Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.31 no.1 Brasília 2022 Epub 13-Abr-2022
http://dx.doi.org/10.1590/s1679-49742022000100013
ORIGINAL ARTICLE
Visceral leishmaniasis in Piauí, Brazil, 2007-2019: an ecological time series analysis and spatial distribution of epidemiological and operational indicators
1Universidade Federal do Piauí, Centro de Ciências da Saúde, Teresina, PI, Brasil
2Universidade Estadual do Piauí, Centro de Ciências da Saúde, Teresina, PI, Brasil
3Universidade Federal do Piauí, Centro de Inteligência em Agravos Tropicais Emergentes e Negligenciados, Teresina, PI, Brasil
Objective
To analyze epidemiological and operational indicators, the temporal trend and spatial distribution of visceral leishmaniasis (VL), as well as VL-HIV co-infection in the state of Piauí, Brazil, from 2007 to 2019.
Methods
This was an ecological time series study. Prais-Winsten regression was used to analyze the trend of VL incidence, case fatality ratio and operational indicators.
Results
Mean VL incidence in the state was 6.03/100,000 inhabitants, with a rising trend in the 40-59 age group [annual percent change (APC) = 3.88; 95%CI 0.49;7.40] and in the regions located in the south of the state: Tabuleiros do Alto Parnaíba (APC = 14.19; 95%CI 3.91;25.50) and Chapada das Mangabeiras (APC = 12.15; 95%CI 6.69;24.96). Mean case fatality ratio was 6.02% and it remained stable. The mean rate of progression to cure was 52.58%, with a falling trend (APC = -5.67; 95%CI -8.05; -3.23).
Key words: Leishmaniasis, Visceral; Epidemiology; Indicators of Morbidity and Mortality; Disease Notification; Health Information Systems; Time Series Studies
INTRODUCTION
| Study contributions | |
|---|---|
| Main results | Visceral Leishmaniose (VL) predominated in males and children, was associated with social vulnerability and was a significantly underreported disease. Moreover, it had a strong endemic trend in most of the state of Piauí. |
| Implications for services | This study offers health services a broad panorama of the VL profile in Piauí, both in relation to the population most affected by the disease and also the locations where the government needs to be more incisive in promoting health actions. |
| Perspectives | Greater efforts are expected from the government to change the epidemiological scenario of VL in Piauí. Actions to combat the disease should be adopted, focusing on the main vulnerabilities presented in this study. |
Visceral leishmaniasis (VL) is a communicable disease with worldwide distribution, typically associated with poor living conditions. According to World Health Organization data, 83 countries or territorial areas are currently considered endemic, or have reported cases of VL; more than 95% of new VL cases globally are concentrated in just ten countries, including Brazil.1 It is estimated that 50,000 to 90,000 new cases of this disease occur each year, although only 25% to 45% of them are reported.2
Historically known as a rural endemic, VL has led to epidemics in several large Brazilian cities in recent decades, making the disease a serious public health problem.3 There is evidence of the relationship between VL and the country’s increasing deforestation and urbanization process, along with human interference in wildlife habitats, leading to the rapid spread of the disease in cities.4 Certain problems such as low income, lack of medical care and the population having little knowledge about the disease, consequences of the lack of public policies or inefficiency thereof, contribute to the persistence of endemic situations.5 Another relevant issue is the high frequency of VL and human immunodeficiency virus (HIV) coinfection, due to the high prevalence of the latter when associated with the increase in VL cases, this being a reason for concern among health professionals, including those who work in epidemiological surveillance, given the severity of the cases and the rapid clinical progression of HIV-positive individuals to manifestation of acquired immunodeficiency syndrome (AIDS).6
VL is caused by parasites of the Leishmania donovani complex, including the species that causes the disease in Brazil, namely Leishmania infantum. The mosquito species Lutzomyia longipalpis and Lutzomyia cruzi, both belonging to the sand fly subfamily, are the two main vectors of VL and transmit the disease to humans during infected female sand fly blood meals.7
Brazil concentrated 97% of VL cases in the Americas in 2019, portraying the seriousness of the problem for the country with regard to its surveillance and control. In the Brazilian territory, during the period from 2007 to 2017, the VL incidence coefficient ranged from 1.7 to 2.0 cases per 100,000 inhabitants, while its case fatality ratio increased from 5.9% to 8.8%: the highest case fatality ratio occurred in 2015 and 2016, and the number of VL deaths corresponded to 9% of total deaths in the 2007-2016 ten-year period.8
The Plan of Action to Control Leishmaniasis in the Americas was approved in 2017. It aims to reduce morbidity and mortality by strengthening the diagnosis, treatment, rehabilitation, prevention, surveillance and control of infection by the year 2022.8
VL is expanding in Brazil. Its occurrence is highest in the Northeast region of the country, where 56.7% of diagnosed cases were recorded in 2019, with the state of Piauí accounting for 9.6% of Brazil’s VL case notifications.9 The VL incidence rate in Piauí was 5.9/100,000 inhab. in 2018, this being three times higher than the national average of 1.85/100,000 inhab. in the same year.10
The objective of this study was to analyze epidemiological and operational indicators, the temporal trend and spatial distribution of visceral leishmaniasis (VL), as well as VL-HIV co-infection in the state of Piauí, in the period from 2007 to 2019.
METHODS
Study design and period
This was an ecological time series study using records of new confirmed cases of VL in people resident in Piauí, notified on the Notifiable Health Conditions Information System (SINAN) between 2007 and 2019. Its unit of analysis was the state of Piauí, divided into 11 health regions, each one aggregating municipalities surrounding an urban center with regional health service referral facilities.
