Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.31 no.1 Brasília 2022 Epub 22-Mar-2022
http://dx.doi.org/10.1590/s1679-49742022000100002
Experience Report
Self-collected nasopharyngeal swab and molecular test using pool testing as strategies to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): feasibility in medical students at the Universidade Federal de Minas Gerais, Brazil, 2021
1Universidade Federal de Minas Gerais, Programa de Pós-Graduação em Ciências da Saúde: Infectologia e Medicina Tropical, Belo Horizonte, MG, Brazil
2Universidade Federal de Minas Gerais, Departamento de Medicina Preventiva e Social, Belo Horizonte, MG, Brazil
3Universidade Federal de Minas Gerais, Centro de Tecnologia de Vacinas, Belo Horizonte, MG, Brazil
4Universidade Federal de Minas Gerais, Faculdade de Medicina, Belo Horizonte, MG, Brazil
5Universidade Federal de Minas Gerais, Departamento de Clínica Médica, Belo Horizonte, MG, Brazil
6Universidade Federal de Minas Gerais, Departamento de Matemática, Belo Horizonte, MG, Brazil
7Universidade Federal de Minas Gerais, Departamento de Pediatria, Belo Horizonte, MG, Brazil
Objective
To show the feasibility of the combined use of self-collected nasopharyngeal swab and pool testing to detect SARS-CoV-2 in epidemiological surveys.
Methods
This experience included a sample of 154 students at the Universidade Federal de Minas Gerais, who performed self-collected nasopharyngeal swab in individual cabins and without supervision. The molecular test was performed using the pool testing technique.
Results
It took each person 5 minutes to collect the sample. An analysis was performed to detect endogenous RNA in 40 samples. The results showed that there were no failures resulting from self-collection. None of the pools detected the presence of viral RNA. The cost of molecular testing (RT-PCR), by pool testing, with samples obtained by self-collection was about ten times lower than the usual methods.
Conclusion
The strategies that were investigated proved to be economically feasible and valid for the research on SARS-CoV-2 in epidemiological surveys.
Keywords: COVID-19; Epidemiological Surveys; Pandemics; RT-PCR; SARS-CoV-2
Study contributions
Main results
The cost of performing self-collected nasopharyngeal swab combined with molecular testing (RT-PCR) for detection of SARS-CoV-2 using the pool testing technique was about ten times lower than that of the usual methods involving individual testing.
Implications for services
Self-collected swab is a strategy that requires minimal infrastructure and had good adherence among the participants. Combined with the pool testing technique, it proved to be economically feasible and valid for the research on SARS-CoV-2 in epidemiological surveys.
Perspectives
The self-collection of nasopharyngeal swabs provides good quality samples and, when it is combined with the pool testing technique it can enable the expansion of testing for SARS-CoV-2 and increase control of outbreaks in schools and work setting.
Introduction
COVID-19 (Coronavirus Disease-19), a disease caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), has affected more than 200 million individuals and accounted for more than 4 million deaths worldwide, 13% of them occurred in Brazil.1 Although vaccination in the country started in January 2021, it is important to maintain an epidemiological surveillance system in order to detect possible outbreaks quickly and promote actions to prevent the transmission of the disease.2
There is concern about groups at high risk of contagion, such as the school community when returning to face-to-face activities.3 In the United States, the pooled testing strategy for screening of asymptomatic individuals was implemented at Duke University and in schools in the state of Massachusetts, when they returned to face-to-face activities, aiming to track and stop the spread of the virus.3,4
The pool testing consists of performing the molecular test RT-PCR (Reverse Transcription Polymerase Chain Reaction) simultaneously, in groups of samples.4 This technique has been used in several countries because it is efficient and cost-effective for population-scale testing.3,5,6 In asymptomatic individuals, pool testing helps in early detection, interrupting the transmission chain, especially in groups with higher exposure to SARS-CoV-2.3.6,9
Self-collection also represents a cost-effective option for mass testing, as it does not require trained professionals and personal protective equipment (PPE). Comparative studies of self-collected nasopharyngeal swab samples and those collected by health professionals showed similar results.8,9
Given the urgency to expand COVID-19 testing coverage, the objective of this study was to demonstrate the feasibility of the combined use of self-collected nasopharyngeal swab and pool testing to detect SARS-CoV-2 in epidemiological surveys.
