in the state of São Paulo, which, since the declaration of Public Health Emergency of National Importance in 2015, has monitored and investigated pregnant women with suspected ZIKV infection (acute rash at any gestational age) and their pregnancy outcomes. The monitoring of these cases is carried out by filling out a specific registration form on CeVeSP, a dynamic platform on the Internet for notification of Public Health Emergency events, with restricted access to epidemiological surveillance groups in the state of São Paulo. This follow-up allows laboratory diagnosis of ZIKV infection, clinical and epidemiological characterization of cases, and follow-up of pregnancy outcome (stillbirth, abortion, neonatal death, healthy newborn, congenital microcephaly and other central nervous system abnormalities).13
All symptomatic pregnant women were included in the study, that is, those with rash regardless of gestational age, from October 2015 to December 2018. Other diagnostic hypotheses reported on CeVeSP in the same period were excluded.
The following case definitions were taken as a reference in this study:12
Confirmed cases
Symptomatic pregnant women, that is, those who presented rash, regardless of gestational age (excluding other diagnostic hypotheses), and had tested positive for ZIKV infection through reverse transcription-polymerase chain reaction (RT-PCR) (urine and/or blood collected in a timely manner).
Discarded cases
Symptomatic pregnant women, that is, those who presented rash, regardless of gestational age, with another diagnostic hypothesis confirmation and/or had tested negative for ZIKV infection through RT-PCR (urine and/or blood collected in a timely manner).
The following variables were analyzed:
a) Date of occurrence of cases among symptomatic pregnant women with suspected ZIKV infection (month and year of occurrence)
b) Sociodemographic data of pregnant women
- Marital status (single; married; stable union; divorced; widow);
- Race/skin color (white; black; brown; others);
- Schooling (without education; complete elementary school; high school; higher education);
- Pregnant women’s age (mean and standard deviation calculated).
c) Clinical characteristics of pregnant women
- Signs and symptoms in pregnant women (headache; myalgia; pruritus; fever);
- STORCH infection (syphilis, toxoplasmosis, rubella, cytomegalovirus or herpes). Regarding the test results for STORCH during pregnancy, there was no information on the differentiation between detection of immunoglobulin M and immunoglobulin G.
d) Current pregnancy data
- Trimesters of pregnancy (1st; 2nd; 3rd);
- Number of prenatal consultations (none; 1-3; 4-6; 7 and more);
- Type of childbirth (C-section; vaginal delivery).
e) Newborn data
- Gender (female; male);
- Newborn’s weight (median and variance calculated);
- Childbirth (full-term; preterm; abortion/stillbirth);
- Conceptus outcome (healthy; death; microcephaly; central nervous system alteration; microcephaly and central nervous system abnormalities; other alterations).
f) Municipality of residence of pregnant women with confirmed ZIKV infection.
The Center for Strategic Information for Health Surveillance (CeVeSP) of the São Paulo State Health Department and Live Birth Information System (Sinasc) were used as data source.
A deterministic linkage between databases was performed, that is, exact identification of the CeVeSP database record on the Sinasc database, to obtain other variables of interest and better characterization of cases. Pregnant women’s full name and date of birth were used as criteria for the selection of true pairs. This linkage was made by a technician who works for the Center for Strategic Information on Health Surveillance. Sinasc nominal data was not provided to the authors of the study.
Absolute and relative frequencies were calculated for categorical, mean and median variables, range of variation and standard deviations for quantitative variables, using Microsoft Excel 2010. Spatial distribution of confirmed cases, in absolute number, presented in a choropleth map, which was elaborated using the QGIS software (version 3.14. 'Pi') was also analyzed. Pearson's chi-square test was used for categorical variables, with a 5% significance level. For the tables with more than 20% of cells with a value lower than 5, Fisher's exact test was used. For the calculation of the p-value, the 'ignored' values were not used. The software RStudio (version 4.0.2) and OpenEpi (version 3.01) were used for statistical calculations.
The study project was approved by the Research Ethics Committee of the University of São Paulo School of Public Health (CEP/FSP/USP). Opinion No. 3,315,552, issued on May 9, 2019, and it was exempted from using the Free and Informed Consent Form.
Results
From October 2015 to December 2018, 3,318 cases of symptomatic pregnant women with suspected Zika virus infection were reported on CeVeSP. 2,842 pregnant women were located in the linkage between databases. Of these, 513 had no report of rash. Thus, 2,329 pregnant women with suspected ZIKV infection were considered in the analysis, and 1,601 (68.7%) of which were discarded cases of ZIKV infection, 683 (29.3%) confirmed cases and 45 (1.9%) were still under investigation until the time of data analysis (Figure 1).
