Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.30 no.4 Brasília dic. 2021 Epub 22-Sep-2021
http://dx.doi.org/10.1590/s1679-49742021000400001
ORIGINAL ARTICLE
COVID-19 in children, adolescents and young adults: a cross-sectional study in Espírito Santo, Brazil, 2020
1Universidade Federal do Espírito Santo, Laboratório de Epidemiologia, Vitória, ES, Brazil
2Organização Pan-Americana da Saúde, Brasília, DF, Brazil
3Secretaria de Estado da Saúde do Espírito Santo, Vitória, ES, Brazil
4Instituto Jones dos Santos Neves, Vitória, ES, Brazil
5Hospital Universitário Cassiano Antônio Moraes, Vitória, ES, Brazil
6Secretaria de Estado da Saúde do Tocantins, Palmas, TO, Brazil
Objective:
To analyze self-reported sociodemographic and clinical characteristics among individuals aged 2 to 22 years and possible associations with SARS-CoV-2 infection in Espírito Santo, Brazil.
Methods:
This was a serial cross-sectional population-based study carried out from May to June 2020. The COVID-19 positivity rate was assessed by serological testing, and associated factors were assessed using Pearson's chi-square test (5% significance level).
Results:
Among 1,693 individuals aged 2 to 22 years, 6.1% tested positive for COVID-19 and, among these, 35.5% did not present any symptoms. Differences were identified between positive and negative cases regarding the number of symptoms (p-value=0.001).
Coughing was reported by 40.4% of positive individuals. Only 14.3% sought health care, namely 29.8% among those who tested positive and 13.3% among those who tested negative (p-value=0.001).
Conclusion:
The percentage of asymptomatic patients can impact the COVID-19 transmission chain in schools and fuel outbreaks of the disease in schools.
Keywords: Coronavirus Infections; Child Health; Adolescent; Cross-Sectional Studies.
Introduction
In December 2019, a series of pneumonia cases of unknown origin emerged in Wuhan, Hubei Province, China. An investigation by the Chinese Center for Disease Control linked most of the cases to the wholesale seafood market, known as the Huanan Market, where many species of live animals are sold. The disease, called COVID-19, has spread rapidly, both in China and globally. The virus responsible for infection has been characterized as a novel member of enveloped ribonucleic acid (RNA) coronavirus: SARS-CoV-2.1-5
Immediately, research aimed at identifying possible risk factors for COVID-19 severity and death was undertaken. Several measures to mitigate the disease were put in place by governments, ranging from the recommendation to wear facemasks to physical distancing, and measures to restrict the movement of people. The first epidemiological data did not identify children and adolescents as the main agents of transmission and illness, which led to low concern about these groups in the context of the pandemic, although studies have shown that younger people also become infected, although they have milder clinical manifestations than adults and the elderly.6,7
The difficulty in estimating the burden of COVID-19 in the younger population may possibly have been accentuated by the closure of schools and daycare centers at the beginning of the pandemic, making it difficult to carry out more robust studies that considered the hypothesis that schools were sites of transmission. A study developed in a tertiary pediatric medical center in the city of Chicago, USA, analyzing 145 individuals, comprising children under 5 years old (n=46), children and adolescents from 5 to 17 (n=51) and adults from 18 to 65 (n=48) who developed moderate symptoms one week after having contracted the virus, showed that preschool children (under 5 years old) had viral loads 10 to 100 times greater in the upper respiratory tract than older children and adults. This finding suggests increased transmission among children under 5 years of age, as is the case of respiratory syncytial virus, with important implications, especially for safety with regard to reopening schools and daycare centers.8
A systematic review of the clinical characteristics and clinical management of children and adolescents with COVID-19 assessed 18 studies, with 1,065 participants (of whom 444 individuals were under 10 years old and 553 were between 10 and 19 years old) with confirmed SARS-CoV-2 infection in the final analysis. The study concluded that children primarily acquire SARS-CoV-2 infection from their family members. Additionally, the results suggested that they develop a less severe form of COVID-19, compared to adults, with mild symptoms, good prognosis and recovery 1 to 2 weeks after disease onset.
The rapid worldwide spread of SARS-CoV-2 and the lack of data on infected children under 10 years of age point to the need for further epidemiological and clinical studies to identify possible preventive and therapeutic strategies for COVID-19.9
With the proposed return to lessons at school, the need exists to understand the main symptoms, severity and evolution of the disease in the 2-22 year age group and how they can indicate better ways to institute biosafety protocols for screening these cases.
The objective of this study was to analyze self-reported sociodemographic and clinical characteristics among individuals aged 2 to 22 years and possible associations with SARS-CoV-2 infection in Espírito Santo State, Brazil.