Methods
This experience report, using a cross-sectional design, carried out in February 2021, is a subproject of a prospective longitudinal study conducted in Belo Horizonte, capital city of Minas Gerais state, Brazil, with the objective of assessing the expansion of RT-PCR for SARS-CoV-2 using pooled testing for people with flu-like syndrome.
All students attending the 9th to 12th semesters at the Medical School of the Universidade Federal de Minas Gerais, in compliance with the mandatory hospital internships, were invited to take part in the study. If they agreed, they would answer a questionnaire to characterize the sample with information about age (in years), sex (male; female) and service where they were attending the hospital internship. The questionnaire was answered using the Google Forms. The study participants consisted of convenience sample, without prior sample size calculation.
The participants provided nasopharyngeal swab samples, obtained by self-collection performed without direct supervision, in individual cabins, where there were posters about the technique. The students also received an instructional video about self-collection before the procedure. The swab was discarded in its own container, in the cabins. The tube containing the sample and viral inactivation and transport solution10 was identified, sealed and delivered to the researchers, who were waiting outside the cabins.
To prepare the pools, 47 microliters of each individual sample were added in 1.5 mL microtubes. The samples were processed for RNA extraction, according to the QIAGEN Inc. protocol (Germany). RT-PCR reactions were performed using probes for the endogenous human RNaseP gene and the E gene that encodes the viral envelope, using QuantStudio 5 thermocycler (Applied Biosystems), according to the Charité protocol.11 Regarding the pool testing strategy, if the result from a pool is detectable, it is necessary to process individually each sample present in that grouping.5 The samples were stowed at 4°C.
As described in the literature, when the prevalence of COVID-19 in the community is 1%, the optimal pool size is 11 samples (Box 1).12,13 Taking into consideration that none of the participants was symptomatic at the time of collection, we opted for testing in pools of ten samples, which would be appropriate to detect viral RNA even with very low viral load.12-14 Fifteen pools containing ten samples and one pool of four samples were prepared.
Box 1 Estimation of optimal pool sizes for the performance of RT-PCR for SARS-CoV-2,a according to the prevalence of COVID-19 in the community
| Prevalence of COVID-19 in the community (%) | Optimal pool size (number of samples per pool) |
|---|---|
| 1 | 11 |
| 2 | 8 |
| 3 | 6 |
| 4 | 6 |
| 5 | 5 |
| 6 | 5 |
| 7 | 4 |
| 8 | 4 |
| 9 | 4 |
| 10 | 4 |
| 20 | 3 |
Source: Costa et al.12 and Cherif et al.13. a) RT-PCR (Reverse Transcription Polymerase Chain Reaction) for SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2).
After processing pooled samples, four pools of ten samples were processed individually in order to evaluate possible failures during the self-collection process. At this stage, we tried to verify whether the samples contained individual endogenous RNA. The calculation of the number of samples/pools required for the aforementioned verification was performed using the MatLab software, estimating a 90% probability of detecting at least one inadequate sample if errors had occurred in 5% of the samples.
The results were presented from absolute and relative frequencies. The costs, in reais (BRL), for extraction and reaction of individual and pooled RT-PCR, considering only plastic materials and reagents, were calculated based on the market values in August 2021 and available on biomedical equipment supplier websites. The calculation included the personal protective equipment (PPE) necessary to perform the collection of nasopharyngeal swab samples, when it was performed by a health professional, instead of self-collection. Cost simulation was also performed when some of the pools presented detectable results, using the Microsoft Excel. The simulation took into consideration the cost of the procedures performed in individual or pooled samples, based on the cost of the materials and supplies calculated in the previous step.
The study project was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (CEP/UFMG): Certificate of Submission for Ethical Appraisal (CAAE) No. 35074720.3.0000.5149. All students included in the sample signed the Free and Informed Consent Form in electronic format.
