Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Epidemiologia e Serviços de Saúde
versão impressa ISSN 1679-4974versão On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.31 no.1 Brasília 2022 Epub 22-Abr-2022
http://dx.doi.org/10.1590/s1679-49742022000100029
Original Article
Pertussis incidence in children under 1 year old and relation with maternal vaccination in Brazil, 2008-2018
1Universidade do Sul de Santa Catarina, Tubarão, SC, Brazil
Objetive:
To analyze the impact of maternal vaccination coverage with diphtheria-tetanus-acellular pertussis (Tdap) adsorbed vaccine for adults on pertussis incidence in children under 1 year old in Brazil from 2008 to 2018.
Methods:
This was a descriptive ecological temporal trend study using data from surveillance systems managed by the Brazilian National Health System Information Technology Department (DATASUS). Incidence rates and incidence rate ratios (IRR) were calculated with respective 95% confidence intervals (95%CI).
Results:
There were 20,650 pertussis cases in the study period. In the post-vaccination period there was a 26.6% reduction (IRR = 0.73; 95%CI 0.66;0.82) in pertussis incidence among children aged from 3 months up to but not including 1 year old, and a 63.6% reduction (IRR = 0.36; 95%CI 0.15;0.58) among children from birth to 2 months old.
Conclusion:
The increase in maternal Tdap vaccination coverage coincided with a reduction in pertussis incidence, especially in the birth to two-month-old age group.
Keywords: Whooping Cough; Immunization Schedule; Child; Primary Health Care; Observational Study
Study contributions
Main results
The reduction in pertussis incidence and pertussis hospitalization incidence coincided with the period when Tdap maternal vaccination was introduced in Brazil, suggesting that vaccination had an effect on that reduction.
Introduction
Pertussis, also known as whooping cough, is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis, the incidence and severity of which is typically highest in neonates and infants. Despite vaccination programs, pertussis remains an important public health problem. According to World Health Organization (WHO) estimates, more than 151,000 cases of the disease occurred worldwide in 2018.1,2
Pertussis is treated using macrolide antibiotics, which are more effective when administered at the onset of symptoms. However, as the initial clinical picture is nonspecific, delays can occur in diagnosis and consequently in starting treatment, leading to the disease progressing to more serious conditions. Therefore, prevention of pertussis through immunization is vital, especially for neonates.3
The first symptoms of the disease appear about 5 to 10 days after infection, and the initial clinical picture is similar to that of common flu-like syndrome, mainly characterized by a mild cough. Infants may not have a cough but present apnea and cyanosis. After 1 or 2 weeks, the disease can progress with fits of continuous coughing, leading to vomiting and exhaustion.4 Infants under 6 months of age are more likely to develop the severe form of the disease, which can lead to dehydration, pneumonia, convulsions, brain damage and death.5
In Brazil, the current vaccination routine for children includes 3 doses of diphtheria-tetanus-pertussis adsorbed vaccine, hepatitis B vaccine (recombinant) and Haemophilus influenzae type b (conjugate) (pentavalent) vaccine, administered at 2, 4 and 6 months of age, with boosters of diphtheria-tetanus-pertussis (DTP) adsorbed vaccine at 15 months and 4 years.6 Thanks to this vaccination schedule, the number of pertussis cases, which reached around 40,000 notifications per year in the 1980s, has been reduced to no more than 1,500 cases per year since 2000.7
As of 2001, there has been a global increase in the number of pertussis cases, mainly in children under 1 year old, especially in those less than 3 months old, possibly due to incomplete vaccination.8 In this context, the primary source of infection has been a family member, often the child’s own mother.9
Pertussis reemerged in Brazil in 2011, and 6,368 cases of the disease were reported in 2013.7 Several hypotheses explaining the increase in the number of cases have been raised, including the occurrence of genetic mutations of the bacterium B. pertussis. Another hypothesis to be considered could be reduced immunogenicity of diphtheria-tetanus-pertussis (acellular) adsorbed vaccine, leading to increased transmission of the bacillus among adolescents and adults, and consequently in infants.8
As of November 2014, in order to reduce pertussis incidence and mortality rates in infants, the Brazilian Ministry of Health included the diphtheria-tetanus-pertussis (acellular) (Tdap) adsorbed vaccine as part of the National Vaccination Schedule for Pregnant Women.9 According to this schedule, it is recommended that pregnant women receive 1 dose of Tdap vaccine as of the 20th week of pregnancy; or in the puerperium in the case of women not vaccinated during pregnancy.10
Tdap vaccination aims to protect pregnant women from infection and prevent vertical transmission of B. pertussis to newborns, besides promoting transplacental transfer of higher levels of maternal antibodies to the fetus and, as a result, inducing protection of infants against infection before the first pentavalent dose when they are 2 months old.10,11
Increased incidence of B. pertussis infection with effect from 2011 and the recent inclusion of Tdap as part of the National Vaccination Schedule for Pregnant Women highlight the relevance of pertussis as a public health issue. In addition, lack of data on the occurrence of pertussis cases after the introduction of Tdap vaccination justifies investigations on the topic.