Methods
A serial cross-sectional population-based study was conducted, the study unit of which was one inhabitant per household.
The State of Espírito Santo is located in Southeast Brazil. In 2019 it had a population of 4,018,650 inhabitants.10 Espírito Santo is comprised of 78 municipalities, distributed between four intermediate regions and eight immediate regions, according to the Brazilian Institute of Geography and Statistics.11
Sentinel municipalities were selected for the study in each of the immediate regions, namely those with the largest populations (Colatina; Linhares; São Mateus; Afonso Cláudio; Nova Venécia; Cachoeiro de Itapemirim; Alegre), as well as the four municipalities that comprise the metropolitan region of Greater Vitória (Vila Velha; Cariacica; Serra; and the state capital, Vitória).
The study included individuals aged 2 to 22 years, chosen at random from each selected househod. No exclusion criteria were established.
The study consisted of four stages. Sample sizes were calculated for each stage based on the calculation for estimating prevalence in a simple random sample, with expected prevalence of 3.0% in the first stage, reaching 20.0% in the last stage. For each stage, the sampling errors ranged from 0.5% (3.0% prevalence) to 1.2% (20.0% prevalence). The final sample of the study, intended to estimate prevalence of around 5%, has a 1.1% sampling error.
Four repeated cross-sectional surveys were conducted. The sampling process of each survey was independent and was conducted in multiple stages, every 15 days, lasting two months: Stage 1, May 10; Stage 2, May 24; Stage 3, June 7; and Stage 4, June 21, 2020.
This study is based on a subsample of the original survey, including all individuals aged 2-22 years, categorized by age groups: 2-5 years old, preschool children; 6-10, elementary education I students; 11-14, elementary education II students; 15-18, high school students; and 19-22, higher education students. A second categorization was also considered: 2-10 years old (children); 11-18 (adolescents); and 19-22 (young adults).
To be interviewed, answer the questionnaire and take the antibody test, the selected household inhabitant had to be over 16 years of age, regardless of whether or not they presented signs or symptoms of the disease. When the household inhabitant was under 16 years old, the survey was based on the replies of the person in charge of them, that is, any inhabitant of the household above this age who was a relative of the under 16-year-old. Children under 2 years old were not included in the study.
Blood sample collection by fingerstick and testing for COVID-19 were performed by trained health professionals. The Celer IgM and IgG antibody rapid immunochromatographic test was used for diagnosis, as registered with the National Health Surveillance Agency (ANVISA) under No. 80537410048. This test has 86.4% sensitivity and 97.6% specificity. The result was considered positive when the test was positive for the detection of SARS-CoV-2 antibodies (IgM and/or IgG) in the blood sample.12
A questionnaire with closed questions was developed to assess variables associated with positive testing. Data were collected on tablets connected to the internet and using a digital platform, with the possibility of operating where or when there was no access to the network. The tablets were provided by the Espírito Santo State Health Department; the team of interviewers consisted of staff provided by the Municipal Health Departments. The field team was trained by the State Health Department, in both face-to-face and online meetings: a pair of researchers was assigned to each census tract and was responsible for 40 interviews.
Regarding sampling, the total number of households for each of the 11 municipalities was proportional to the estimated total size of the urban population. Census tracts were selected at random, with 40 households per census tract. We only considered census tracts measuring less than 100 hectares, with an urban population, and more than 200 households, based on 2010 Demographic Census data.
The households were selected systematically, one in every five, from a randomly generated starting point. A list was made of the inhabitants of each selected household, only one of whom was randomly selected to participate in the study. The sample included the same census tracts in all stages of the survey, but not the same households as those included in the previous stages. Individuals were randomly selected at each household, regardless of whether or not they presented signs and symptoms of COVID-19.
Besides testing for COVID-19, the following information about the participants was collected: sex (male; female); age group I, according to distribution in school categories (in years: 2-5; 6-10; 11-14; 15-18; 19-22); age group II, grouped in three age ranges (in years: 2-10, children; 11-18, adolescents; 19-22, young adults); schooling of the individual with the highest level of education in the household (illiterate; elementary education; high school education; complete higher education; incomplete higher education); self-reported race/skin color (White; brown; Black; yellow; Indigenous); number of inhabitants in the household (1, 2, 3, 4, 5 or more); seeking health care services in the event of COVID-19 symptoms (yes; no); and presence of WHO-listed symptoms of COVID-19 (cough, fever, tiredness, aching body, difficulty breathing, changes in taste and smell) in the 15 days prior to interview and other unrelated symptoms.13 Participants were asked about symptoms one symptom at a time and had to reply 'yes' or 'no' for each symptom. We also studied self-reported comorbidities (asthma, systemic arterial hypertension, obesity, diabetes mellitus, cardiovascular disease, cancer, and kidney disease), for which the answer was either "yes" or "no" for each comorbidity.