Results
Of the 492 students enrolled in the 9th to 12th semesters, 207 (42.1%) were present on the days previously scheduled by the Education Council to perform academic procedures, and 154 agreed to take part in the study (74.4% of those who were present on the scheduled days). More than half of the participants were male (54.7%) and aged between 20 and 24 years (56.0%). All participants attended a mandatory curricular internship in public hospitals in the city.
Of the 207 students attending the Medical School during the study, 53 refused to perform self-collected swab (25.6%). The main reasons for refusal were: poor experience in performing previous testing (n=12), fear of self-collection (n=12), lack of availability to participate at that time (n=10), recent RT-PCR (n=10) and other reasons (n=9). Figure 1 presents the sample composition steps.
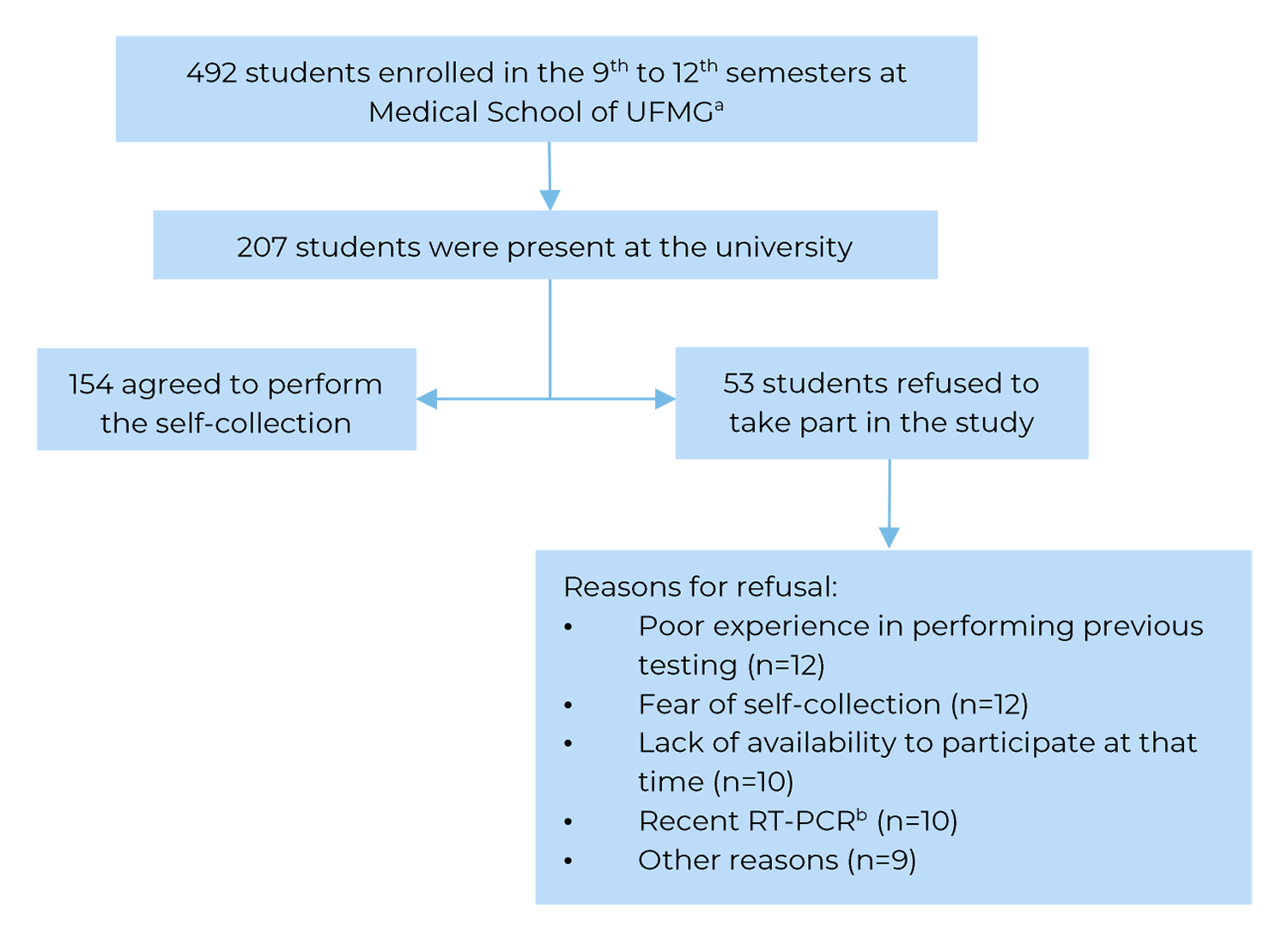
a) UFMG: Universidade Federal de Minas Gerais; b) RT-PCR: Reverse Transcription Polymerase Chain Reaction for SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2).