The objective of this study was to analyze the impact of maternal Tdap vaccination on pertussis incidence in children under 1 year old in Brazil from 2008 to 2018.
Methods
This was a descriptive observational ecological temporal trend study. The data of interest for study came from secondary databases provided by the Brazilian National Health System Information Technology Department (DATASUS).12
All pertussis cases in children under 1 year old, deaths and Tdap vaccination records in Brazil from 2008 to 2018 were included in the study.
Data on confirmed pertussis cases per age group (in months), in children under 1 year old, according to year of notification, were obtained from the Notifiable Health Conditions Information System (SINAN), available on the DATASUS website.12,13
Data on vaccination coverage were retrieved from the National Immunization Program online information system, also available on the DATASUS website. The data selected were Immunizations/Tdap for pregnant women/Vaccination coverage, per year, for the period studied.14
Information on pertussis hospitalizations was retrieved from the SUS Hospital Information System (SIH/SUS), also available on the DATASUS website. We selected pertussis hospitalization cases, according to age group and year of occurrence, based on the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems - ICD-10: A37.0.15
Data retrieval and tabulation was performed using free TabWin software after downloading the tabulation program, tabulation files and data files from the DATASUS information systems. The tabulated data were then transferred to Microsoft Excel spreadsheets in order to calculate the indicators and the build graphs and tables.
The reference population used when calculating incidence rates was obtained by dividing the total number of children under 1 year old by 12 and multiplying by 3 for the population from zero to 2 months old, and by 9 for the population aged from 3 months up to but not including 1 year old. This calculation was performed because there were no data on the target population stratified by months of life.13,16
For the purpose of analysis, the study population was divided into 2 age groups: from zero to 2 months old; and from 3 months up to but not including 1 year old. The 0 to 2-month age range was defined considering the average circulation time of maternal-transferred anti-pertussis antibodies in infants, which confer protection against infection in this period until the 1st dose of pentavalent vaccine, scheduled for 2 months of age.11
Annual pertussis incidence was calculated by dividing the number of confirmed cases by the reference population for their respective age group, according to place and year of notification, and the quotient of the division was multiplied by 100,000, in the period from 2008 to 2018. The hospitalization rate among confirmed cases was also calculated.
In order to evaluate the impact of maternal vaccination on pertussis incidence in the study population, we selected the periods 2010-2013 (pre-vaccination) and 2015-2018 (post-vaccination), given that the maternal Tdap vaccination campaign started in 2014. The analysis of the differences in the annual pertussis and hospitalization incidence rates between the pre- and post-vaccination periods was performed by calculating the absolute reduction (pre-vaccination incidence minus post-vaccination incidence) and the relative difference, obtained by calculating percentage reduction, that is, incidence in the pre-vaccination period was divided by the sum of the incidence rates in the 2 periods, and the quotient of the division was multiplied by 100. We calculated the incidence rate ratios (IRR) and their 95% confidence intervals (95%CI).
Since this study is based on data from a public domain and open access database, with no identification of participants or any personal information enabling individual identification or putting data confidentiality at risk, the study project did not need to be submitted to a Human Research Ethics Committee.