Tables of absolute and relative frequency were compiled in the statistical analysis. Bivariate analysis of the COVID-19 test results and the study variables was also performed using Pearson's chi-square test. A 5% significance level was used. The data were analyzed using SPSS (Statistical Package for the Social Sciences), version 20.0.
The study project was approved by the Federal University of Espírito Santo 'Cassiano Antônio Moraes' University Hospital Research Ethics Committee. The Certificate of Submission for Ethical Appraisal was registered under No. 31417020.3.0000.5064 and received approval through Opinion No. 4.317.264, issued on April 30, 2020. All individuals selected for the population survey sample were informed about the objectives of the study, its risks and benefits. The blood samples and information were collected after participants had signed a Free and Informed Consent form; or an Assent form in the case of underage participants. All individuals tested in the field were given a registered telephone number to get their test results. Seropositive cases were notified to the municipal health service for the necessary measures to be taken. Appropriate biosafety measures were taken to ensure the health of field workers responsible for data and blood sample collection.
In households where the selected individual tested positive, as well as in households with symptomatic residents, the test was offered to all residents of the household. These results were not computed in the prevalence study, nor were they included in the sample we assessed.
Results
The original survey was comprised of a sample of 18,791 participants, of which a subsample was analyzed in this study, corresponding to 1,693 (9.0%) children, adolescents, and young adults aged 2 to 22 years old. Of the total 1,693 individuals in the analyzed age group (2-22 years) who were tested, 104 (6.1%) tested positive for SARS-CoV-2 antibodies. All participants were tested regardless of whether or not they had signs and symptoms of COVID-19.
Figure 1 shows the evolution of positivity over the four stages of the survey for the total age group (2-22 years) and by three age categories (2-10, 11-18, and 19-22 years). The chi-square test between stages indicated a difference in positivity, with an increasing trend for all categories (p-value=0.001). The percentage of COVID-19 positive results in the 2 to 10 years age group increased from 1.0% to 11.0% between the first and the last stage of the study , while the percentage of positives increased from 2. 6% to 9.3% and 2.2% to 11.6% in the 11 to 18 and 19 to 22 years age groups, respectively, between the first and third phase.
Table 1 shows the sociodemographic profile of the participants. 63.7% of the sample were found to be in the 15-22 year age group, 56.6% were female, 64.6% self-reported their race/skin color as Black or brown, and 49.8% lived with more than 4 people in their household. With regard to higher education, 10.6% had incomplete higher education and 20.2% had complete higher education. No statistically significant differences were found between the groups studied (positive or negative anti-SARS-CoV-2 antibodies).
We found that 35.5% of the sample that tested positive for SARS-CoV-2 had no symptoms, 16.3% had one symptom, 9.6% had two symptoms, 9.7% had three symptoms, while 29.8% had four or more symptoms. The differences between those testing positive and negative with regard to the number of symptoms were statistically significant (p-value=0.001).
In the study population, only 242 (14.3%) sought health care because they had COVID-19 symptoms. They accounted for 29.8% of those with a positive result for COVID-19 and 13.3% of those with a negative result, with statistical significance (p-value=0.001).
Figure 2 shows the frequency of self-reported symptoms in those who tested positive. 'Coughing' stands out as the most prevalent symptom, accounting for 40.4% of the individuals identified with COVID-19, followed by anosmia (33.7%), fever (26.0%) and myalgia (24.0%).