The cases were concentrated between 2015 and 2016 (epidemic period), and peaked in February 2016 (a total of 473; 211 confirmed and 2 under investigation) and also in March (a total of 464; 179 confirmed and 9 under investigation). From 2017, there was a decrease in the number of cases (post-epidemic period), and there was not a single month, in which, a number of more than 50 cases was recorded. In November and December 2018, there were no cases recorded (Figure 2).
Pregnant women who had tested positive for ZIKV infection (confirmed cases) with a mean age of 27 years old (standard deviation: ± 6), most of them were single (44.8%), the majority of them were white women (74.2%) and with complete high school (53.6%), followed by those with complete elementary school (25.3%). A greater proportion of pregnant women who tested positive for ZIKV infection contracted the infection during the third trimester of pregnancy (43.6%), compared to those who had contracted the infection during the first two trimesters. The vast majority of pregnant women who tested positive for ZIKV infection had seven or more prenatal consultations (83.7%), and only two did not get any prenatal visits during pregnancy. More than half of the pregnant women who had tested positive for ZIKV infection underwent cesarean section (53.6%) (Table 1).
Among pregnant women who tested negative for ZIKV infection (discarded cases), with a mean age of 28 years old (standard deviation: ± 6), most of them were white (75.9%), the majority of woman were married (45.0%) and with complete high school (49.3%), followed by those with complete higher education (23.3%). The proportion of symptomatic pregnant women who tested negative for Zika virus infection during the third trimester of pregnancy (39.4%) was higher, compared to symptomatic pregnant women who tested negative during the first two trimesters. The vast majority of pregnant women who tested negative for ZIKV infection (discarded cases) had seven or more prenatal consultations (87.1%) and, only two of them did not get any prenatal visits during pregnancy. Most pregnant women who had tested negative for ZIKV infection, underwent cesarean section (69.1%) (Table 1).
The variables that presented statistical significance in the comparison between confirmed and discarded cases were: marital status; schooling; trimesters of pregnancy; and type of childbirth (Table 1).
The main signs and symptoms presented, in addition to rash, were: headache (40.0% in confirmed cases; 39.2% in discarded cases), myalgia (37.8% in confirmed cases; 32.8% in discarded cases) and pruritus (37.0% in confirmed cases; 50.4% in discarded cases). Fever was reported in only 210 (30.7%) confirmed cases and 553 (34.5%) (p<0.001) (data not shown in table).
As for the conceptuses, there was no statistical difference regarding gender, gestational age at birth and birth weight. Most of them were male (54.6% of confirmed cases and 52.0% of discarded cases of ZIKV infection) and were full-term infants (76.1% of confirmed cases and 64.4% of discarded cases) (Table 2). The average birth weight was 3.2kg (290g to 4,855g) for confirmed cases and 3,180g (735g to 4,650g) for discarded cases of ZIKV infection (data not shown in table).
Regarding pregnant women with confirmed ZIKV infection, 59 (8.7%) had conceptuses with some type of impairment (abortion, stillbirth, death, microcephaly and/or central nervous system abnormalities, other alterations), while among pregnant women who tested negative for ZIKV infection (discarded cases), 54 (3.4%) had some type of impairment (p<0.001). The proportion of newborns with microcephaly and/or central nervous system abnormalities whose mothers tested positive for Zika virus infection was 3.95% (27/683), and among discarded cases, 0.69% (11/1,601) (p<0.001). The proportion of deaths (abortion, stillbirth and postnatal death) among confirmed cases was 2.49% (17/683), and in those discarded, 1.50% (24/1,601) (p=0.109).
Of the 27 conceptuses born to mothers testing positive for Zika virus infection, who were born with microcephaly and/or central nervous system abnormalities, in 17, ZIKV infection occurred during the first trimester, in eight during the second trimester and in two during the third trimester of pregnancy. And among the 17 conceptuses who died (abortion, stillbirth or post-birth death), in nine, viral infection occurred in the first trimester, in five in the second trimester and in two in the third trimester of pregnancy; regarding only one death, there is no information about this outcome (data not shown in table).
Of the 2,284 cases among those discarded and confirmed, 1,517 (66.4%) had a record of performing STORCH tests during prenatal care. Of the 1,007 discarded cases of ZIKV infection that underwent STORCH tests, 60 (6.0%) had positive result in the test of some STORCH, that is, 15 (25.0%) for syphilis, 12 (20.0%) for toxoplasmosis, four (6.7%) for rubella, eight (13.3%) for cytomegalovirus and 21 (35.0%) for herpes. Of the 510 confirmed cases of ZIKV infection that underwent STORCH tests, 26 (5.1%) also tested positive for some of the infections (STORCH+Zika co-infection): five (19.2%) syphilis, eight (30.8%) for toxoplasmosis, three (11.5%) for rubella, six (23.1%) for cytomegalovirus, three (11.5) for herpes and one (3.8%) parvovirus (data not shown in table).
