Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Epidemiologia e Serviços de Saúde
versión impresa ISSN 1679-4974versión On-line ISSN 2237-9622
Epidemiol. Serv. Saúde vol.31 no.2 Brasília 2022 Epub 30-Jun-2022
http://dx.doi.org/10.1590/s2237-96222022000200016
Research note
Diagnosis of vaccination rooms in Brazilian primary health care centers taking part in the PlanificaSUS project, 2019 PlanificaSUS, 2019
1 Hospital Israelita Albert Einstein, Centro de Estudos, Pesquisa e Prática em APS e Redes, São Paulo, SP, Brazil
2 Centro Colaborador da Planificação da Atenção à Saúde, Uberlândia, MG, Brazil
Methods:
This was a cross-sectional study with secondary data of convenience sampling comprised of 25 rooms. Results of a checklist adapted from the Vaccine Room Supervision Tool of the National Immunization Program in 2019 regarding the dimensions ‘general organization’, ‘general aspects’, ‘technical procedures’, ‘cold chain’, ‘information system’, ‘adverse events following vaccination’, ‘special immunobiological agents’, ‘epidemiological surveillance’ and ‘health education’, were used. Percentages of scores, both overall and by dimensions were described in median, interquartile range, minimum and maximum values.
Results:
The overall median was 77.1%, higher for ‘health education’ (100.0%) and ‘cold chain’ (86.7%), and lower for ‘special immunobiological agents’ (50.0%) and ‘general organization’ (58.3%).
Conclusion:
Using the checklist enabled the diagnosis in different macro-regions, inter- and intra-regional differences were found in the dimensions, and positive results and opportunities for improvement in the general plan.
Keywords: Vaccination; Primary Health Care; Immunization Programs; Quality of Health Care
Study contributions
Main results
A greater strengthening in the dimensions ‘health education’ and ‘cold chain’ stands out, with scores of at least 87%; ‘special immunobiological agents’ and ‘general organization’ obtained median scores lower than 60%. It can be seen inter- and intra-regional heterogeneity.
Introduction
The National Immunization Program (PNI) coordinates immunization activities in Brazil and has contributed to the history of high vaccination coverage, in addition to ensuring universal and free access to immunobiological agents.1 However, currently there has been a decrease and heterogeneity in vaccination coverage in the country.2-5 Given the reemergence of vaccine-preventable diseases,7-10 the need to strengthen the evaluation and organization actions of the PNI and health services stands out.6
Regarding the so-called Health Care Planning, a methodology that aims at the organization and integration of services in health care networks,11,12 the strengthening of these actions includes storage organization, logistics, dose administration and local coverage monitoring, contributing to the adequacy of vaccination rooms to meet the standards recommended by the PNI.6
Few studies address the diagnosis of vaccination rooms at the local level and there are no studies addressing this subject at the national level.13-17 Therefore, the objective of this Research Note was to describe the diagnosis of vaccination rooms in Primary Healthcare Centers (PHC) that executed the Health Care Planning in 2019.
Methods
Study design
This was a cross-sectional study based on secondary data from a convenience sampling of vaccination rooms distributed over the five Brazilian macro-regions.
Setting
The universe of this study was comprised of 26 PHCs, each of them was located in a regional health department in 20 Federative Units. These PHCs were selected as a model for the execution of the Planning process in the first stage of the PlanificaSUS project, whose official name is ‘Organization of Specialized Outpatient Care in Network with Primary Health Care’,18 carried out between 2018 and 2020.
Participants
Among the 26 PHCs selected, 25 had a vaccination room, therefore they were eligible to take part in this study.
Data source and measurement
Vaccine Room Supervision Tool, developed by the PNI19 and adapted as a checklist by means of Microsoft Excel Spreadsheet, was used during PlanificaSUS activities aiming at performing the diagnosis and organizing vaccination rooms. The nursing professional who was responsible for the vaccination room filled out the checklist, pointing out whether or not the items were implemented (yes; no). PlanificaSUS tutor of the respective PHC was responsible for inserting the completed checklist file, in Excel, into the project platform (e-Planifica), where it was stored for consultation. This study used the files available in e-Planifica.
The checklist is comprised of 131 items, subdivided into nine dimensions (Box 1). The diagnosis was made by analyzing continuous measurements of the scores, both overall score and by dimension, as detailed in statistical methods.