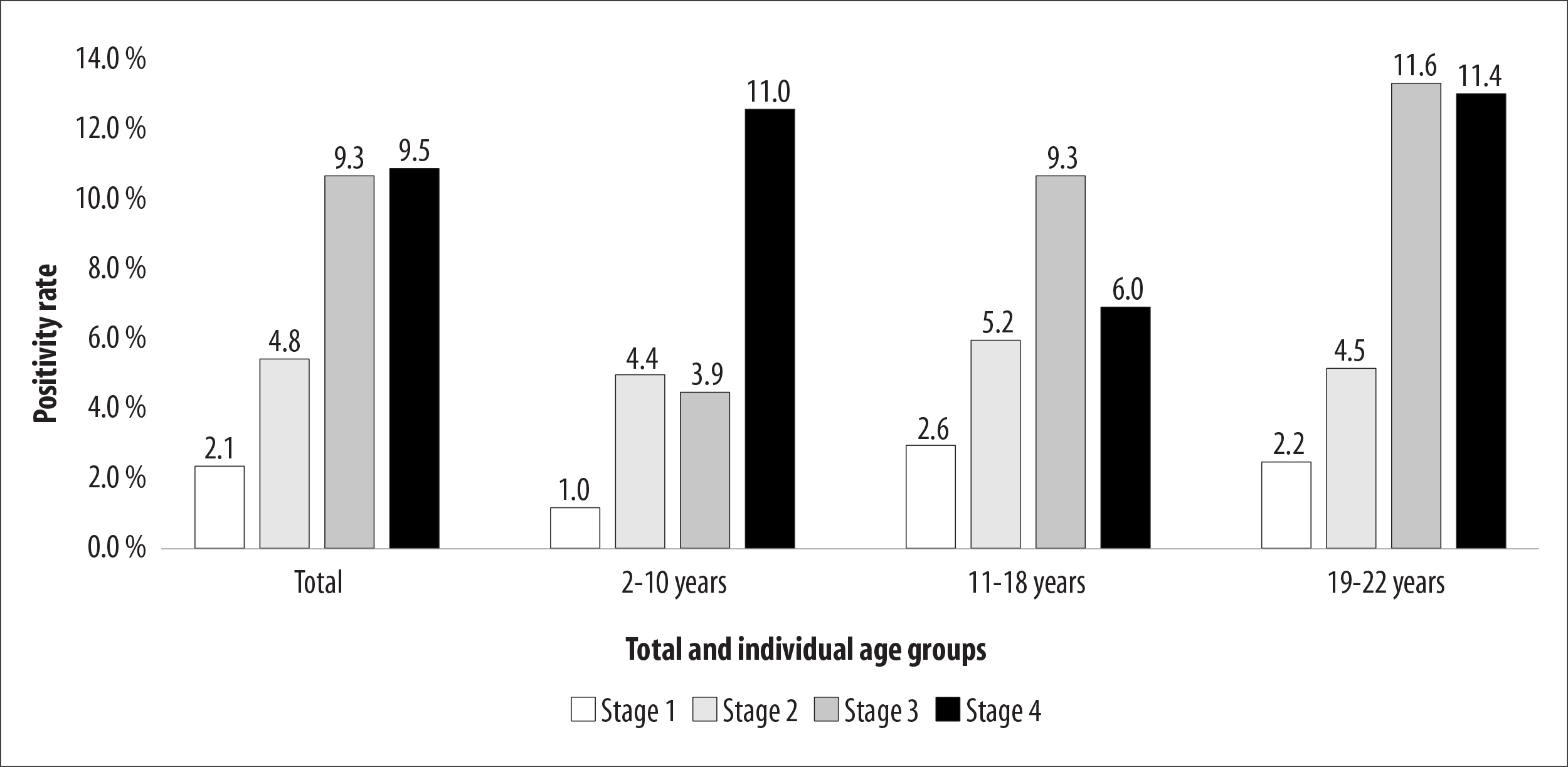
Note: The tests were performed on the following dates: Stage 1, May 10; Stage 2, May 24; Stage 3, June 7; and Stage 4, June 21, 2020.
Figure 1 - Percentage distribution of individuals aged between 2 and 22 years old (n=1,693) with positive test results for total SARS-CoV-2 antibodies, by age groups and survey stages, Espírito Santo, Brazil, 2020
Table 1 - Absolute and relative frequencies of the sociodemographic characteristics of individuals age between 2 and 22 years old (n=1,693), according to SARS-CoV-2 antibody test positivity, Espírito Santo, Brazil, 2020
| Variable | Category | Totala | Positiveb | Negativeb | p-valuec | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||||
| Age group I (in years) | ≤5 | 136 | 8.0 | 9 | 6.6 | 127 | 93.4 | 0.195 | ||
| 6-10 | 212 | 12.5 | 8 | 3.8 | 204 | 96.2 | ||||
| 11-14 | 268 | 15.8 | 11 | 4.1 | 257 | 95.9 | ||||
| 15-18 | 361 | 21.3 | 23 | 6.4 | 338 | 93.6 | ||||
| 19-22 | 716 | 42.4 | 53 | 7.4 | 663 | 92.6 | ||||
| Age group II (in years) | 2-10 | 348 | 20.6 | 17 | 10.4 | 331 | 89.6 | 0.172 | ||
| 11-18 | 629 | 37.2 | 34 | 10.5 | 595 | 89.5 | ||||
| 19-22 | 716 | 42.2 | 53 | 7.4 | 663 | 92.6 | ||||
| Sex | Female | 958 | 56.6 | 63 | 6.6 | 895 | 93.4 | 0.397 | ||
| Male | 735 | 43.4 | 41 | 5.6 | 694 | 94.4 | ||||
| Race/skin color | White | 572 | 33.7 | 30 | 5.2 | 542 | 94.8 | 0.611 | ||
| Brown | 799 | 47.2 | 52 | 6.5 | 747 | 93.5 | ||||
| Black | 294 | 17.4 | 21 | 7.1 | 273 | 92.9 | ||||
| Yellow or indigenous | 28 | 1.7 | 1 | 3.6 | 27 | 96.4 | ||||
| Number of inhabitants in household | 1 | 86 | 5.1 | 6 | 7.0 | 80 | 93.0 | 0.483 | ||
| 2 | 281 | 16.6 | 19 | 6.8 | 262 | 93.2 | ||||
| 3 | 482 | 28.5 | 24 | 5.0 | 458 | 95.0 | ||||
| 4 | 456 | 26.9 | 25 | 5.5 | 431 | 94.5 | ||||
| ≥5 | 388 | 22.9 | 30 | 7.7 | 358 | 92.3 | ||||
| Highest household level of schooling | Illiterate | 24 | 1.4 | 2 | 8.3 | 22 | 91.7 | 0.157 | ||
| Elementary education | 358 | 21.1 | 25 | 7.0 | 333 | 93.0 | ||||
| High school education | 790 | 46.7 | 56 | 7.1 | 734 | 92.9 | ||||
| Complete higher education | 342 | 20.2 | 16 | 4.7 | 326 | 95.3 | ||||
| Incomplete higher education | 179 | 10.6 | 5 | 2.8 | 174 | 97.2 | ||||
a) Sum of total percentages in column; b) Sum of positive and negative category percentages in row; c) Pearson chi-square test p-value.
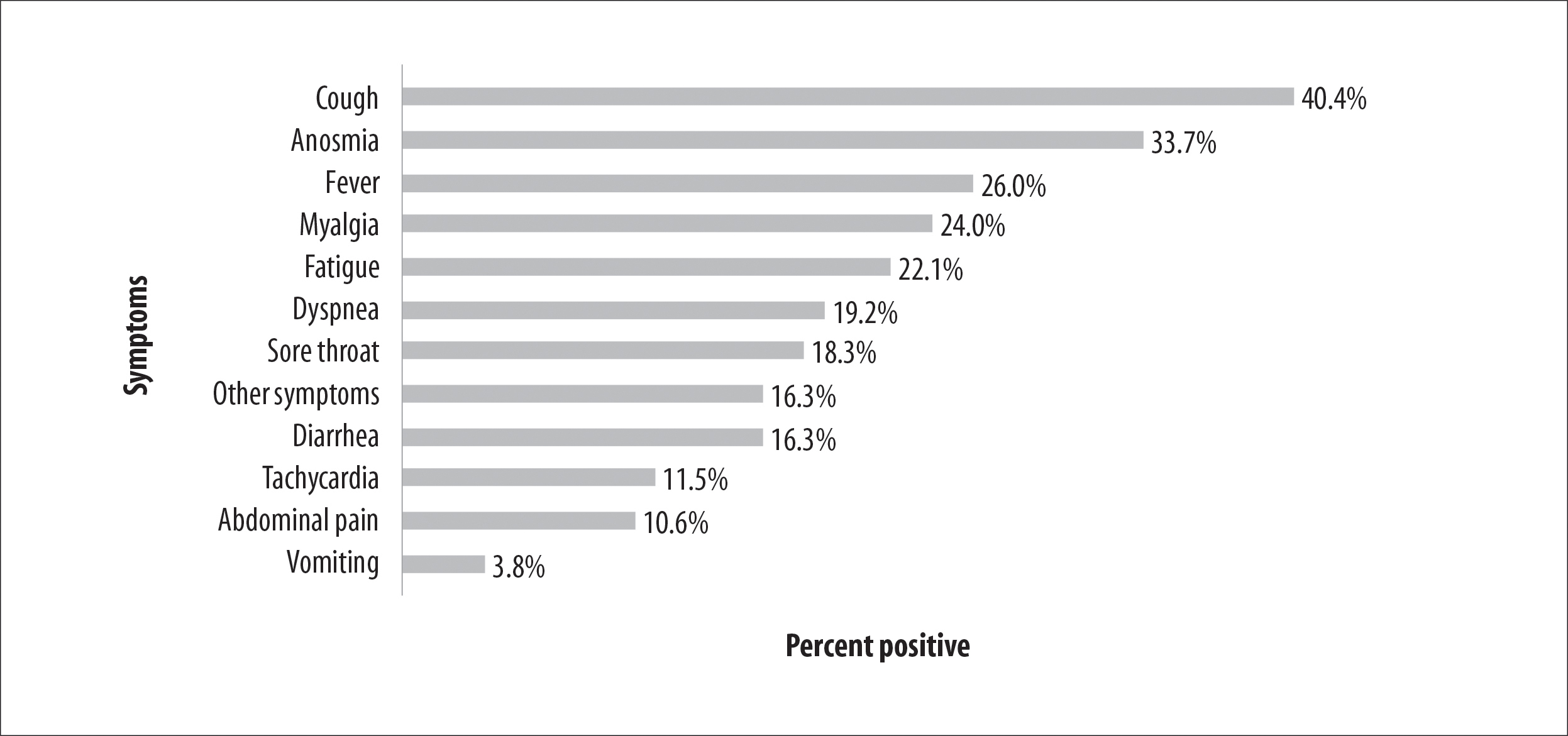
Figure 2 - Percentage distribution of symptoms self-reported by individuals aged between 2 and 22 years old (n=1,693) with positive test results for SARS-CoV-2 antibodies, Espírito Santo, Brazil, 2020
Table 2 - Absolute and relative frequencies of comorbidities self-reported by individuals aged between 2 and 22 years old (n=1,693), according to SARS-CoV-2 antibody test results, Espírito Santo, Brazil, 2020