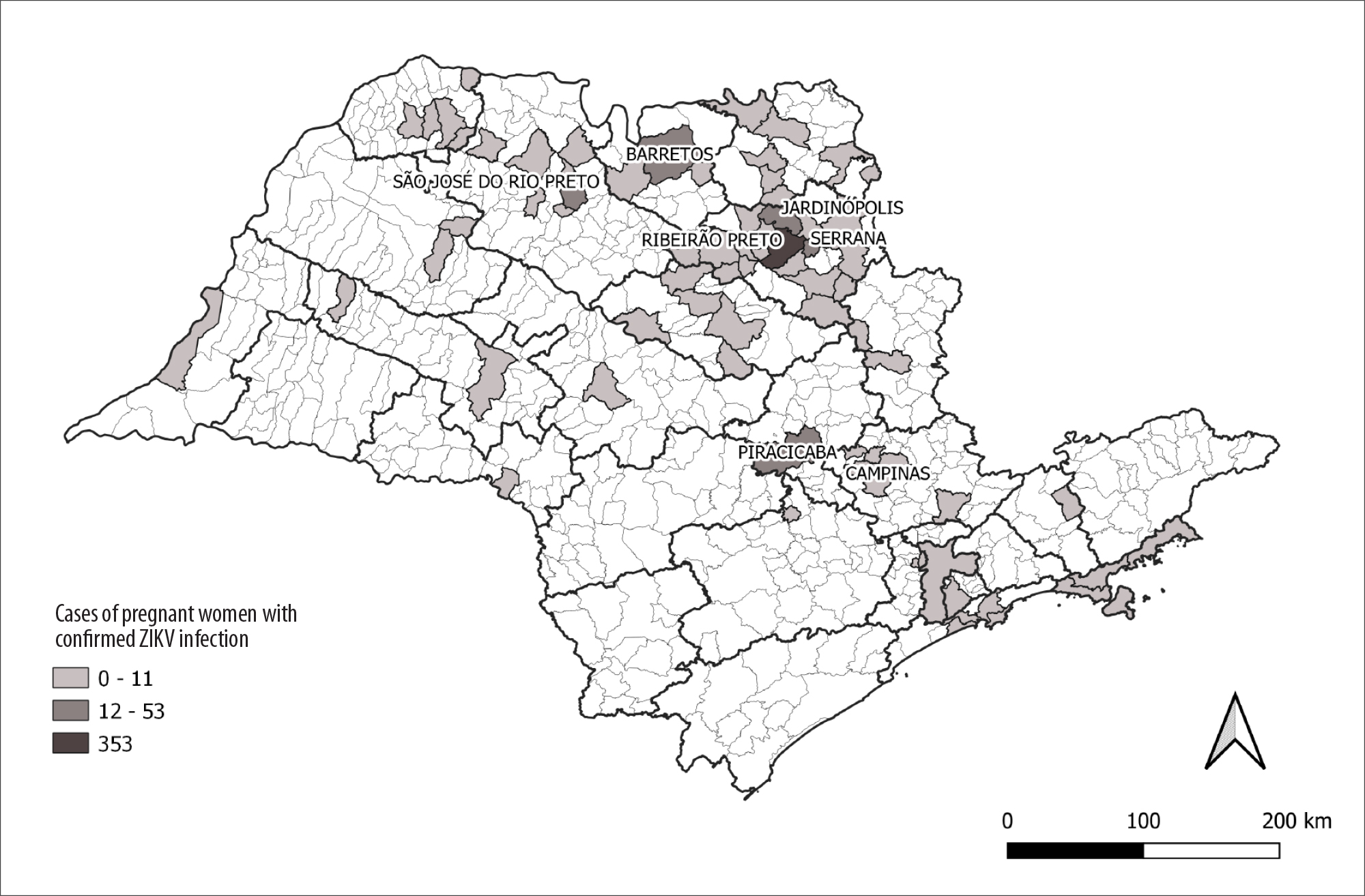
The majority of confirmed cases were concentrated in the northeast region of the state of São Paulo. The municipality of Ribeirão Preto stood out with the highest number of cases in the state: 353 confirmed cases (Figure 3).

CeVeSP: Center for Strategic Information for Health Surveillance; Sinasc: Live Birth Information System.