Results
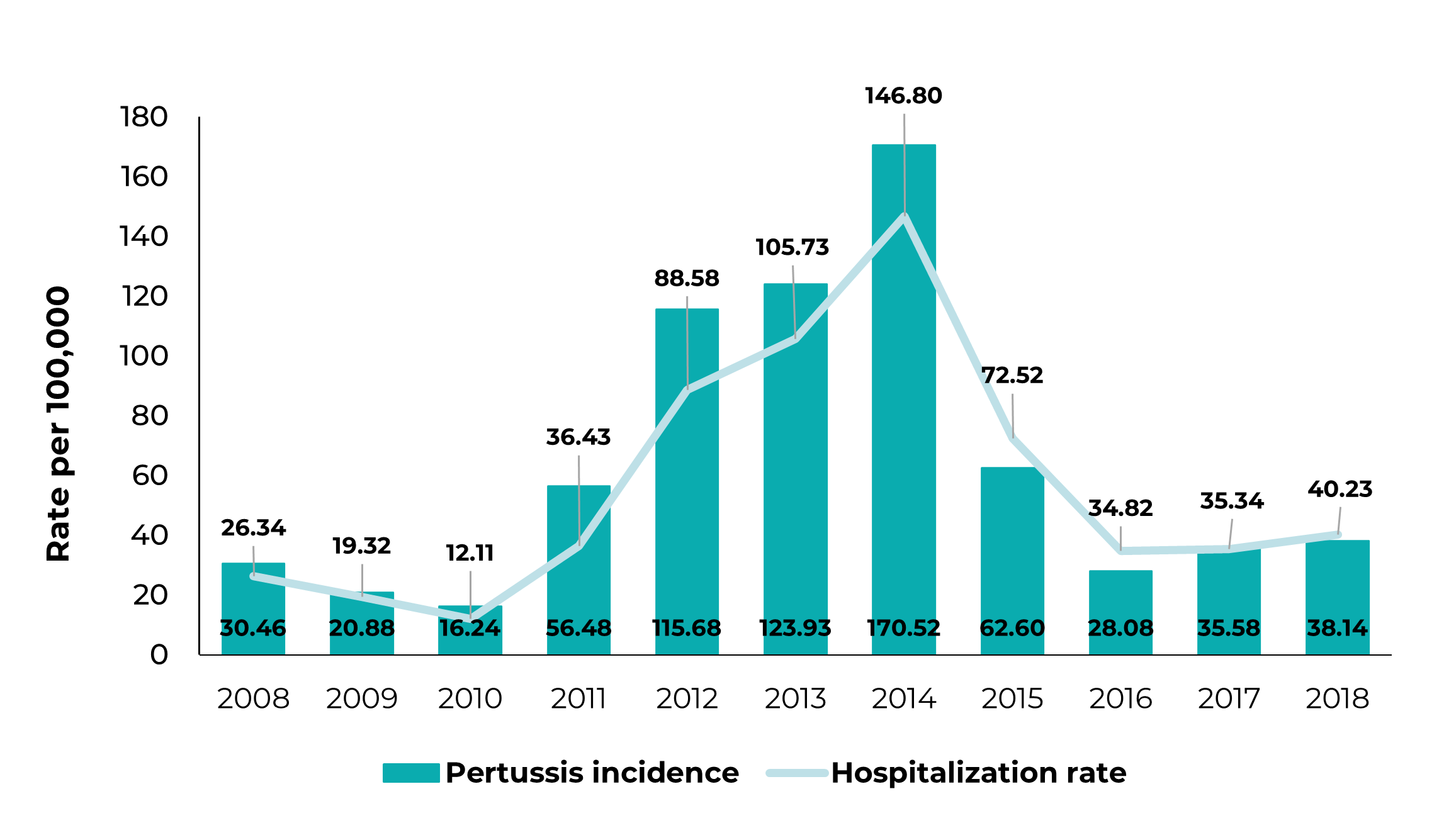
Between January 2008 and December 2018, 20,650 confirmed pertussis cases in children under 1 year of age were reported in Brazil, 10,760 (52%) of which corresponded to children under 3 months old. In 2008, the first year of the series analyzed, 967 cases were reported in children under 1 year old. A peak occurred in 2014, with 5,033 cases, 2,845 of which were children from zero to 3 months old, corresponding to 56% of cases. The incidence rate in children under 1 year old was 30.46 cases per 100,000 in 2008, rising to 170.52 cases/100,000 in 2014 (Table 1).