| Comorbidities | Total (n=1,693) | Positive (n=104) | Negative (n=1,589) | p-valuea | |||||
| n | % | n | % | n | % | ||||
| Asthma | 257 | 15.2 | 13 | 12.5 | 244 | 15.4 | 0.432 | ||
| Systemic arterial hypertension | 193 | 11.4 | 21 | 20.2 | 172 | 10.8 | 0.004 | ||
| Obesity | 133 | 7.9 | 15 | 14.4 | 118 | 7.4 | 0.010 | ||
| Diabetes mellitus | 86 | 5.1 | 9 | 8.7 | 77 | 4.8 | 0.086 | ||
| Cardiovascular disease | 63 | 3.7 | 5 | 4.8 | 58 | 3.7 | 0.546 | ||
| Neoplasia | 20 | 1.2 | 3 | 2.9 | 17 | 1.1 | 0.097 | ||
| Kidney disease | 14 | 0.8 | 1 | 1.0 | 13 | 0.8 | 0.876 | ||
a) Pearson chi-square test p-value.
Note: All percentages shown relate to total presence of comorbidity, both positive and negative.
Table 2 shows the distribution of the study sample according to investigated comorbidities: 15.2% had asthma, 11.4% had systemic arterial hypertension and 7.9% were obese. Regarding comorbidities, significant differences were found in the frequency of arterial hypertension (p value=0.004) and obesity (p value = 0.010) according to infection status (SARS-CoV-2 positive or negative).
Discussion
The percentage of positivity for SARS-CoV-2 IgM and IgG antibodies was 6.1% in the age group from 2 to 22 years, considering all four stages of the epidemiological survey in Espírito Santo. There were no statistically significant differences between the groups studied, according to their sociodemographic profile. However, some data deserve attention, given their relevance for the return of this group to lessons at school. Firstly, the percentage of people who tested positive and had no symptoms (35.5%); secondly, the number of participants who sought health care and were not tested because they were not in the age groups indicated for testing; and thirdly, the fact that the 'fever' symptom, invariably present in the screening protocols, was found in approximately a quarter of the participants who tested positive.
In this sense, we must also point out the existence of individuals who sought health services but were not submitted to diagnostic assessment (testing), either because of the scarcity of tests in Brazil in 2020, or because the norms in force in Espírito Santo, at that time, did not include this age group as a priority for diagnostic testing in the event of clinical suspicion of COVID-19.11 This finding highlights the difficulty of getting tested in Brazil, and its possible impact on case underreporting in this age group.14