Figure 1 - Flowchart to obtain study data
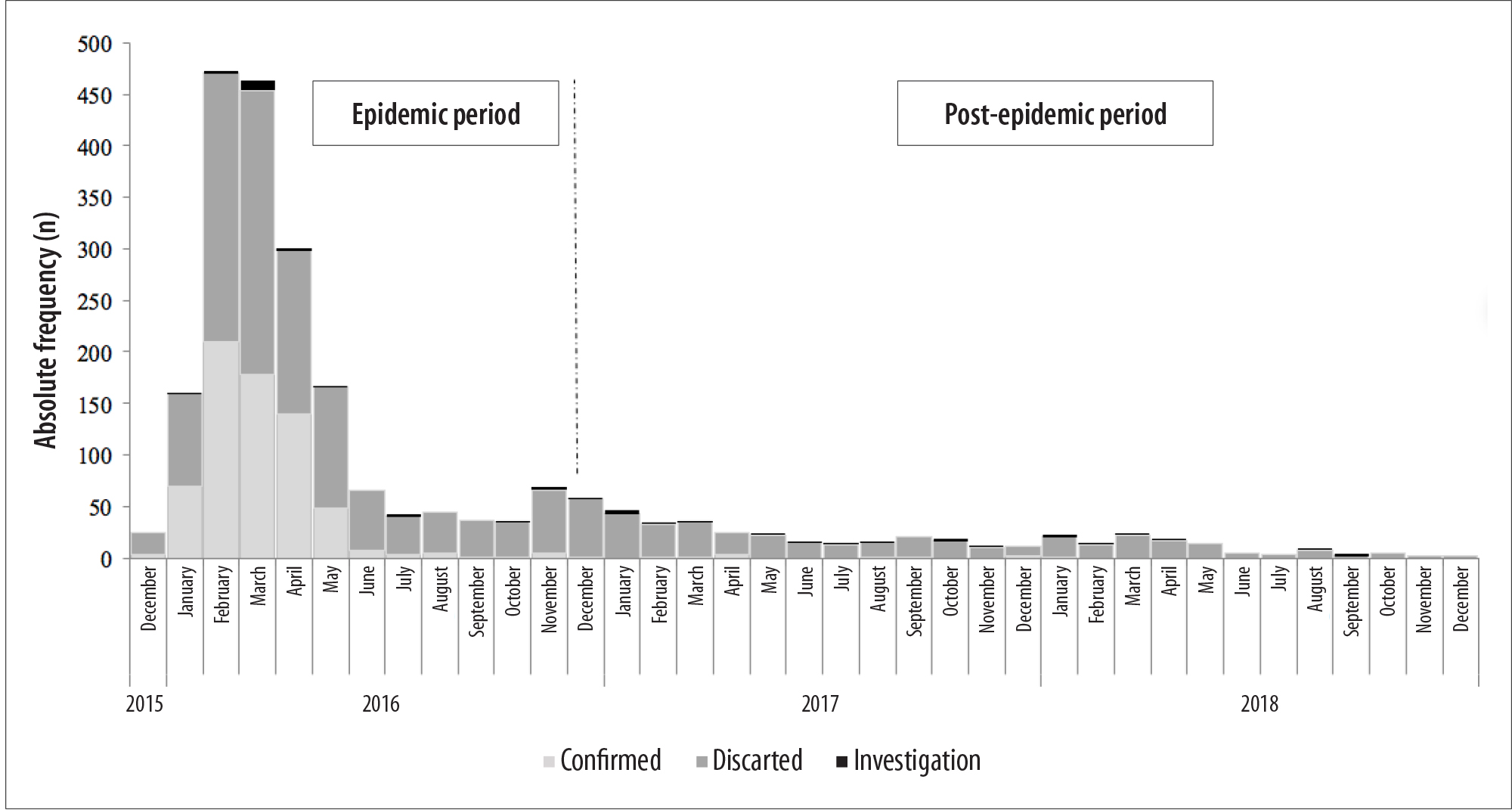
Figure 2 - Distribution of cases of symptomatic pregnant women with suspected Zika virus infection (N=2,329), second year and month of notification and final classification, São Paulo, state of São Paulo, Brazil, 2015-2018
Table 1 - Distribution of cases of pregnant women with suspected Zika virus infection (n=2,284), according to trimester of pregnancy, schooling, race/skin color, type of delivery and number of prenatal visits, São Paulo, São Paulo state, Brazil, 2015-2018
| Variables | Confirmed (N=683) | Discarded (N=1,601) | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Marital status | |||||
| Single | 306 | 44.8 | 617 | 38.5 | 0.013g |
| Married | 254 | 37.2 | 721 | 45.0 | |
| Stable union | 105 | 15.4 | 223 | 13.9 | |
| Divorced | 12 | 1.8 | 31 | 1.9 | |
| Widow | 2 | 0.3 | 3 | 0.2 | |
| Race/skin colorb | |||||
| White | 507 | 74.2 | 1,215 | 75.9 | 0.442g |
| Black | 22 | 3.2 | 35 | 2.2 | |
| Brown | 149 | 21.8 | 334 | 20.9 | |
| Other | 2 | 0.1 | 7 | 0.4 | |
| Schooling | |||||
| Without education | 4 | 0.6 | 1 | 0.1 | <0.001h |
| Complete elementary school | 173 | 25.3 | 227 | 17.2 | |
| High school | 366 | 53.6 | 789 | 49.3 | |
| Higher education | 97 | 14.2 | 374 | 23.3 | |
| Trimesters of pregnancyd | |||||
| 1st | 113 | 16.5 | 401 | 25.0 | <0.001g |
| 2nd | 261 | 38.2 | 550 | 34.4 | |
| 3rd | 298 | 43.6 | 630 | 39.4 | |
| Number of Prenatal visits | |||||
| None | 2 | 0.3 | 2 | 0.1 | 0.589h |
| 1-3 | 10 | 1.5 | 20 | 1.2 | |
| 4-6 | 80 | 11.7 | 176 | 11 | |
| ≥7 | 572 | 83.7 | 1,394 | 87.1 | |
| Type of childbirth | |||||
| Cesarian section | 366 | 53.6 | 1,107 | 69.1 | <0.001g |
| Vaginal delivery | 316 | 46.3 | 494 | 30.9 | |
a) Four confirmed cases with ignored information; six discarded cases with ignored information; b) Three confirmed cases with ignored information; ten discarded cases with ignored information; c) 43 confirmed cases with ignored information; 210 discarded cases with ignored information; d) 11 confirmed cases with ignored information; 20 discarded cases with ignored information; e) 19 confirmed cases with ignored information; nine discarded cases with ignored information; f) One confirmed case with ignored information; g) Pearson chi-square test; h) Fisher's exact test.