Table 1 Total number of cases and incidence rate per 100,000 children with pertussis under 1 year old, Brazil, 2008-2018
| Year | Total | <3 months | 3 months to <1 year |
|---|---|---|---|
| n (incidence per 100,000) | n (incidence rate) | n (incidence rate) | |
| 2008 | 967 (30.46) | 554 (69.81) | 413 (17.35) |
| 2009 | 653 (20.88) | 358 (45.80) | 295 (12.58) |
| 2010 | 472 (16.24) | 268 (36.89) | 204 (9.36) |
| 2011 | 1,645 (56.48) | 966 (132.66) | 679 (31.08) |
| 2012 | 3,380 (115.68) | 1,825 (249.85) | 1,555 (70.96) |
| 2013 | 3,610 (123.93) | 2,044 (280.67) | 1,566 (71.68) |
| 2014 | 5,033 (170.52) | 2,845 (385.55) | 2,188 (98.84) |
| 2015 | 1,885 (62.60) | 976 (129.64) | 909 (40.25) |
| 2016 | 828 (28.08) | 109 (14.78) | 719 (32.51) |
| 2017 | 1,041 (35.58) | 408 (55.78) | 633 (28.85) |
| 2018 | 1,136 (38.14) | 407 (54.66) | 729 (32.64) |
| Total | 20,650 (63.01) | 10,760 (113.33) | 9,890 (40.23) |
Maternal Tdap vaccination coverage, which was 0% until 2012, grew progressively from that year on, reaching 60% in 2018 (Figure 1). Starting in 2014, there was a drop in pertussis incidence rates until 2016. In 2017 and 2018, there was an increase in pertussis incidence among those under 3 months old, reaching 55.78/100,000 in 2017; however, the incidence rates were lower than the rate recorded in 2014, namely 385.55/100,000 (Table 1).
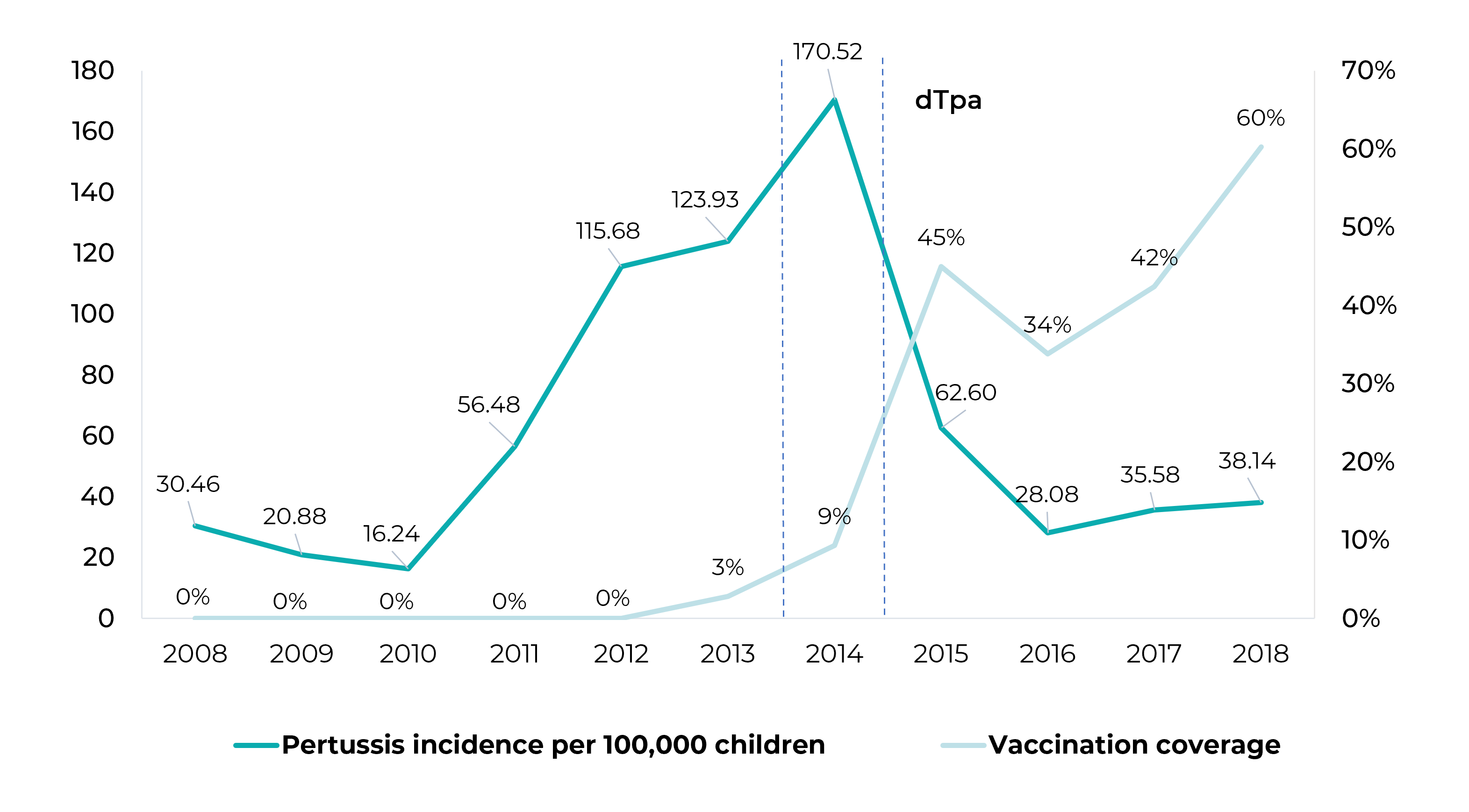
Figure 1 Pertussis incidence rate per 100,000 children under 1 year old and maternal vaccination coverage with diphtheria-tetanus-acellular pertussis (Tdap) adsorbed vaccine for adults, Brazil, 2008-2018