Figure 1 Flowchart for composition of the study sample, comprised of students enrolled in the 9th to 12th semesters at Medical School of the Universidade Federal de Minas Gerais, attending mandatory hospital internship, Belo Horizonte, Minas Gerais state, Brazil, 2021
It took each person about five minutes to collect the sample. All participants received the test result after two working days. The presence of viral RNA was not detected in any of the pools. Endogenous RNA was properly detected in the 40 samples examined individually in order to analyze the quality of the material obtained by self-collection.
Table 1 presents the costs of the procedure adopted in this study, compared to the costs of the usual procedure (swabs collected by a health care worker and individual sample processing) and other hypothetical scenarios. The final value per sample in this study was about ten times lower than that of usual procedure. The cost per sample increased progressively as more pools presented detectable results. Even if half of the pools needed to be processed individually, performing the self-collection of swab and initial testing in pools of ten samples, the value per sample would correspond to half of the estimated value for the usual procedure.
Table 1 Costs, in reais (BRL), of materials and reagents to perform the procedure adopted in this study,a compared to the usual procedureb and other hypothetical scenarios, for testing students enrolled in the 9th to 12th semesters at the Medical School of the Universidade Federal de Minas Gerais, attending mandatory hospital internship, Belo Horizonte, Minas Gerais state, Brazil, 2021
| Materials and reagents, per procedure | Unit pricee (BRL) | Number of samples | Value of materials and reagents (BRL) | Total (pool + individual tests) (BRL) | Final value per sample (BRL) |
|---|---|---|---|---|---|
| Procedure adopted in the studya | |||||
| Pooled RT-PCR | 36.96 | 16 | 591.36 | 591.36 | 3.84 |
| Usual procedurec | |||||
| PPE for swab collectiond | 3.00 | 154 | 462.00 | 6,153.84 | 39.96 |
| RT-PCR processed individually | 36.96 | 154 | 5,691.84 | ||
| Other hypothetical scenarios using self-collected swab + 150 samples that were processed, initially in pools of ten samples | |||||
| Scenario 1: 14 undetectable pools + 1 detectable pool | 36.96 | 15 | 554.40 | 924.00 | 6.16 |
| 10 samples that were processed individually | 36.96 | 10 | 369.60 | ||
| Scenario 2: 13 undetectable pools + 2 detectable pools | 36.96 | 15 | 554.40 | 1,293.60 | 8.62 |
| 20 samples that were processed individually | 36.96 | 20 | 739.20 | ||
| Scenario 3: 12 undetectable pools + 3 detectable pools | 36.96 | 15 | 554.40 | 1,663.20 | 11.09 |
| 30 samples that were processed individually | 36.96 | 30 | 1,108.80 | ||
| Scenario 4: 11 undetectable pools + 4 detectable pools | 36.96 | 15 | 554.40 | 2,032.80 | 13.55 |
| 40 samples that were processed individually | 36.96 | 40 | 1,478.40 | ||
| Scenario 5: 10 undetectable pools + 5 detectable pools | 36.96 | 15 | 554.40 | 2,402.40 | 16.02 |
| 50 samples that were processed individually | 36.96 | 50 | 1,848.00 | ||
| Scenario 6: 9 undetectable pools + 6 detectable pools | 36.96 | 15 | 554.40 | 2,772.00 | 18.48 |
| 60 samples that were processed individually | 36.96 | 60 | 2,217.60 | ||
| Scenario 5: 8 undetectable pools + 7 detectable pools | 36.96 | 15 | 554.40 | 3,141.60 | 20.94 |
| 70 samples that were processed individually | 36.96 | 15 | 554.40 | 3,141.60 | 20.94 |
a) Self-collected nasopharyngeal swab + RT-PCR (Reverse Transcription Polymerase Chain Reaction for SARS-CoV-2) performed using pool testing; b)Collection of Nasopharyngeal swab by a health professional + individually processed RT-PCR; c) 15 pools with 10 samples and 1 pool with 4 samples (total = 154 participants), all pools showed undetectable result; d) Considering all the personal protective equipment (PPE) necessary for a professional to work 8 hours/day and collect 154 nasopharyngeal swabs (PFF2 mask, face shield, gloves, head, cap and aprons), excluding professional costs for collection; e) Considering the values in August 2021.