Study site
In 2020 Piauí had an estimated population of 3,281,480 inhabitants, population density of 12.4 inhab. per km2 and a human development index of 0.646, ranking 24th among the 27 Brazilian states, below the national average of 0.765.
Data source
The SINAN data on notification of confirmed VL cases were obtained between September and October 2020, while the data on the resident population in September 2020 came from projections made by the Brazilian Institute of Geography and Statistics. Both databases are available on the website of the Brazilian National Health System Information Technology Department (DATASUS) and can be accessed using the TabNet tool.11
A ‘new VL case” is defined as confirmation of VL, either by laboratory or clinical-epidemiological criteria, reported for the first time in an individual, or by exacerbation of symptoms after 12 months of clinical cure, provided there is no evidence of immunodeficiency.12 We selected new confirmed leishmaniasis cases notified between 2007 and 2019. The indicators were calculated according to definitions provided in the Indicator Booklet, prepared by the Ministry of Health Technical Group on Leishmaniasis.13
Epidemiological indicators
The VL incidence rate was calculated by dividing the number of new VL cases by the resident population during the study period, multiplied by 100,000 inhabitants. The case fatality ratio was calculated by dividing the number of VL deaths by the total number of new VL cases of visceral leishmaniasis multiplied by 100. Percentage laboratory confirmation was calculated by dividing the number of laboratory-confirmed VL cases by the total number of new VL cases multiplied by 100.
Variables
The following sociodemographic variables were analyzed: sex (male; female); age group (in years: 0-4; 5-9; 10-19; 20-39; 40-59; 60 or over); race/skin color (white; black; yellow; brown; indigenous; no information); schooling (illiterate/elementary education; high school education; higher education); zone of residence (urban; rural/periurban; no information); and health region in the state (Carnaubais; Chapada das Mangabeiras; Cocais; Entre Rios; Planície Litorânea; Serra da Capivara; Tabuleiros do Alto Parnaíba; Vale do Canindé; Vale do Rio Guaribas; Vale do Sambito; Vale dos Rios Piauí e Itaueiras).
The following clinical variables were analyzed: VL-HIV coinfection (yes; no; no information); confirmation criterion (laboratory; clinical-epidemiological); diagnosis via parasitology (positive; negative; not performed); diagnosis via indirect immunofluorescence (positive; negative; not performed); and clinical progression (cure; death from VL; transfer; death from other cause; dropout; no information).
Operational indicators
The proportion of VL cases coinfected with HIV was obtained by dividing the total number of new VL cases in HIV coinfected individuals by the total number of new VL cases multiplied by 100. Progression to clinical cure was calculated by dividing the total number of new VL cases that progressed to clinical cure by the total number of new VL cases multiplied by 100. The proportion of VL cases with unknown progression was calculated according to the quotient between the total number of VL cases (new and recurrent) with unknown progression and the total number of VL cases (new and relapsed) with unknown progression multiplied by 100.
Case characterization was presented in terms of absolute and relative frequencies, with association according to sex being verified by the Mantel-Haenszel chi-square test. The Prais-Winsten linear regression model was applied for the trend analysis, calculating annual percent change (APC) and its 95% confidence intervals (95%CI) using Stata version 14 (StataCorp LP, College Station, USA). The trend of the indicators we analyzed was interpreted as rising (p-value<0.05 and positive regression coefficient), falling (p-value<0.05 and negative regression coefficient), and stable (p-value>0.05); in all cases, statistical significance was attested by a p-value<0.05. The indicators were disaggregated by health region and presented in the form of maps, prepared using the Tabwin application.