Among children aged from 3 months up to but not including 1 year old, mean annual pertussis incidence was 45.8/100,000 in the period before the start of maternal Tdap vaccination (2010-2013), while mean annual pertussis incidence in the post-vaccination period (2014-2018) was 33.6/100,000, representing 26.6% decrease in incidence (IRR = 0.73; 95%CI 0.66;0.82). The largest drop in incidence rates occurred among children under 3 months old, whereby the relative difference in incidence between the pre- and post-vaccination periods was 63.6% (IRR = 0.36; 95%CI 0.15;0.58), as shown in Table 2.
Table 2 Number of confirmed pertussis cases in children under 1 year old, incidence rate and absolute and relative differences, by age group, Brazil, 2008-2018
| Age | n (incidence per 100,000/year) | Absolute difference in the rate (pre and post-vaccination) | Relative difference (pre and post-vaccination) | ||
|---|---|---|---|---|---|
| 2010-2013 | 2015-2018 | % | IRRa (95%CIb) | ||
| <3 months | |||||
| Mean | 1,276 (175.0) | 475 (63.7) | 111.3 | 63.6 | 0.36 (0.15;0.58) |
| Total | 5,103 (700.1) | 1,900 (254.9) | 445.2 | 63.6 | |
| 3 months to <1 year | |||||
| Mean | 1,001 (45.8) | 747 (33.6) | 12.2 | 26.6 | 0.73 (0.66;0.82) |
| Total | 4,004 (183.1) | 2,990 (134.2) | 48.9 | 26.7 | |
a) IRR: Incidence rate ratio; b) 95%CI: 95% confidence interval.
There was a 24.7% reduction (RR = 0.75; 95%CI 0.55;0.96) in the pertussis hospitalization rate between the pre- and post-vaccination periods in children under 1 year old (Table 3). In 2014, the peak in pertussis incidence was found to coincide with the peak in hospitalization incidence, namely 5,033 pertussis cases and 4,333 hospitalizations (Figure 2).