Box 1 Dimensions and items of the diagnostic checklist of vaccination rooms
| Dimension 1: General organization |
| The municipality has a vaccine coordinator |
| The municipal vaccine coordinator conducts periodic supervision of the vaccination room, verifying the standard procedures, compliance with indicators and training for professionals |
| The primary healthcare center has a nurse responsible for the vaccination room |
| The vaccination room provides services throughout the unit opening hours, including lunch time, every day |
| All vaccines from the current schedule of the National Immunization Program are administered during the entire period in which the vaccination room is open |
| There are technical standards related to vaccination and they are made available to all professionals: vaccine administration protocols; cold chain; epidemiological surveillance of adverse events; reference center for special immunobiological agents; and personnel training in the vaccination room |
| All professionals are aware of the technical standards |
| The set of standard operating procedures for the vaccination room is implemented and updated by the team, according to the current standards of epidemiological and health surveillance |
| All nurses and nursing technicians/assistants who work and are responsible for the vaccination process know the standard operating procedures for vaccination |
| All nurses and nursing technicians/assistants who work and are responsible for the vaccination process are up to date on vaccination and immunization procedures for the population in the coverage area |
| Specific training sessions are periodically carried out for all professionals who work in the vaccination room |
| The nurse in charge periodically supervises all procedures performed by the nursing technicians/ assistants who work in the vaccination room |
| Dimension 2: General aspects of the vaccination room |
| The room is exclusively used for vaccination |
| The room is easily accessible to the population |
| The room is properly identified |
| The physical area of the vaccination room meets the standards recommended by the General Coordination of the National Immunization Program/Agência Nacional de Vigilância Sanitária |
| Light-colored, waterproof, and easy-to-clean walls |
| Resistant and non-slip floor |
| Waterproof and easy-to-clean floor |
| The room has an easy-to-clean sink with a tap and a countertop |
| The room has adequate protection against direct sunlight |
| The room has adequate lighting |
| The room has adequate ventilation |
| The room is in ideal preservation conditions |
| The room is in ideal cleaning conditions |
| General cleaning (walls, ceiling, etc.) is done every 15 days |
| The room temperature is maintained between 18º C and 20º C |
| There are no decorative objects in the room (paintings, vases, etc.) |
| The furniture has a good functional distribution |
| Printed matters and information materials are organized |
| Syringes and needles of daily use are properly packed (in clean and capped containers) |
| Syringes and needles in the stock are packed in sealed packages and in place with no humidity |
| The room has dispenser with liquid soap and alcohol |
| The room has a medical examination table (stretcher) for vaccination, with a waterproof mattress or similar and protected with disposable material |
| The room has a chair to accommodate the user during his/her vaccination |
| Dimension 3: Technical procedures |
| Age and interval between doses are checked, at each vaccine administration |
| Adverse events from previous doses are investigated, at each vaccine administration |
| Temporary or permanent contraindication to the indicated vaccine is evaluated, at each vaccine administration |
| The technician in charge advises the user or guardian about the vaccine to be administered |
| The technician in charge instructs the user or guardian about the record of scheduling |
| The expiration date of the vaccine is observed, at each vaccine administration |
| Vaccine preparation is performed according to technical standards |
| The nurse responsible for the vaccination room periodically supervises the preparation of the vaccine performed by the various technicians of the team |
| Date and time of bottle opening are properly recorded |
| The expiration date after the bottle opening is observed |
| The vaccine administration technique follows the defined standards |
| The nurse responsible for the vaccination room periodically supervises the vaccine administration performed by the various technicians of the team |
| Perforating and cutting materials are packed according to the biosafety standards, in a container used to pack perforating and cutting materials |
| Vaccines with live microorganisms are treated before disposal, according to biosafety standards |
| Active search for susceptible individuals among users in the coverage area is conducted |
| Control cards (mirror cards) are used for vaccination in children |
| Control cards (mirror cards) are used for vaccination in adolescents |
| Control cards (mirror cards) are used for adult vaccination |
| Control cards (mirror cards) are used for older adult vaccination |
| Control cards (mirror cards) are used for vaccination in pregnant women |
| The control cards are organized by return date |
| Active search of defaulters is conducted |
| The number of vaccines is sufficient to meet the demand of the population |
| There is a vaccine stock management in the unit |
| The number of syringes and needles is sufficient to meet the demand |
| Expiration date of syringes and needles is observed |
| Different types of waste are packed separately |
| Final destination of the waste is in accordance with the norms of health surveillance |
| Dimension 4: Cold chain |
| Switches are available for the exclusive use of each equipment |
| On the electrical distribution box there is a warning not to turn off the vaccination room circuit breaker |
| The refrigerator is exclusively used for immunobiological agents |
| The capacity of the refrigerator is equal to or greater than 280 liters |
| The refrigerator is in good condition |
| The refrigerator is an ideal state of cleanliness |
| The refrigerator is away from heat sources, out of direct sunlight and at least 20 cm from the wall |