RESULTS
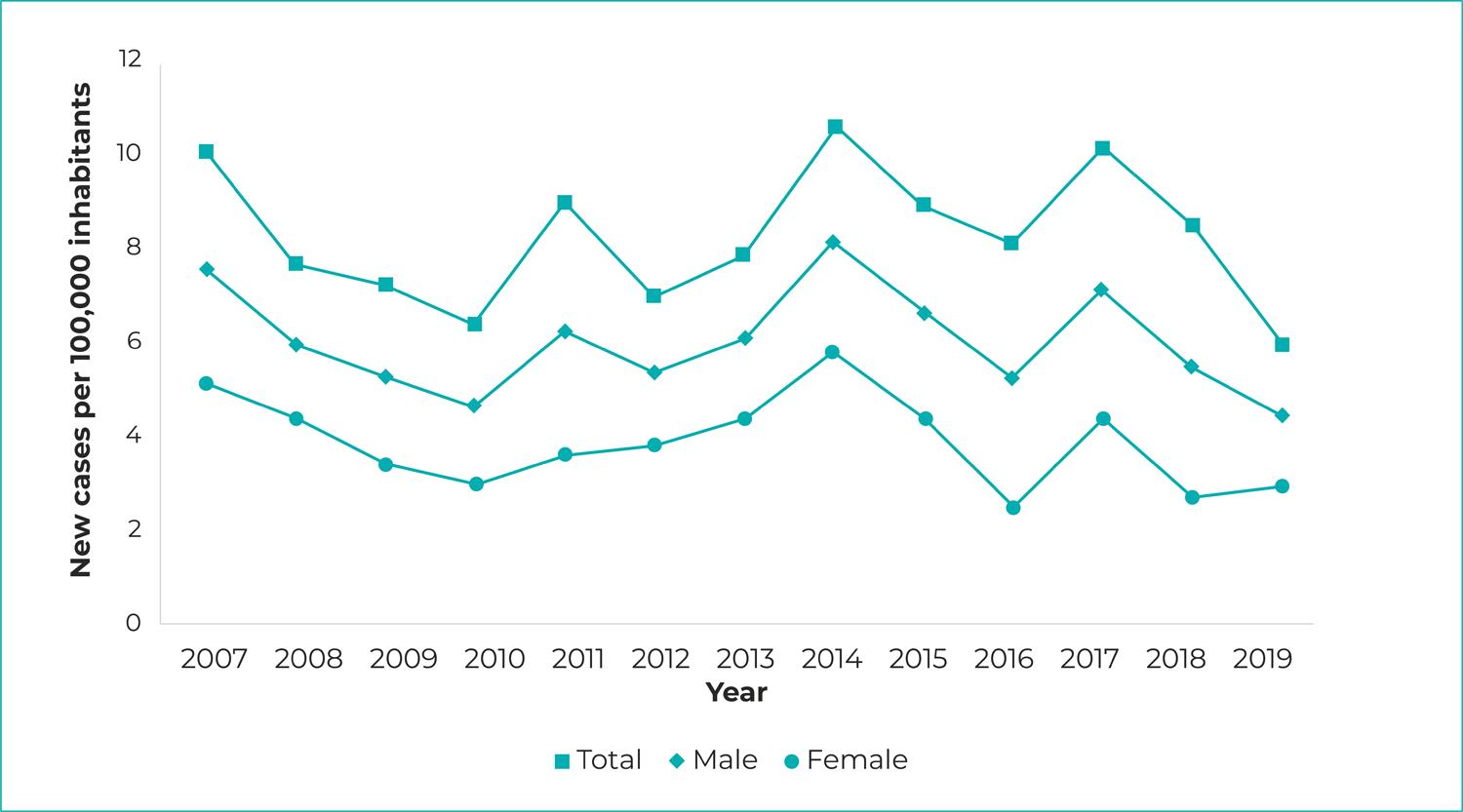
A total of 2,521 new confirmed visceral leishmaniasis cases were notified in Piauí between 2007 and 2019. The year with the highest number of cases in the time series analyzed was 2007, accounting for 237 registered VL cases, while the lowest number of occurrences (145 registered cases) was in 2019. The average number of cases recorded per year was 193.9, with an increase or decrease in notifications from one year to another, with greater positive variation observed in the 2013-2014 two-year period (+66 cases), and greater negative variation in the 2007-2008 two year period (-47 cases).
Among children under 9 years of age, most cases were female (62.4%), while in the 20-59 year age group there was a greater proportion of male cases (50.9%) (p<0.001). There was a predominance of people of black/brown race/skin color (91.7%), people who were illiterate or had elementary education (80.1%) and those living in urban areas (67.9%). With regard to clinical aspects, laboratory-confirmed VL cases prevailed (87.4%), and the percentage of positive parasitology tests was higher among males (56.2%). Regarding diagnosis by indirect immunofluorescence test, a higher proportion of positive results was found in females (27.0%), compared to males (21.5%). Moreover, attention is drawn to the percentage of indirect immunofluorescence tests that were not performed, both in the case of males (67.8%) (p=0.002) and females (62.1%) (p=0.002). The proportion of cases of VL-HIV co-infection was 12.4% (p<0.001) in males, this being higher than that found in females (6.1%). Finally, the proportion of individuals with a record of progression to clinical cure was 52.4% in both sexes. However, there was a considerable percentage of records with no information about this variable (35.9%) (Table 1).
Table 1 – Sociodemographic and clinical characteristics of new visceral leishmaniasis cases (n=2,521), Piauí, 2007-2019
| Variables | Total | Male | Female | p-valuea | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age group (in years) | <0.001 | ||||||
| 0-9 | 1,077 | 42.7 | 557 | 33.0 | 520 | 62.4 | |
| 10-19 | 212 | 8.4 | 143 | 8.5 | 69 | 8.3 | |
| 20-39 | 646 | 25.6 | 529 | 31.3 | 117 | 14.0 | |
| 40-59 | 415 | 16.5 | 331 | 19.6 | 84 | 10.1 | |
| ≥60 | 171 | 6.8 | 128 | 7.6 | 43 | 5.2 | |
| Race/skin color | 0.150 | ||||||
| Black/brown | 2,311 | 91.7 | 1,562 | 92.5 | 749 | 89.9 | |
| White | 115 | 4.6 | 67 | 4.0 | 48 | 5.8 | |
| Yellow | 18 | 0.7 | 10 | 0.6 | 8 | 1.0 | |
| Indigenous | 7 | 0.2 | 6 | 0.4 | 1 | 0.1 | |
| No information | 70 | 2.8 | 43 | 2.5 | 27 | 3.2 | |
| Schoolingb | 0.678 | ||||||
| Illiterate/elementary education | 1,065 | 80.1 | 825 | 79.8 | 240 | 81.1 | |
| High school education | 246 | 18.5 | 200 | 19.3 | 46 | 15.5 | |
| Higher education | 19 | 1.4 | 9 | 0.9 | 10 | 3.4 | |
| Zone of residence | 0.438 | ||||||
| Urban | 1,713 | 67.9 | 1,149 | 68.1 | 564 | 67.7 | |
| Rural/periurban | 726 | 28.8 | 492 | 29.1 | 234 | 28.1 | |
| No information | 82 | 3.3 | 47 | 2.8 | 35 | 4.2 | |
| Confirmation criterion | 0.603 | ||||||
| Laboratory | 2,203 | 87.4 | 1471 | 87.1 | 732 | 87.9 | |
| Clinical-epidemiological | 318 | 12.6 | 217 | 12.9 | 101 | 12.1 | |
| Diagnostic parasitology | 0.002 | ||||||
| Positive | 1,374 | 54.5 | 949 | 56.2 | 425 | 51.0 | |
| Negative | 464 | 18.4 | 315 | 18.7 | 149 | 17.9 | |
| Not performed | 683 | 27.1 | 424 | 25.1 | 259 | 31.1 | |
| Diagnosis using indirect immunofluorescence | 0.002 | ||||||