Discussion
Regarding the self-collection method, the results showed that (i) most participants adhered to that procedure, (ii) minimal infrastructure was required and (iii) self-collection could be performed quickly, resulting in samples with acceptable quality. The detection of endogenous RNA in the 40 samples evaluated individually suggests that there were no failures during the self-collection of nasopharyngeal swabs.
With regard to the self-collection strategy, a study conducted in the United States, Guest et al. demonstrated that most of the oropharyngeal swab samples collected by the participants were adequate for testing for SARS-CoV-2 RNA.15 The absence of significant failures during the self-collection process favors the performance of population surveys or those conducted with specific groups, without the presence of a trained professional, expanding testing capacity, reducing health care worker exposure and PPE costs, required for this procedure.8 Therefore, the results of this study show that self-collection is a useful resource for surveillance of COVID-19 infection in asymptomatic individuals.
There was resistance to performing the procedure on the part of the university students, either due to the fear of performing self-collection or the memory of previous unpleasant experiences. These elements suggest the need for greater awareness among the target population, in order to promote adherence to self-collection.9 However, most participants adhered to the self-collection and were able to perform the procedure based on the instructions they had received.
The average cost of procedures depends on the prevalence of COVID-19 in the community: the higher the prevalence, the greater the probability of the samples having to be processed individually.12-14 The analysis of the samples obtained by self-collection using pool testing proved to be more cost-effective than if they had been analyzed individually, including hypothetical scenarios, in which some pools showed detectable results.
It took us two working days to collect, process and present the results. The rapid result release allows the adoption of adequate epidemiological surveillance actions, which is particularly relevant for the control of outbreaks in closed communities, such as schools and work setting.3,4,7-9 The quality of the samples was verified, thus validating the result found. The pool testing technique enabled the performance of rapid testing in a relatively large number of people, with significant cost reduction.
As a limitation of the study, it is worth mentioning that the sample size was smaller than expected. The research was conducted in two days, when all medical students attending the mandatory internship should be present at the university for academic procedures. However, many students chose to perform such procedures remotely, thus reducing the number of students eligible for the study. Nevertheless, the number of participants was sufficient for the proposed design. Feasibility studies are relevant to public health, as they can contribute to the planning and conduction of larger studies. It would be important to keep monitoring these students, adopting the strategies described, as long as they continue to act in scenarios with higher risk of contamination by SARS-CoV-2.