Oligosymptomatic and asymptomatic individuals can be relevant for the transmission chain, when the possibility of returning to school activities is being considered, in view of the difficulty in identifying signs and symptoms. Non-identification of infection can lead to these infected individuals remaining in classrooms and, added to this, the eventual lack of sufficient ventilation in these environments and the difficulty in ensuring adequate physical distance between students, and between them and teachers and support staff, can be factors that facilitate the dissemination of the disease in schools. Another issue to highlight, among the signs and symptoms reported, was that only a small percentage had fever, but this is precisely the indicator most used in the protocols for returning to school. Coughing and anosmia, as the main symptoms, should also be taken into consideration. In addition, random testing protocols in schools could contribute to minimizing outbreaks.15
Growth of positivity in all age groups from 2 to 22 years closely resembled the overall results of the main survey16 and indicates that children, adolescents, and young adults tend to represent the prevalence of COVID-19 in the community of which they are part. Therefore, a safe return to lessons at school must take into account the incidence of the disease in the community where the school is located.17
No socio-demographic differences were observed between cases with positive or negative test results. However, the percentage of comorbidities in the age group studied is striking, especially the higher percentage of individuals with systemic arterial hypertension and obesity in the group that tested positive. A meta-analysis found severe COVID-19 in 5.1% of children with comorbidities and in 0.2% of those without comorbidities. Random effects analysis revealed that this difference represented a relative risk of 1.79 (95% CI 1.27;2.51) for severe COVID-19 among children with comorbidities.18 Concern about these children must be reflected in a policy of greater protection and care, with a view to preserving their health, through intersectoral action programs, between the Health and Education sectors, to achieve better control of the disease, avoiding severe or fatal cases. Investing in educational strategies that do not involve going to school in person can be an alternative, when there are still no vaccines for this group, nor specific treatments against COVID-19.19
The results of this study reveal that the 2-22 age group needs to be better assessed regarding prevalence of infection, illness, comorbidities, and deaths. There is insufficient data to support and guide public policies for this age group. However, when the participation of younger people in the COVID-19 transmission chain is not minimized, without scientific evidence to justify that participation, this allows cases to continue and to accumulate, and provides opportunities for the emergence of outbreaks of the disease in schools.