| Positive | 588 | 23.3 | 363 | 21.5 | 225 | 27.0 | |
| Negative | 271 | 10.7 | 180 | 10.7 | 91 | 10.9 | |
| Not performed | 1,662 | 65.9 | 1,145 | 67.8 | 517 | 62.1 | |
| VLc-HIVd coinfection | <0.001 | ||||||
| Yes | 261 | 10.4 | 210 | 12.5 | 51 | 6.1 | |
| No | 1,906 | 75.6 | 1,265 | 74.9 | 641 | 77.0 | |
| No information | 354 | 14.0 | 213 | 12.6 | 141 | 16.9 | |
| Clinical progression | 0.071 | ||||||
| Cure | 1,321 | 52.4 | 873 | 51.7 | 448 | 53.8 | |
| Death from VLc | 153 | 6.1 | 95 | 5.6 | 58 | 7.0 | |
| Transfer | 110 | 4.4 | 68 | 4.0 | 42 | 5.0 | |
| Death from other cause | 23 | 0.9 | 17 | 1.0 | 6 | 0.7 | |
| Dropout | 9 | 0.3 | 8 | 0.5 | 1 | 0.1 | |
| No information | 905 | 35.9 | 627 | 37.2 | 278 | 33.4 | |
a) Mantel-Haenszel chi-square test; b) Records filled in with ‘not applicable’ (n=990) or ‘no information’ (n=201) were excluded; c) VL: Visceral leishmaniasis; d) HIV: Human immunodeficiency virus.
The mean VL incidence rate in the study period was 6.03/100,000 inhab., ranging from 7.57 in 2007 to 4.43 in 2019. A rising trend in this indicator was observed in the Chapada das Mangabeiras region (APC = 12.15; 95%CI 6.69;24.96) and the Tabuleiros do Alto Parnaíba region (APC = 14.19; 95%CI 3.91;25.50), located in southern Piauí. A falling trend was only found in the Planície Litorânea region (APC = -8.77; 95%CI -14.45;-2.71). The mean case fatality ratio was 6.02%, ranging from 6.33% in 2007 to 4.14% in 2019, so that the trend was found to be stable (Table 2; Figure 1).
Table 2 – Visceral leishmaniasis incidence rate trend (per 100,000 inhabitants), case fatality ratio trend (%) and operational indicator trend (%), Piauí, 2007-2019
| Variables | Annual percent change (%) | 95%CIa | p-value | Trend | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Epidemiological indicators | |||||
| Overall incidence rate | -1.16 | -4.47 | 2.27 | 0.468 | Stable |
| Incidence rate per health region | |||||
| Carnaubais | 3.52 | -5.81 | 13.77 | 0.437 | Stable |
| Chapada das Mangabeiras | 12.15 | 6.69 | 24.96 | 0.040 | Rising |
| Cocais | -2.47 | -6.76 | 2.01 | 0.246 | Stable |
| Entre Rios | -1.99 | -5.61 | 1.76 | 0.263 | Stable |
| Planície Litorânea | -8.77 | -14.45 | -2.71 | 0.009 | Falling |
| Serra da Capivara | -1.90 | -12.15 | 9.54 | 0.709 | Stable |
| Tabuleiros do Alto Parnaíba | 14.19 | 3.91 | 25.50 | 0.010 | Rising |
| Vale do Canindé | 2.15 | -11.21 | 17.53 | 0.745 | Stable |
| Vale do Rio Guaribas | -1.85 | -6.83 | 3.40 | 0.448 | Stable |
| Vale do Sambito | -2.56 | -21.26 | 20.59 | 0.794 | Stable |
| Vale dos Rios Piauí e Itaueiras | -4.14 | -11.50 | 3.82 | 0.268 | Stable |
| Casa fatality ratio (%) | -2.62 | -6.72 | 1.66 | 0.202 | Stable |
| Operational indicators (%) | |||||
| Laboratory confirmation | 0.58 | 0.29 | 0.87 | 0.001 | Rising |
| HIVb,c testing | 0.66 | -0.20 | 1.53 | 0.120 | Stable |
| Parasitology | -1.48 | -2.94 | 0.00 | 0.050 | Stable |
| Immune testing | -10.69 | -18.59 | -2.03 | 0.021 | Falling |
| VLd-HIVb,c coinfection | 1.90 | -2.34 | 6.33 | 0.347 | Stable |
| Progression to clinical cure | -5.67 | -8.05 | -3.23 | <0.001 | Falling |
| Progression unknown | 7.02 | 2.18 | 12.09 | 0.008 | Rising |
a) 95%CI; 95% confidence interval (LL = lower limit; UL = upper limit); b) Data with effect from 2008; c) HIV: Human immunodeficiency virus; d) VL: Visceral leishmaniasis.
Regarding the VL operational indicators, there was an increase in the percentage of cases with laboratory confirmation (APC = 0.58; 95%CI 0.29;0.87) and in the percentage of cases with unknown information about clinical progression (APC = 7.02; 95%CI 2.18;12.09). There was a downward trend in the percentage of cases with immune testing (APC = -10.69; 95%CI -18.59;-2.03) and in progression to clinical cure (APC = -5.67; 95%CI -8.05;-3.23) (Table 2; Figure 2).