Note: For the calculation of the p-value, 'ignored' values were not used.
Table 2 - Distribution of conceptuses born to symptomatic pregnant women with suspected Zika virus infection (n=2,284), according to sex, childbirth and outcome, São Paulo, state of São Paulo, Brazil, 2015-2018
| Variables | Confirmed (N=683) | Discarded (N=1,601) | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sexa | |||||
| Female | 310 | 45.4 | 767 | 47.9 | 0.270d |
| Male | 373 | 54.6 | 834 | 52.1 | |
| Childbirthb | |||||
| Full term | 520 | 76.1 | 1,031 | 64.4 | 0.312d |
| Preterm birth | 48 | 7.0 | 109 | 6.8 | |
| Abortion/stillbirth | 15 | 2.2 | 19 | 1.2 | |
| Conceptus’ outcomec | <0.001e | ||||
| Healthy | 565 | 82.7 | 1,217 | 76.0 | |
| Death | 17 | 2.5 | 24 | 1.5 | |
| Microcephaly | 12 | 1.8 | 3 | 0.2 | |
| Central nervous system abnormalities | 4 | 0.6 | 5 | 0.3 | |
| Microcephaly and central nervous system abnormalities | 11 | 1.6 | 3 | 0.2 | |
| Other alterations | 15 | 2.2 | 19 | 1.2 | |
a) One discarded case with ignored information; b) 100 confirmed cases with ignored information; 442 discarded cases with ignored information; c) 59 confirmed cases with ignored information; 330 discarded cases with ignored information; d) Pearson chi-square test; e) Fisher's exact test.
Note: For the calculation of the p-value, 'ignored' values were not used.
Discussion
In the state of São Paulo, ZIKV infection in pregnant women was predominant in white young women, with complete high school and concentrated in the northeastern region of the state. The proportion of newborns with microcephaly and/or central nervous system abnormalities among pregnant women with confirmed ZIKV infection was higher than the proportion of microcephaly and/or central nervous system abnormalities among discarded cases.
Regarding the methodological limitations of the study, incomplete documentation inherent to the routine surveillance systems should be taken into account, given that it may have compromised data accuracy in a descriptive study. Another type of limitation to consider was the high laboratory specificity, using only RT-PCR for ZIKV infection diagnosis in pregnant women, given the unavailability of serology tests at the time of laboratory investigation.