A study conducted in the United States evaluated how school closures impacted the COVID-19 incidence curve.20 Between March and May 2020, school closure was associated with reduced COVID-19 incidence and mortality. Although the authors point out the difficulty of separating this measure from other non-pharmacological interventions, the findings indicate the importance of schools in the transmission of infection.
Recently, the emergence of new SARS-CoV-2 variants has reignited the debate in Europe about the centrality of Education over other activities, and closing schools in cases of accelerated transmission. COVID-19 incidence in school settings appears to be affected primarily by levels of transmission in the community.21,22 Widespread transmission of highly transmissible variants of SARS-CoV-2 would increase the likelihood of COVID-19 cases appearing in school settings, not least because these variants are more transmissible among young people, with profound negative health impacts for students, teachers, and staff. The consequent closure of schools in localities where other non-pharmacological measures are unable to control local transmission is recommended by the European Center for Disease Control and Prevention as a last resort, a time-bound supplementary decision. Under these conditions, school closure is expected to lead to further reductions in the rate of disease transmission.
This study has limitations. The sample size was not calculated for the prevalence of seropositive individuals according to age groups, which is why the percentages of seropositive individuals by age groups fluctuate. Only the total age group of the study (2-22 years) presented more consistent data. Added to this, there is the limitation of the research design itself, which only allows children at home to be identified at the time of data collection, which may lead to selective survival bias, excluding severe cases that may have been hospitalized.
The survey does, however, have important strengths: its large sample size in the age group in question and its assessment of data on symptoms, health care seeking, and the presence of comorbidities, which can serve to inform the definition of public policy guidelines for COVID-19.
We conclude that the percentage of asymptomatic cases can impact the infection transmission chain in schools, and fuel disease outbreaks. To avoid these outbreaks, it is important to plan the timing of the return to school, combining the negative impact of missing school activities with the need to maintain the downward curve of cases and deaths for a period of several weeks. It is also necessary to include new approaches for capturing signs and symptoms other than just checking a person’s temperature, which is insufficient to predict the disease, as the results suggested. Another important action to recommend is health education with specific guidance for children and adolescents not to attend school in the event of either them or any of their family members having any signs or symptoms of COVID-19.
Monitoring students - children, adolescents and young adults - and education workers upon their return to school, with rapid action for effective isolation and prompt diagnosis of infection using molecular methods, should any of these individuals show symptoms of COVID-19, is a fundamental strategy at this pandemic time.