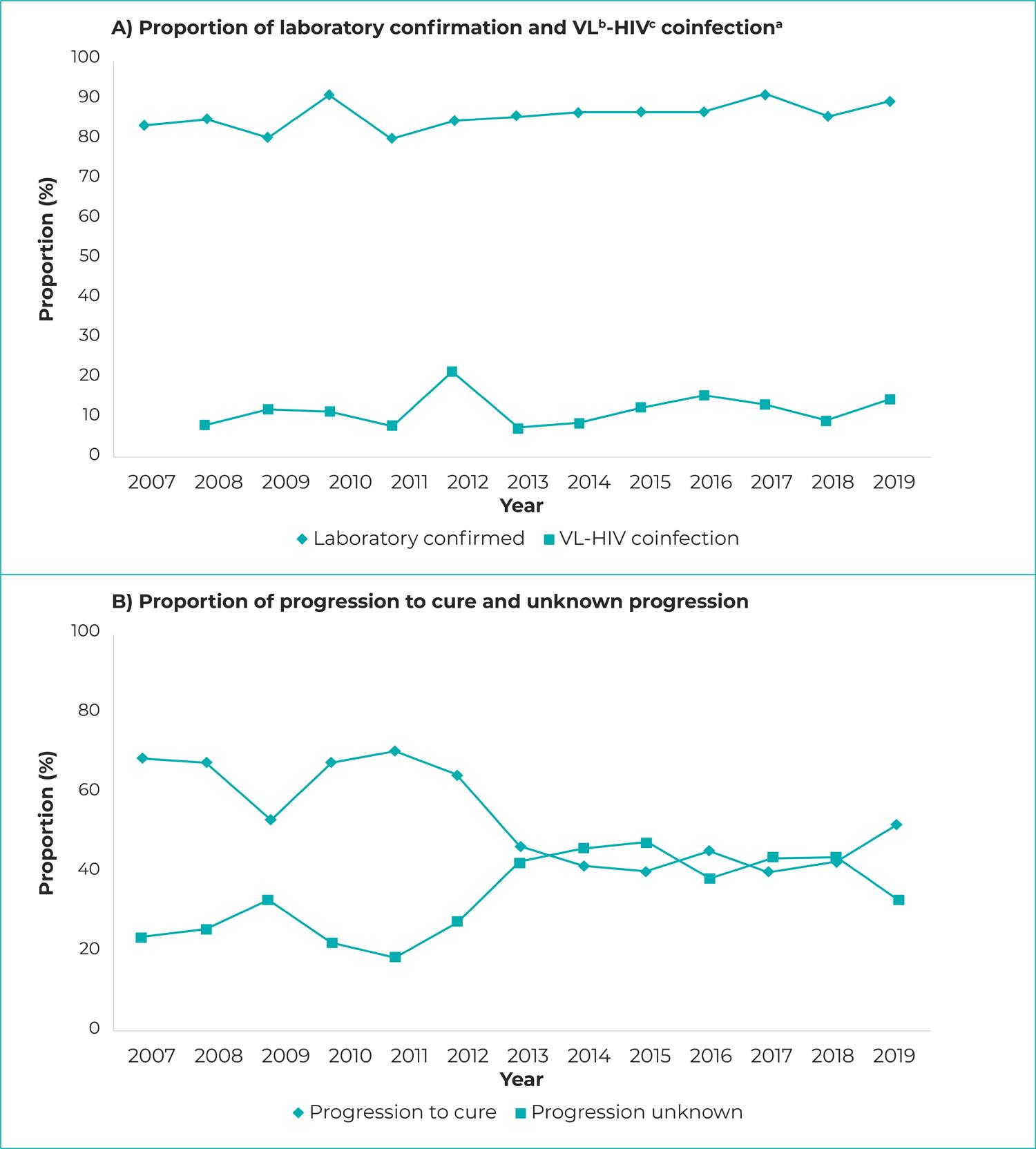
Figure 2 – Visceral leishmaniasis operational indicator proportion progression and VL-HIV coinfection case proportion progression, Piauí, 2007-2019a) Data with effect from 2008; b) VL: Visceral leishmaniasis; c) HIV: Human immunodeficiency virus.