The number of cases of pregnant women with ZIKV infection reported in the state of São Paulo peaked in early 2016 (second wave in Brazil), unlike the Northeast region of the country, where the peak of data recorded occurred in the second half of 2015 (first wave in Brazil).6 The reasons for these differences over time and in different regions, however, are not fully elucidated; notwithstanding, some possible explanations are (i) environmental and social conditions, (ii) the control actions of Aedes aegypti and (iii) the adoption of individual or household protection measures (repellents, long clothes, mosquito netting and screens), especially for the protection of pregnant women during the second wave of ZIKV infection.4.6
Symptomatic pregnant women with suspected ZIKV infection were characterized as being young, single white women, and with complete high school. These findings differ from national studies,4.5.14.15 mainly when these studies refer to the North and Northeast regions, where there was a predominance of non-white pregnant women who were married/in stable union. However, the findings of this study are similar to a study conducted in the city of São José do Rio Preto, in the state of São Paulo,16 probably because it refers to a municipality in the same state and with a similar population profile. In addition, 54 pregnant women and children showed by Nogueira et al. in São José do Rio Preto16 are in the population group described in this study.
ZIKV infection can affect individuals of any social, economic or ethnic class. Nevertheless, studies have suggested poverty situation as a social determinant in the configuration of ZIKV epidemic in the country, being race/skin color and schooling indicative of this social condition.17 The cases studied on screen presented a sociodemographic profile similar to the population profile of the state of São Paulo,18 different from the cases and population profile of the North and Northeast regions, which may have caused the lowest proportion of cases of congenital Zika virus syndrome in the state of São Paulo.17
Although most symptomatic pregnant women underwent seven or more prenatal consultations, and thus complied with the recommendation of the Ministry of Health,19 as observed in other national studies,5.14.15 there were pregnant women who did not attended any consultations or with one to three prenatal visits. Given the magnitude of Zika virus infection among the mother-child binomial, it is important that 100% of pregnant women get recommended prenatal care.
The majority of confirmed cases of ZIKV infection occurred during the second and third trimesters of pregnancy. However, when analyzing the trimester of ZIKV infection in children who were born with microcephaly and/or central nervous system abnormalities, it could be seen that most of them were infected during the first trimester of pregnancy. This finding is compatible with the literature, given that infection in the first trimester implies a higher risk of microcephaly and other central nervous system abnormalities in conceptuses, as well as more severe cases.20
The main sign of infection among pregnant women was rash, and the most common symptoms were myalgia, headache and pruritus; fever was not a frequent symptom, a profile similar to that identified in other studies.8.21 This characterization has a direct impact on the suspicion of ZIKV infection, given that the restriction in case definition, as being a febrile illness, may delay the diagnosis and, consequently, its treatment.
Regarding the occurrence of outcome such as 'abortion' or 'stillborn', there was no statistical difference between the groups of pregnant women confirmed with ZIKV infection and those discarded cases. In a cohort study, no significant difference was observed in fetal loss rate between symptomatic ZIKV-positive and negative mothers.8 The same cannot be said about the presence of malformations, for which this difference was statistically significant - six-fold higher in confirmed cases - which corroborates the characteristic of Zika virus of causing multiple malformations, and its predilection for neural cells.21
In this study, the proportion of microcephaly and/or central nervous system abnormalities in infants born to ZIKV-positive pregnant women was approximately 4.0%, similar to that found in national8.23 and international studies.10.24.25 However, it differs from the findings of studies conducted in the Northeast region, where it is four-fold higher (16.3%),4 showing different behavior of the disease according to regions of the country. This characterization reveals the necessity of developing studies that deepen the knowledge about these regional differences and their impact on population health, given that the country has great territorial, population, social, cultural and economic diversity. ZIKV infection during pregnancy is only part of a group of infectious diseases that can be transmitted to the fetus and cause microcephaly. Other congenital infections such as cytomegalovirus, rubella, herpes or toxoplasmosis26 are potential causes of microcephaly and central nervous system abnormalities, therefore the need for studies evaluating factors other than ZIKV infection with regard to malformations. In this study, this evaluation was limited, due to the small percentage of pregnant women whose STORCH test results were recorded together with the notification of Zika virus infection. However, it could be seen that one out of 20 women had co-infection - simultaneous ZIKV infection and STORCH - a result similar to studies conducted in French territories,9 and a study conducted using secondary data from all over Brazil.4
In São Paulo, ZIKV had the greatest impact in the northeast region of the state,27 possibly because it is a region with a high infestation rate of Aedes aegypti and where climatic conditions are more favorable for the spread of the mosquito.28 Geolocation of ZIKV cases in São Paulo allows a strategic direction for disease control actions in the state.