Taking these results, it can be concluded that the combined use of self-collected nasopharyngeal swabs and pool testing as strategies, provides time savings, material and human resources and it is economically feasible, either for conducting population surveys or implementing measures to contain COVID-19 outbreaks
Referências
1. Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) [Internet]. Baltimore: Johns Hopkins University & Medicine, 2021 [cited 2021 Aug 09]. Available from: https://coronavirus.jhu.edu/map.html [ Links ]
2. Hallal PC, Horta BL, Barros AJD, Dellagostin OA, Hartwig FP, Pellanda LC, et al. Evolução da prevalência de infecção por COVID-19 no Rio Grande do Sul, Brasil: inquéritos sorológicos seriados. Cad Saude Colet. 2020; 25(Suppl 1): 2395-401. doi: 10.1590/1413-81232020256.1.09632020 [ Links ]
3. Denny TN, Andrews L, Bonsignori M, Cavanaugh K, Datto MB, Deckard A, et al. Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections on a College Campus - Duke University, Durham, North Carolina. MMWR Morb Mortal Wkly Rep. 2020; 69(46): 1743-7. doi: 10.15585/mmwr.mm6946e1externalicon [ Links ]
4. Paykamian B. Massachusetts Schools Try Pool Testing for COVID-19. California: Government technology: technology for state and local government, 2021 [cited 2021 Feb 25]. Available from: https://www.govtech.com/education/k-12/Massachusetts-Launches-COVID-19-Pool-Testing-for-Schools-After-Pilot.html [ Links ]
5. Hogan CA, Sahoo MK, Pinsky BA. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA. 2020; 323(19): 1967-9. doi: 10.1001/jama.2020.5445 [ Links ]
6. Ben-Ami R, Klochendler A, Seidel M, Sido T, Gurel-Gurevich O, Yassour M, et al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin Microbiol Infect. 2020; 26(9): 1248-53. doi: 10.1016/j.cmi.2020.06.009 [ Links ]
7. Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baião F, Hamacher S, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021; 9(4): 407-18. doi: 10.1016/S2213-2600(20)30560-9 [ Links ]
8. Tan SY, Tey HL, Lim ETH, Toh ST, Chan YH, Tan PT, et al. The accuracy of healthcare worker versus self-collected (2-in-1) Oropharyngeal and Bilateral Mid-Turbinate (OPMT) swabs and saliva samples for SARS-CoV-2. Plos One 2020; 15(12): e0244417. doi: 10.1371/journal.pone.0244417 [ Links ]
9. Tu YP, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, et al. Patient-collected tongue, nasal, and mid-turbinate swabs for SARS-CoV-2 yield equivalent sensitivity to health care worker collected nasopharyngeal swabs. MedRxiv. 2021. doi: 10.1101/2020.04.01.20050005 [ Links ]
10. Carvalho AF, Rocha RP, Gonçalves AP, Silva TBS, Sato HI, Vuitika L, et al. The use of denaturing solution as collection and transport media to improve SARS-CoV-2 RNA detection and reduce infection of laboratory personnel. Braz J Microbiol. 2021; 52: 531-9. doi: 10.1007/s42770-021-00469-4 [ Links ]
11. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25(3): 2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045 [ Links ]
12. Costa MS, Sato HI, Rocha RP, Carvalho AF, Guimarães NS, Machado EL, et al. Adjusting the cut-off and maximum pool size in RT-qPCR pool testing for SARS-CoV-2. Viruses 2021; 13(4): 557. doi: 10.3390/v13040557 [ Links ]
13. Cherif A, Grobe N, Wang X, Kotanko P. Simulation of pool testing to identify patients with coronavirus disease 2019 under conditions of limited test availability. JAMA Netw Open. 2020; 3(6): e2013075. doi: 10.1001/jamanetworkopen.2020.13075 [ Links ]
14. Regen F, Eren N, Heuser I, Hellmann-Regen J. A simple approach to optimum pool size for pooled SARS-CoV-2 testing. Int J Infect Dis. 2020; 100: 324-6. doi: 10.1016/j.ijid.2020.08.063 [ Links ]
15. Guest JL, Sullivan PS, Valentine-Graves M, Valencia R, Adam E, Luisi N, et al. Suitability and sufficiency of telehealth clinician-observed, participant-collected samples for SARS-CoV-2 testing: the iCollect cohort pilot study. JMIR Public Health Surveill. 2020; 6(2): e19731. doi: 10.2196/19731 [ Links ]
Received: June 26, 2021; Accepted: December 20, 2021











 texto en
texto en 