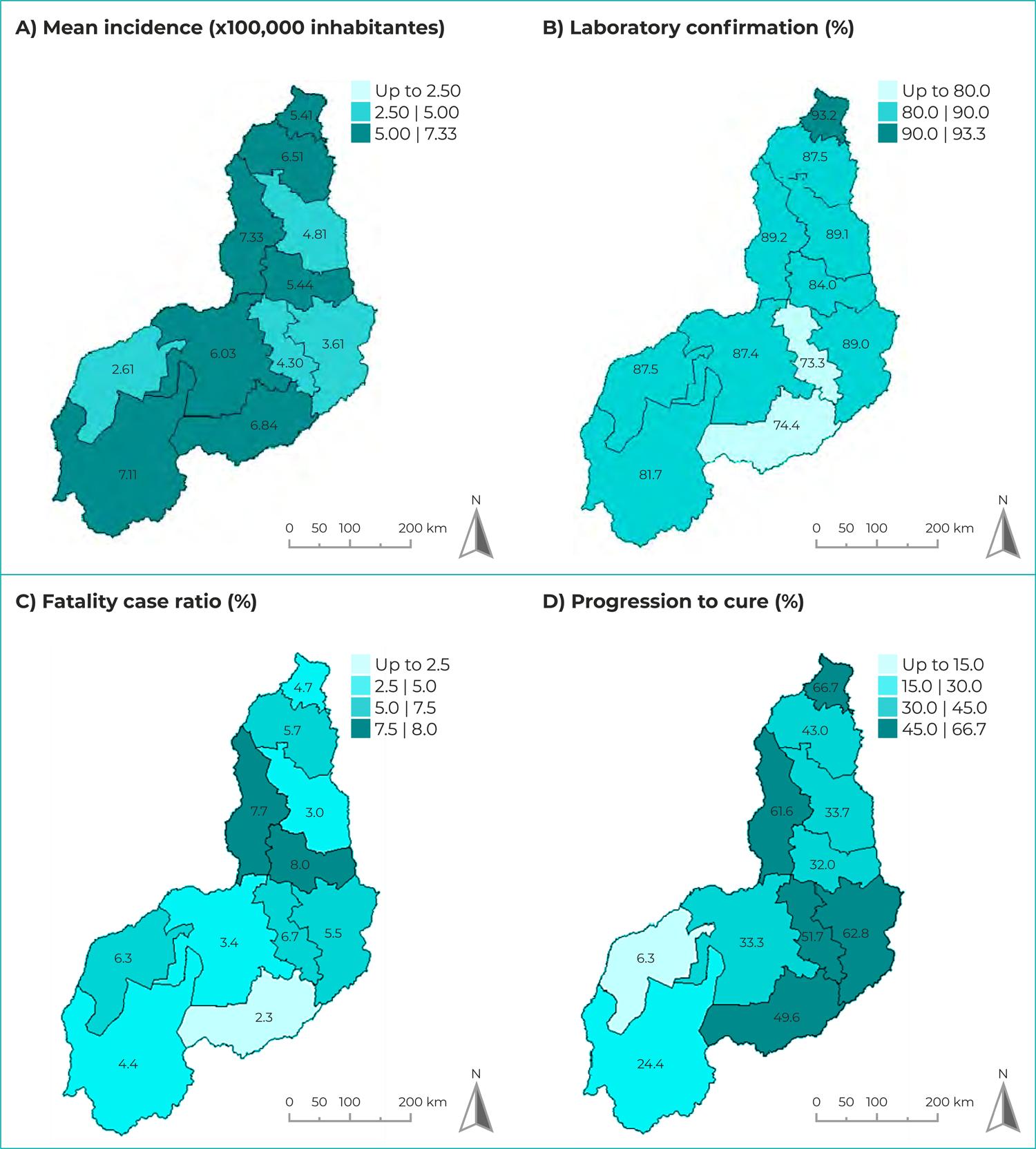
Figure 3 shows the spatial distribution of the VL epidemiological and operational indicators according to the state of Piauí’s health regions. The regions with the highest mean VL incidence rates were Entre Rios, where the capital Teresina is located (7.33/100,000 inhab.), Chapada das Mangabeiras (7.11/100,000 inhab.) and Serra da Capivara (6.84/100,000 inhab.) in the south, and Cocais (6.51/100,000 inhab.) in the northern region of the state. The highest proportion of laboratory confirmed cases corresponded to the Planície Litorânea region (93.2%), while only two regions had laboratory confirmation lower than 80%: Serra da Capivara and Vale do Canindé. The highest mean case fatality ratios were found in the Vale dos Rios Piauí e Itaueiras region (8.0%) and the Entre Rios region (7.7%), with the lowest ratio corresponding to the Serra da Capivara region (2.3%). A higher proportion of progression to cure was found in the northern region of the state, mainly in the Planície Litorânea region (66.7%) and the Entre Rios region (61.6%). Piauí’s health region with the lowest cure rate was Tabuleiros do Alto Parnaíba (6.3%).
DISCUSSION
The study analyzed the epidemiological context of VL in Piauí over a 13 year period. The state has high occurrence of VL cases, and factors such as socioeconomic inequalities and environmental conditions have contributed to the increase in cases of the disease.14
A higher proportion of VL was observed in males and in people aged between 20 and 59 years. These data corroborate results of studies conducted in the city of Teresina,15 and in the northern region of the state of Minas Gerais,16 where higher proportions of VL were identified, 67% and 64% respectively, in males and in people in this age group. Regarding higher VL occurrence among males, there is a hypothesis that the disease is related to hormonal factors.17 However, other authors suggest that occurrence of VL may be associated with occupational risks.14 It should be noted that both sexes can be affected by VL, and that from the analysis made, one cannot conclude that being of the male sex is a risk factor for infection.