The results found can contribute to clinical and epidemiological characterization of pregnant women possibly exposed to ZIKV, as well as the description of their pregnancy outcome, either the evolution to abortion and/or stillbirth, or characterization of clinical conditions of the newborn exposed to ZIKV (microcephaly, central nervous system abnormalities or healthy newborn). The findings also enable the identification of regions with the highest occurrence and circulation of ZIKV in the state of São Paulo.
It is recommended further studies, in the state of São Paulo, aiming to monitor these children with malformations due to ZIKV infection during pregnancy, and to evaluate the consequences of these malformations in family, social, psychological and financial contexts. It is also recommended to evaluate the impact of dengue epidemics in the state of São Paulo and the risk of malformations due to ZIKV infection. For example, a nationwide study suggests an inverse correlation between dengue epidemic and the risk of malformations due to ZIKV infection in Brazil.29
It can be concluded that the characterization of Zika virus infection in pregnant women in the state of São Paulo, can support guidelines for Public Health actions toward regions and places with a higher risk of ZIKV transmission and, consequently, contribute to the prevention of malformations due to vertical transmission of the virus.
REFERENCES
1. World Health Organization. Zika epidemiology update [Internet]. [Geneva]: WHO; Jul. 2019 [acesso 24 jul. 2021]. Disponível em: Disponível em: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1 [ Links ]
2. Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552-63. doi: https://doi.org/10.1056/NEJMra1602113. [ Links ]
3. Zanluca C, Melo VCA, Mosimann ALP, Santos GIV, Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569-72. doi: https://doi.org/10.1590/0074-02760150192. [ Links ]
4. Oliveira WK, França GVA, Carmo EH, Duncan BB, Kuchenbecker RS, Schmidt MI. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet. 2017 Aug 26;390(10097):861-70. doi: https://doi.org/10.1016/S0140-6736(17)31368-5 [ Links ]
5. Marinho F, Araújo VEM, Porto DL, Ferreira HL, Coelho MRS, Lecca RCR, et al. Microcephaly in Brazil: prevalence and characterization of cases from the information system on live births (Sinasc), 2000-2015. Epidemiol Serv Saude. 2016;25(4):701-12. doi: http://doi.org/10.5123/S1679-49742016000400004. [ Links ]
6. Garcia LP. Epidemia do vírus Zika e microcefalia no Brasil: emergência, evolução e enfrentamento. Texto Discussao. 2018;(2368):7-54. [ Links ]
7. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016 May 19;374(20):1981-7. doi: http://doi.org/10.1056/NEJMsr1604338. [ Links ]
8. Brasil P, Pereira Junior JP, Moreira ME, Nogueira RMR, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016 Dec 15;375(24):2321-34. doi: http://doi.org/10.1056/NEJMoa1602412. [ Links ]
9. Araújo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, Melo APL, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis. 2018;18(3):328-36. doi: http://doi.org/10.1016/S1473-3099(17)30727-2. [ Links ]
10. Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabie A, et al. Pregnancy outcomes after ZIKV infection in french territories in the Americas. N Engl J Med. 2018 Mar 15;378(11):985-94. doi: http://doi.org/10.1056/NEJMoa1709481. [ Links ]
11. Ministério da Saúde (BR). Orientações integradas de vigilância e atenção à saúde no âmbito da emergência de saúde pública de importância nacional [Internet]. Brasília: MS; 2017 [acesso 15 dez. 2019]. Disponível em: Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/orientacoes_integradas_vigilancia_atencao_emergencia_saude_publica.pdf . [ Links ]
12. Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014 Apr 3;19(13):20751. [ Links ]
13. Secretaria da Saúde do Estado de São Paulo. Protocolo de vigilância para gestantes com exantema: orientação para as vigilâncias epidemiológicas do estado de São Paulo frente à investigação e o acompanhamento de gestante suspeita [e] confirmada para ZIKA vírus. [São Paulo: SSESP]; abr. 2016. [ Links ]
14. Vanderlei JS, Franchi EPLP, Gomes NS, Oliveira AKR, Monteiro LD. Perfil de gestantes confirmadas para zika vírus e assistência pré-natal na atenção primária à saúde de Palmas, Tocantins, 2016. Rev Patol Tocantins. 2018;5(3):12-7. doi: http://doi.org/10.20873/uft.2446-6492.2018v5n3p12. [ Links ]