Referências
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199-207. doi: https://doi.org/10.1056/NEJMoa2001316. [ Links ]
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: https://doi.org/10.1016/S0140-6736(20)30183-5. [ Links ]
3. Tan W, Zhao X, Ma XJ, Wang W, Niu P Xu W, et al. A novel coronavirus genome identified in a cluster of pneumonia cases, Wuhan, China 2019-2020. China CDC Wkly. 2020;2(4):61-2. doi: https://doi.org/10.46234/ccdcw2020.017. [ Links ]
4. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565-74. doi: https://doi.org/10.1016/S0140-6736(20)30251-8. [ Links ]
5. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-33. doi: https://doi.org/10.1056/NEJMoa2001017. [ Links ]
6. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shoa J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 Jul 1;174(7):722-5. doi: https://doi.org/10.1001/jamapediatrics.2020.0878. [ Links ]
7. Milani GP, Bottino I, Rocchi A, Marchiosio P, Elli S, Agostinni C, et al. Frequency of children vs adults carrying severe acute respiratory syndrome coronavirus 2 asymptomatically. JAMA Pediatr. 2021 Feb 1;175(2):193-194. doi: https://doi.org/10.1001/jamapediatrics.2020.3595. [ Links ]
8. Heald-Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr. 2020 Sep 1;174(9):902-3. doi: https://doi.org/10.1001/jamapediatrics.2020.3651. [ Links ]
9. Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlin S, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 Sep 1;174(9):882-9. doi: https://doi.org/10.1001/jamapediatrics.2020.1467. [ Links ]
10. Instituto Brasileiro de Geografia e Estatística. Censo brasileiro de 2010. Rio de Janeiro: IBGE; 2012 [acesso 26 maio 2021]. Disponível em: Disponível em: https://censo2010.ibge.gov.br/ [ Links ]
11. Secretaria do Estado do Espírito Santo. Nota técnica covid-19 n. 05/2020 - GEVS/SESA/ES. Define sobre a indicação de coleta de exames. [Vitória (ES)]: SESA; maio 2020 [acesso 26 ago 2021]. Disponível em Disponível em https://coronavirus.es.gov.br/Media/Coronavirus/NotasTecnicas/NOTA%20T%C3%89CNICA%20COVID.19%20N.%2005.20.%20Coleta%20de%20Exames.pdf [ Links ]
12. Ministério da Saúde (BR). Acurácia dos testes diagnósticos registrados na ANVISA para a COVID-19. Brasília, DF: MS; maio 2020 [acesso 8 mar 2021]. Disponível em: https://portalarquivos2.saude.gov.br/images/pdf/2020/June/02/AcuraciaDiagnostico-COVID19-atualizacaoC.pdf. [ Links ]
13. World Health Organization. WHO COVID-19 case definition. [Geneva]: WHO; 2020 Dec [acesso 8 mar 2021]. Disponível em: Disponível em: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 . [ Links ]
14. Sola AM, David AP, Rosbe KW, Baba A, Ramirez-Avila L, Chan DK. Prevalence of SARS-CoV-2 infection in children without symptoms of coronavirus disease 2019. JAMA Pediatr. 2021 Feb 1;175(2):198-201. doi: https://doi.org/10.1001/jamapediatrics.2020.4095. [ Links ]
15. Center for Health Security. Public health principles for a phased reopening during COVID-19: guidance for governors. [Baltimore (MD): Johns Hopkins University; 2020 Apr 17 [acesso 26 ago 2020]. Disponível em Disponível em https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2020/200417-reopening-guidance-governors.pdf [ Links ]
16. Gomes CG, Cerutti Júnior C, Zandonade E, Maciel ELN, Alencar FEC, Almada GL, et al. A population-based study of the prevalence of COVID-19 infection in Espírito Santo, Brazil: methodology and results of the first stage. medRxiv 2020.06.13.20130559. doi: https://doi.org/10.1101/2020.06.13.20130559. [ Links ]
17. Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021 Mar;21(3):344-353. doi: https://doi.org/10.1016/S1473-3099(20)30882-3. Epub 2020 Dec 8. [ Links ]
18. Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauvé LJ, Vallance BA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. 2021 Feb;103:246-256. doi: https://doi.org/10.1016/j.ijid.2020.11.163. Epub 2020 Nov 20. [ Links ]
19. Auger KA, Shah SS, Richardson T, Hartley D, Hall M, WarnimentA, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020 Sep 1;324(9):859-70. doi: https://doi.org/10.1001/jama.2020.14348. [ Links ]
20. European Centre for Disease Prevention and Control. COVID-19 in children and the role of school settings in transmission: first update. Solna: ECDC; 23 Dec 2020 [acesso 26 dez 2020]. Disponível em: Disponível em: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-COVID-19-transmission [ Links ]
21. World Health Organization. Schooling in the time of COVID-19: opening statement at high-level meeting on keeping schools open and protecting all children amid surging COVID-19 cases. Copenhagen: 8 Dec 2020 [acesso 15 jan 2021]. Disponível em: Disponível em: https://www.euro.who.int/en/media-centre/sections/statements/2020/schooling-in-the-time-of-COVID-19-opening-statement-at-high-level-meeting-on-keeping-schools-open-and-protecting-all-children-amid-surgingCOVID-19-cases [ Links ]
22. European Centre for Disease Prevention and Control. Risk related to the spread of new SARS-CoV-2 variants of concern in the EU/EEA: first update. Stockholm; 2021 Jan 21 [acesso 15 jan 2021]. Disponível em: Disponível em: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-risk-related-to-spread-of-new-SARS-CoV-2-variants-EU-EEA-first-update.pdf [ Links ]
Received: December 17, 2020; Accepted: April 08, 2021











 texto en
texto en 