People of black/brown race/skin color were the most affected by the disease, corroborating data from another study carried out in Teresina, in which the authors attributed this result to (i) the difficulty in defining race/skin color in the Brazilian population and (ii) the fact that the largest local population is low-income and of black or brown race/skin color, and coincidentally the group most affected by the disease.17
Most (80%) of the cases occurred in people who were illiterate or had only elementary schooling, thus confirming poverty as one of the determinants of the higher occurrence of VL, since (i) low schooling is a marker of low income and (ii) socioeconomic inequality is high in the state of Piauí. Moreover, the results of the present study confirm the evidence that individuals with low schooling tend to have a lower level of knowledge about VL prevention measures, thus favoring higher incidence of VL cases.18
There was a higher proportion of occurrence of VL in urban areas, which accounted for the greatest growth in cases in relation to other areas. Underreporting in rural areas, as a consequence of lack of resources, infrastructure and availability of diagnostic tests, along with the migratory process, precarious socioeconomic conditions and rising deforestation to build houses, roads and factories, are factors that possibly contributed to the increase of cases in urban regions.19Shortcomings in surveillance activities, such as active tracing and identification of suspected cases, added to the scarcity of qualified financial and human resources, are important factors for the lack of VL control in urban centers.20
Part of the registered cases had VL-HIV coinfection, with higher occurrence among males. Antiretroviral therapy (ART) leads to increased immune response in people living with HIV, reducing opportunistic infections and complications arising from HIV infection. However, low adherence of the HIV-positive population to ART and low demand for care are factors that possibly explain the high frequency of people with this coinfection.21It is therefore important to carry out detailed investigation of cases in order to confirm and/or rule out VL-HIV coinfection, including testing people with suspected or confirmed VL for HIV. Equally important is ensuring treatment of both diseases, considering that leishmaniasis favors the worsening of HIV infection in individuals with VL.10
According to this study, of Piauí’s 11 health regions, Chapada das Mangabeiras and Tabuleiros do Alto Parnaíba are those that have a rising trend, being regions of agrarian expansion located in the southern part of the state. These results are possibly related to the fact that this rural population has greater social vulnerability and lower levels of knowledge about prevention measures.14
A study conducted in one of Piauí’s municipalities suggested that expansion of VL in urban-rural transition areas suggests that expansion of peripheral neighborhoods in cities may be a determining factor for the persistence of the disease in urban areas.22Furthermore, occupying recently deforested areas means that humans can come into closer contact with the breeding environment of the vector that causes the disease and with wildlife reservoirs bearing the parasite.23
The high rates of VL in different regions of the state of Piauí can also be explained by the absence of prevention and control measures in part of the municipalities, contrary to Ministry of Health recommendations, besides the lack of trained human resources at the local level.16Sousa et al.24 emphasized that according to studies conducted in Brazil, the VL control measures implemented thus far have not been capable of eliminating the transmission of the disease nor preventing the occurrence of new endemics.
The Vale dos Rios Piauí e Itaueiras region and the Entre Rios region had the highest VL case fatality ratios. In relation to Brazil as a whole,20 the highest VL case fatality ratio (9%) in the last ten years was registered in 2019. This data is ratified by a study conducted in Piauí,14 between 2015 and 2017, which found a case fatality ratio greater than 7%. The case fatality ratio in the Norte de Minas health macro-region,4 between 2011 and 2015, of around 8%, was equally high. Late diagnosis and the increase in the number of cases in people who have comorbidities are factors that can explain the high case fatality ratio found, with infectious complications and hemorrhages being the main causes of death from VL.16
We found a reduction in cases that progressed to cure over the years studied, reaching the lowest percentage (33.1%) in 2019. Otherwise, there was an increase in the frequency of notifications with unknown information about this outcome, which also reached a higher percentage in 2019. These data suggest that this parameter is distorted because of these information losses. Another study conducted in Piauí,14 between 2015 and 2017, found similar results to this research, having reported a cure rate above 39%. Another study points out that there is a falling trend in progression to cure among children under 1 year old and is significantly low in individuals over 60 years old.26 Furthermore, the proportional increase in unknown information can contribute to a reduction in the clinical cure rate.
The limitations of this study relate to it being based on data from secondary sources, which are subject to inaccurate information and underreporting, duplicate records and/or errors in filling out the forms.
Based on the results presented, we conclude that VL remains a neglected disease in Piauí, with a worrisome rising incidence trend and a high percentage of cases under treatment with unknown progression, suggesting failures in actions for VL care, surveillance and control in the state.