Table 3 Number of deaths and number of hospitalizations due to pertussis and rate differences in children under 1 year old, by age group, Brazil, 2008-2018
| Age | n (hospitalization per 100,000/year) | Absolute difference in the rate (pre and post-vaccination) | Relative difference (pre and post-vaccination) | ||
|---|---|---|---|---|---|
| 2010-2013 | 2015-2018 | % | IRRa (95%CIb) | ||
| <1 year | |||||
| Mean | 1,770 (60.7) | 1,361 (45.7) | 15.00 | 24.7 | 0.75 (0.55;0.96) |
| Total | 7,081 (242.8) | 5,443 (182.9) | 59.90 | 24.7 | |
a) IRR: Incidence rate ratio; b) 95%CI: 95% confidence interval.
Discussion
The study indicated a reduction in pertussis incidence rates among children under 1 year old in Brazil between 2015 and 2018, this being the period following the start of maternal Tdap vaccination, compared to the period from 2010 to 2013 prior to the implementation of the measure, suggesting a relationship between both events.
As pertussis incidence increases in a cyclical epidemiological pattern, in this study it was not possible to conclude that there was a direct relationship between maternal Tdap vaccination and infant pertussis incidence; however, other research, such as that conducted by Fabricius et al. in 2018 in Argentina, demonstrated this relationship based on epidemiological data and mathematical modeling over the period from 2010 to 2016,16 corroborating the hypothesis of the present study.
Regarding hospitalizations due to pertussis in Brazil, a reduction in the mean annual hospitalization rates per 100,000 was found in children under 1 year old between the pre-vaccine and post-vaccine periods. This finding was corroborated by Desai et al. in a study conducted in Canada in 2018, when they found a decrease in mean annual pertussis hospitalizations, from 165.1 per 100,000 in the period prior to vaccination implementation (1981-1995), to 33.6 per 100,000 in the period from 2006 to 2016, following vaccination implementation, and that reduction was even more pronounced than the 1 we found in our study.17 The results are suggestive of the protective effect of maternal Tdap vaccination in reducing pertussis severity, possibly greater in Canada due to the higher vaccination coverage achieved there.
Other countries have demonstrated the relationship. In Israel, for example, after routine Tdap vaccination in pregnancy was introduced, there was a 59.7% reduction in pertussis incidence and a 49.5% reduction in pertussis hospitalizations. According to the same Israeli study,18 even greater reductions, i.e. 71.2% in pertussis incidence and 58.4% in pertussis-related hospitalizations, were seen in the group aged under 2 months old.
Pertussis is usually especially severe in children under 3 months old, since i) children in this age group are not yet able to produce antibodies against pertussis, and ii) the vaccination schedule only includes anti-pertussis vaccination at 2 months old. In this sense, maternal vaccination is especially important for this population.19
Vaccination of pregnant women with Tdap allows maternal antibodies to be transferred to the fetus via the placenta. Barug et al. demonstrated that maternal vaccination ensures high concentration of circulating pertussis antibodies in the infant until it is 3 months old.20 From this age on, the child’s immune system is more developed and able to produce its own antibodies following administration of the first dose of the vaccine at 2 months old.
Regarding safety, Vygen-Bonnet et al. conclude that maternal vaccination is indeed safe for the fetus and the mother and has a positive benefit-risk ratio.21 According to that study, efficacy of maternal vaccination ranges from 69% to 91% in preventing childhood pertussis, and from 91% to 94% in preventing pertussis-related hospitalization in this population, as well as preventing 95% of fatal outcomes. Pertussis vaccine is recommended for pregnant women at 26 to 36 weeks of pregnancy, the earlier the better.
A study conducted in Brazil by Friedrich et al. with a similar design and adopting the same target period as the present study, also reported a reduction in the pertussis incidence rate in the post-vaccination period.22 However, Friedrich et al. took 1 month of age as the limit for dividing the 2 groups and found a 47.7% decrease in mean annual pertussis incidence in the group aged from zero up to but not including 1 month, while the reduction in the group aged from 1 month up to but not including 1 year was 54.8%.
In order to obtain more reliable results on the impact of maternal vaccination on children who have not yet been vaccinated, this study used 3 months of age as the limit for dividing the groups. Thus, even more promising results were obtained, indicating the greater effectiveness of maternal vaccination in preventing pertussis in children up to 3 months old, precisely the age group most affected by pertussis and for whom maternal vaccination is intended.