15. Vargas A, Saad E, Dimech GS, Santos RH, Sivini MAVC, Albuquerque LC, et al. Características dos primeiros casos de microcefalia possivelmente relacionados ao vírus Zika notificados na Região Metropolitana de Recife, Pernambuco. Epidemiol Serv Saude. 2015;25(4): 691-700. doi: http://doi.org/10.5123/s1679-49742016000400003. [ Links ]
16. Nogueira ML, Nery Júnior NRR, Estofolete CF, Terzian ABC, Guimarães GF, Zini N, et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin MicrobiolInfect. 2018;24(6):646-52. doi: http:// doi.org/10.1016/j.cmi.2017.11.004. [ Links ]
17. Lowy I. Zika no Brasil: história recente de uma epidemia [e-book]. Rio de Janeiro: Editora Fiocruz; 2019. (Temas em saúde). doi: http://10.7476/9786557080634. [ Links ]
18. Instituto Brasileiro de Geografia e Estatística. Censo demográfico 2010: características da população e dos domicílios: resultados do universo[Internet].Rio de Janeiro: IBGE; 2011. [acesso 18 nov. 2020]. Disponível em: Disponível em: https://biblioteca.ibge.gov.br/visualizacao/periodicos/93/cd_2010_caracteristicas_populacao_domicilios.pdf [ Links ]
19. Cruz RSBLC, Batista Filho M, Caminha M, Souza ES. Protocolos de atenção pré-natal à gestante com infecção por Zika e crianças com microcefalia: justificativa de abordagem nutricional. Rev Bras Saude Mater Infant. 2016.16(suppl1):S95-S102. doi: http://doi.org/10.1590/1806-930420160s100008. [ Links ]
20. Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):E1587-E1596. doi: http://doi.org/10.1073/pnas.1616097114. [ Links ]
21. Wiwanitkit S, Wiwanitkit V. Afebrile, asymptomatic and non-thrombocytopenic Zika virus infection: Don't miss it. Asian Pac J Trop Med. 2016;9(5):513. doi: http://doi.org/10.1016/j.apjtm.2016.03.036. [ Links ]
22. Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016 May 5;18(5):587-90. doi: http://doi.org/10.1016/j.stem.2016.02.016. [ Links ]
23. Coelho AVC, Crovella S. Microcephaly prevalence in infants born to Zika virus-infected women: a systematic review and meta-analysis. IntJ Mol Sci. 2017 Aug 5;18(8):1714. doi: http://doi.org/10.3390/ijms18081714. [ Links ]
24. Pomar L, Malinger G, Benoist G, Carles G, Ville Y, Rousset D, et al. Association between Zika virus and fetopathy: a prospective cohort study in French Guiana. Ultrasound Obstet Gynecol. 2017;49(6):729-36. doi: http://doi.org/10.1002/uog.17404. [ Links ]
25. Rodriguez-Morales AJ, Cardona-Ospina JA, Ramirez-Jaramillo V, Gaviria JA, González-Moreno GM, Castrillón-Spitia JD. Diagnosis and outcomes of pregnant women with Zika virus infection in two municipalities of Risaralda, Colombia: second report of the ZIKERNCOL study. Travel Med Infect Dis. 2018;25:20-5. doi: http://doi.org/10.1016/j.tmaid.2018.06.006. [ Links ]
26. Devakumar D, Bamford A, Ferreira MU, Broad J, Rosch RE, Groce N, et al. Infectious causes of microcephaly: epidemiology, pathogenesis, diagnosis, and management. Lancet Infect Dis. 2018;18(1):e1-e13. doi: http://doi.org/10.1016/S1473-3099(17)30398-5. [ Links ]
27. Secretaria da Saúde do Estado de São Paulo. Distribuição dos casos notificados e confirmados de febre pelo vírus Zika, por mês de início de sintomas. Estado de São Paulo [Internet]. [São Paulo: SSESP] ; 2016 [acesso dez. 2019]. Disponível em: Disponível em: http://www.saude.sp.gov.br/cve-centro-de-vigilancia-epidemiologica-prof.-alexandre-vranjac/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/agravos/zika-virus/dados-estatisticos [ Links ]
28. Fonseca Junior DP, Serpa LLN, Barbosa GL, Pereira M, Holcman MM, Voltolini JC, et al. Vetores de arboviroses no estado de São Paulo: 30 anos de Aedes aegypti e Aedes albopictus. Rev Saude Publica. 2019;53:84. doi: http://doi.org/10.11606/s1518-8787.2019053001264. [ Links ]
29. Carvalho MS, Freitas LP, Cruz OG, Brasil P, Bastos LS. Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci Rep. 2020;10:1752. doi: http:// doi.org/10.1038/s41598-020-58407-7. [ Links ]
*Article derived from a doctoral thesis entitled 'Association between congenital syndrome and Zika virus infection during pregnancy: cohort study in the state of São Paulo, 2015 to 2018', to be submitted by Renata Soares Martins to the Postgraduate Program in Epidemiology of the School of Public Health of the University of São Paulo.
Received: September 21, 2020; Accepted: March 02, 2021











 texto em
texto em