Studies that have analyzed VL in the state of Piauí are still scarce. In this sense, this analysis contributes to greater knowledge of the epidemiological situation of VL in the state of Piauí, and consequently, to the identification of areas at risk of vector transmission and needing entomological and zoonotic surveillance. Considering the rising trend of VL in some of the state’s health regions, further studies are needed in these regions in order to identify the conditioning factors of this health condition and ultimately eliminate transmission of the disease.
REFERENCES
1. World Health Organization. Leishmaniasis. Key facts. 20 May 2021. Geneva: World Health Organization; 2021 [citado 2021 jul 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis [ Links ]
2. Sevá AP, Mao L, Galvis-Ovallos F, Lima JMT, Valle D. Risk analysis and prediction of visceral leishmaniasis dispersion in São Paulo State, Brazil. Plos Negl Trop Dis. 2017;11(2).e0005353. doi: 10.1371/journal.pntd.0005353 [ Links ]
3. Moreira CM, Segundo AS, Carvalhosa AA, Estevam LS, Pereira SA, Moreira AM. Comportamento geoespacial da leishmaniose tegumentar americana no município de Tangará da Serra–MT. J Health Sci. 2016; 18(3):171-6. doi: 10.17921/2447-8938.2016v18n3p171-6 [ Links ]
4. Negrão GN, Ferreira MEMC. Considerações sobre a leishmaniose tegumentar americana e sua expansão no território brasileiro. Rev Percurso. 2014;6(1):147-68. doi: 10.4025/revpercurso.v6i1.21375 [ Links ]
5. Araújo TME, Félix ELS, Araújo OD, Chaves AFCP, Sousa ECCL. Coinfecção leishmaniose visceral-HIV em um estado brasileiro: aspectos sociodemográficos, clínicos e laboratoriais Rev Interd. 2020 [citado 2021 abr 01];13(1):1-13. Disponível em: https://revistainterdisciplinar.uninovafapi.edu.br/index.php/revinter/article/view/1752/pdf_466 [ Links ]
6. Cavalcante FRA, Cavalcante KKS, Florêncio CMGD, Moreno JO, Correia FGS, Alencar CH. Human visceral leishmaniasis: epidemiological, temporal and spacial aspects in Northeast Brazil, 2003-2017. Rev Inst Med Trop Sao Paulo. 62:e12. doi: 10.1590/S1678-9946202062012 [ Links ]
7. Organização Pan-Americana da Saúde. Leishmanioses: informe epidemiológico das Américas [Internet]. Brasília: Organização Pan-Americana de Saúde; 2020 [citado 2021 jun 1]. Disponível em: https://www.paho.org/leishmaniasis [ Links ]
8. Ministério da Saúde (BR). Departamento de Informática do SUS. Datasus: Tabnet - informações de saúde, epidemiológicas e morbidade [Internet]. Brasília: Ministério da Saúde; 2021 [citado 2021 mar 08] Disponível em: http://www2.datasus.gov.br/DATASUS/index.php?%20area=0203 [ Links ]
9. Mendes JR, Lopes AS, Sousa MSC, Silva MJM, Sousa PB, Chagas NS, et al. O Piauí como coadjuvante da leishmaniose visceral brasileira. Braz J Dev. 2020;6(3):11210-9. doi: 10.34117/bjdv6n3-114 [ Links ]
10. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de vigilância em saúde [Internet]. 3. ed. Brasília: Ministério da Saúde; 2019 [citado 2021 fev 10]. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf [ Links ]
11. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças Transmissíveis. Coordenação-Geral de Doenças Transmissíveis. Caderno de indicadores: leishmaniose tegumentar e leishmaniose visceral [Internet]. Brasília: Ministério da Saúde. 2018 [citado 2021 jun 1]. Disponível em: http://portalsinan.saude.gov.br/images/documentos/Agravos/LTA/Indicadores_Leishmanioses_2018.pdf [ Links ]
12. Lemos MHS, Silva WC, Gomes FCS, Lages LP, Costa JO, Assis Júnior JDP, t. al. Epidemiologia das leishmanioses no estado do Piauí. Braz J Surg Clin Res. 2019;25 (2):53-7 [citado 2021 abr 07] Disponível em: https://www.mastereditora.com.br/periodico/20190103_214829.pdf [ Links ]
13. Correia AVGM. Perfil clínico-epidemiológico da leishmaniose visceral em Teresina-PI [dissertação]. Rio de Janeiro (RJ): Fundação Oswaldo Cruz; 2015. Disponível em: https://www.arca.fiocruz.br/bitstream/icict/13944/1/angela_correia_ioc_mest_2015.pdf [ Links ]
14. Farias HMT, Gusmão JD, Aguilar RV, Barbosa SFA. Perfil epidemiológico da leishmaniose visceral humana nas regiões de saúde do norte de Minas Gerais. Enferm Foco. 2019;10(2):90-6. doi: 10.21675/2357-707X.2019.v10.n2.1887 [ Links ]
15. Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8(4): e62390. doi: 10.1371/journal.pone.0062390 [ Links ]
16. Pontes DS, Moraes LCA, Batista MHJ, Luz PK, Silva RS. Aspectos epidemiológicos da leishmaniose visceral humana em Teresina, Piauí. Temas em Saúde. 2020;20(4):110-36. doi: 10.29327/213319.20.4-5 [ Links ]
17. Rocha MBM. Investigação epidemiológica da leishmaniose visceral no município de Sobral, Ceará de 2014 a 2018. SANARE. 2020;19(1):18-25. doi: 10.36925/sanare.v19i1.1283 [ Links ]
18. Sousa NA, Linhares CB, Pires FGB, Teixeira TC, Lima JS, Nascimento MLO. Perfil epidemiológico dos casos de leishmaniose visceral em Sobral-CE, de 2011 a 2015. SANARE. 2018;17(1):51-7. doi: 10.36925/sanare.v17i1.1222 [ Links ]
19. Menezes EG, Santos SRF, Melo GZS, Torrente G, Pinto AS, Goiabeira YNLA. Fatores associados à não adesão dos antirretrovirais em portadores de HIV/AIDS. Acta Paul Enferm. 2018;31(3):299-304. doi: 10.1590/1982-0194201800042 [ Links ]
20. Nascimento L, Andrade EB. Epidemiologia da leishmaniose canina no município de Pedro II, Piauí, entre os anos de 2013 e 2019. Pesqui Ensino Ciênc Exatas Nat. 2021;5:e1623. doi: 10.29215/pecen.v5i0.1623 [ Links ]
21. Batista FMA, Machado FFOA, Silva JMO, Mittmann J, Barja PR, Simioni AR. Leishmaniose: perfil epidemiológico dos casos notificados no estado do Piauí entre 2007 e 2011. Revista Univap. 2014;20(35):44-55. doi: 10.18066/revunivap.v20i35.180 [ Links ]
22. Sousa RLT, Nunes MI, Freire SM. Perfil epidemiológico de pacientes com leishmaniose visceral notificados em hospital de referência em Teresina – PI. RIES. 2019;8(1):126-35. doi: 10.33362/ries.v8i1.1475 [ Links ]
23. Santos JP, Silva TPD, Lima DWG, Mendonça IL. Leishmaniose visceral no município de Bom Jesus, Piauí, Brasil. Acta Vet Bras. 2014;8(4):236-41. doi: 10.21708/avb.2014.8.4.4302 [ Links ]
24. Cavalcante IJM, Vale MR. Aspectos epidemiológicos da leishmmaniose visceral (calazar) no Ceará no período de 2007 a 2011. Rev Bras Epidemiol. 2014;17(4):911-24. doi: 10.1590/1809-4503201400040010 [ Links ]
Received: June 17, 2021; Accepted: December 15, 2021











 texto em
texto em