This study has limitations. It depends on correct notification by epidemiological surveillance services with regard to identifying suspected and confirmed pertussis cases, as well as correct diagnosis by attending physicians. These variables contribute to increased underreporting of data. Furthermore, as mentioned above, pertussis is characterized by cyclical occurrence and, as Brazilian vaccination coverage is still low, the need exists to carry out studies with longer monitoring time, in order to prove the associations found. Finally, this is an ecological study, and thus the relationship between maternal vaccination and non-occurrence of pertussis cannot be analyzed at the individual level. However, the reduction in pertussis incidence and in pertussis hospitalization incidence, coincided with the introduction of maternal Tdap vaccination, suggesting the effect of vaccination on this reduction, as reported in the medical literature and in accordance with the global trend presented by pertussis.
This study suggests the promising effect of the maternal Tdap vaccination policy in Brazil, especially in children under 3 months old, and this practice should be encouraged in prenatal care. It is expected that dissemination of the results found will contribute to greater adherence to prenatal vaccination policies, both by attending physicians and also by pregnant women.
Referências
1. World Health Organization. Pertússis [Internet]. Geneva: World Health Organization; 2020 [cited 2021 mar 19]. Available from: https://www.who.int/health-topics/pertússis#tab=tab_1 [ Links ]
2. Vaz-de-Lima LRA, Sato HK, Fernandes EG, Sato APS, Pawloski LC, Tondella ML, et al. Association between the timing of maternal vaccination and newborns' anti-pertússis toxin antibody levels. Vaccine. 2019;37(36):5474-80. doi: 10.1016/j.vaccine.2019.04.079 [ Links ]
3. Argondizo-Correia C, Rodrigues AKS, Brito CA. Neonatal immunity to Bordetella pertússis infection and current prevention strategies. J Immunol Res. 2019;2019:7134168. doi: 10.1155/2019/7134168 [ Links ]
4. Centers for Disease Control and Prevention. Summary of notifiable diseases - United States, 2008 [Internet]. Atlanta: Centers for Disease Control and Prevention; 2008 [update 2010 june 25; cited 2021 mar 19]. (Morbidity and Mortality Weekly Report, vol. 57, nº 54). Available from: https://www.cdc.gov/mmwr/pdf/wk/mm5754.pdf [ Links ]
5. Fundação Oswaldo Cruz. Coqueluche: sintomas, transmissão e prevenção [Internet]. Rio de Janeiro: Fundação Oswaldo Cruz; 2018 [atualizado 2018 jun 14; citado 2020 mar 20]. Disponível em: https://www.bio.fiocruz.br/index.php/br/coqueluche-sintomas-transmissao-e-prevencao#:~:text=É%20causada%20pela%20bactéria%20Bordetella,acessos%20de%20tosse%20seca%20cont%C3%ADnua [ Links ]
6. Ministério da Saúde (BR). Calendário nacional de vacinação [Internet]. Brasília: Ministério da Saúde; 2020 [citado 2021 mar 19]. Disponível em: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z-1/c/calendario-de-vacinacao [ Links ]
7. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Coordenação Geral do Programa Nacional de Imunizações. Secretaria de Estado da Saúde Santa Catarina. Informe Técnico para implantação da vacina adsorvida difteria, tétano e coqueluche (Pertússis Acelular) tipo adulto - dTpa (Adaptado pelo Programa Estadual de Imunização SC) [Internet]. Florianópolis: Secretaria de Estado da Saúde Santa Catarina; 2019 [citado 2021 mar 19]. Available from: https://www.saude.sc.gov.br/index.php/informacoes-gerais-documentos/redes-de-atencao-a-saude-2/rede-aten-a-saude-materna-e-infantil-rede-cegonha/acervo-e-e-books/10411-nota-tecnica-vacinacao-para-gestantes-dtpa-prevencao-de-coqueluche-em-menores-de-1-ano/file [ Links ]
8. Guimarães LM, Carneiro ELNC, Carvalho-Costa FA. Increasing incidence of pertússis in Brazil: a retrospective study using surveillance data. BMC Infect Dis. 2015;15:442. doi: 10.1186/s12879-015-1222-3 [ Links ]
9. Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of Infant pertússis infection in the United States. Pediatrics. 2015;136(4):635-41. doi: 10.1542/peds.2015-1120 [ Links ]
10. Sociedade Brasileira de Imunização. Calendário de vacinação da gestante [Internet]. [São Paulo]: Sociedade Brasileira de Imunização; 2021 [citado 2021 mar 19]. Disponível em: http://sbim.org.br/images/calendarios/calend-sbim-gestante.pdf [ Links ]
11. Gkentzi D, Katsakiori P, Marangos M, Hsia Y, Amirthalingam G, Heath PT, et al. Maternal vaccination against pertússis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F456-F463. doi: 10.1136/archdischild-2016-312341 [ Links ]
12. Ministério da Saúde (BR). Departamento de Informática do Sistema Único de Saúde do Brasil. Coqueluche - Casos confirmados notificados no Sistema de Informação de Agravos de Notificação - Brasil. [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2021 mar 20]. Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/coquebr.def [ Links ]
13. Instituto Brasileiro de Geografia e Estatística. Censo populacional [Internet]. Brasília: Instituto Brasileiro de Geografia e Estatística; 2021 [citado 2021 mar 20]. Disponível em: https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html?edicao=21830&t=resultados [ Links ]
14. Ministério da Saúde (BR). Sistema de Informação do Programa Nacional de Imunização (SI-PNI). dTpa para gestantes [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2021 mar 20] Disponível em: http://tabnet.datasus.gov.br/cgi/dhdat.exe?bd_pni/cpnibr.def [ Links ]
15. Ministério da Saúde (BR). Departamento de Informática do Sistema Único de Saúde do Brasil. Morbidade Hospitalar do SUS - por local de internação - Brasil [Internet]. Brasília: Ministério da Saúde; 2018 [citado 2021 mar 20]. Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/niuf.def [ Links ]
16. Fabricius G, Martin Aispuro P, Bergero P, Bottero D, Gabrielli M, Hozbor D. Pertússis epidemiology in Argentina: TRENDS after the introduction of maternal immunisation. Epidemiol Infect. 2018;146(7), 858-66. doi: 10.1017/S0950268818000808 [ Links ]
17. Desai S, Schanzer DL, Silva A, Rotondo J, Squires SG. Trends in Canadian infant pertússis hospitalizations in the pre- and post-acellular vaccine era, 1981-2016. Vaccine. 2018;36(49):7568-73. doi: 10.1016/j.vaccine.2018.10.047 [ Links ]
18. Langsam D, Anis E, Haas EJ, Gosinov R, Yechezkel M, Grotto I, et al. Tdap vaccination during pregnancy interrupts a twenty-year increase in the incidence of pertússis. Vaccine. 2020;38(12):2700-6. doi: 10.1016/j.vaccine.2020.01.095 [ Links ]
19. Faucette AN, Pawlitz MD, Pei B, Yao F, Chen K. Immunization of pregnant women: future of early infant protection. Hum Vaccin Immunother. 2015;11(11):2549-55. doi: 10.1080/21645515.2015.1070984 [ Links ]
20. Barug D, Pronk I, van Houten MA, Versteegh FGA, Knol MJ, van de Kassteele J, et al. Maternal pertússis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392-401. doi: 10.1016/S1473-3099(18)30717-5 [ Links ]
21. Vygen-Bonnet S, Hellenbrand W, Garbe E, von Kries R, Bogdan C, Heininger U, et al. Safety and effectiveness of acellular pertússis vaccination during pregnancy: a systematic review. BMC Infect Dis. 2020;20(1):136. doi: 10.1186/s12879-020-4824-3 [ Links ]
22. Friedrich F, Valadão MC, Brum M, Comaru T, Pitrez PM, Jones MH, et al. Impact of maternal dTpa vaccination on the incidence of pertússis in young infants. PLoS One. 2020;15(1):e0228022. doi: 10.1371/journal.pone.0228022 [ Links ]
Received: July 06, 2021; Accepted: February 07, 2022











 texto em
texto em