| There is a maximum and minimum thermometer and/or an extension cable in the refrigerator |
| Recyclable ice coils are kept in the evaporator in the recommended quantity |
| The refrigerator has a water drip tray |
| On the first shelf of the refrigerator, in perforated trays, only the vaccines that can be submitted to negative temperature are kept |
| On the second shelf of the refrigerator, in perforated trays, only the vaccines that cannot be submitted to negative temperature are kept |
| On the third shelf of the refrigerator, vaccine stocks, serum and diluents are stored |
| Immunobiological agents are organized by type, batch and expiration date |
| Distance between immunobiological agents and the walls of the refrigerator is kept in order to allow air circulation |
| Dyeing water bottles are kept in the entire lower internal space of the refrigerator |
| There is some material stored in the refrigerator inner door panel |
| The correct reading and recording of the temperature at the beginning and end of the workday is routinely conducted |
| Daily Temperature Control Chart is displayed in a visible place |
| Defrosting and cleaning of the refrigerator is performed every 15 days or when the ice layer reaches 1.0 cm |
| There is a preventive and/or corrective maintenance program for the vaccination room refrigerator |
| The service has a cooler box (polyurethane and/or expanded polystyrene - Styrofoam) or other equipment for daily use, in sufficient number to meet routine activities |
| The service has sufficient number of recyclable ice coils to meet routine activities |
| The service has a sufficient number of maximum and minimum thermometers and extension cables to meet routine activities |
| The service has sufficient number of polyvinyl chloride (PVC) tapes/crepe tapes to meet routine activities |
| The recyclable ice coils are stored in the organization of the cooler box |
| The temperature of the cooler boxes or daily use equipment is monitored |
| When, for any reason, the immunobiological agents are submitted to temperatures that are not recommended, the hierarchically superior authority is immediately communicated |
| When, for any reason, immunobiological agents are submitted to non-recommended temperatures, the evaluation form of the immunobiological agents under suspicion is filled out and sent to the hierarchically superior authority |
| When, for any reason, immunobiological agents are submitted to non-recommended temperatures, the vaccines under suspicion are kept at a temperature between +2ºC and +8ºC until the decision of the hierarchically superior authority |
| Dimension 5: Information system |
| The National Immunization Program Information System is implemented in the unit |
| All professionals know and routinely use the National Immunization Program Information System |
| The unit provides the forms and instruments for recording vaccination-related care |
| Child’s card |
| Adult card |
| Pregnant woman’s card |
| Older adult card |
| Daily Bulletin of Vaccine Doses Administered |
| Monthly Bulletin of Vaccine Doses Administered |
| Control Card (schedule) |
| Daily Temperature Control Chart |
| Adverse Event Investigation Form |
| Form for the Evaluation of Vaccines Under Suspicion |
| Monthly tendency of immunobiological agents |
| The technicians in charge know how to fill out the forms and tools properly |
| The nurse in charge periodically supervises the record of vaccination-related care |
| There is a routine monitoring and evaluation of vaccine indicators |
| The whole team takes part in the monitoring and evaluation moments |
| The vaccination coverage indicator is monitored |
| The dropout rate indicator is monitored |
| Professionals know and periodically discuss the available information |
| Dimension 6: Adverse events following vaccination |
| Information about the occurrence of adverse events is shared with all professionals |
| Professionals know the possible adverse events following vaccination for each type of vaccine |
| Professionals identify adverse events that should be referred for medical evaluation |
| Professionals report adverse events following vaccination |
| Investigation of occurrence of adverse events is carried out |
| Dimension 7: Special immunobiological agents |
| All professionals know about the existing Reference Center for Special Immunobiological Agents |
| Professionals know about the list of immunobiological agents available at the Reference Center for Special Immunobiological agents |
| Professionals are aware of the indications of these immunobiological agents |
| All professionals are aware of the flow to request these immunobiological agents and/or referrals for vaccination |
| Dimension 8: Epidemiological surveillance |
| Information about the occurrence of any vaccine-preventable disease cases in their area of coverage (measles, rubella, diphtheria, pertussis, tetanus, polio, rabies and others) is shared immediately with the whole team, generating the necessary alert |
| The team evaluates the information about the incidence of vaccine-preventable diseases, comparing it with vaccination coverage |
| The team takes part in transmission blocking vaccination, when indicated |
| The team reports suspected cases of diseases under epidemiological surveillance that come to their knowledge |
| Dimension 9: Health education |
| The team develops partnerships with social entities and community groups for dissemination and mobilization of the target population for immunization actions |
| The team uses educational programs in the primary healthcare center in order to suggest the theme of vaccination and immunization |
| All users attending the vaccination room are guided and informed about the importance of vaccines and compliance with the vaccination schedule |
| All staff members are informed about the available vaccines, the importance of being vaccinated, and referring users to the vaccination room |
Source: Adapted from the Vaccine Room Supervision Tool.19
Bias control
In order to reduce biases, the checklist was presented in a standard way by the project mentoring team, and the completion of all services occurred in the same operationalization phase of the Planning process.
Study size
The study was comprised of 25 eligible rooms that had completed the checklist and inserted it into the e-Planifica.
Statistical methods
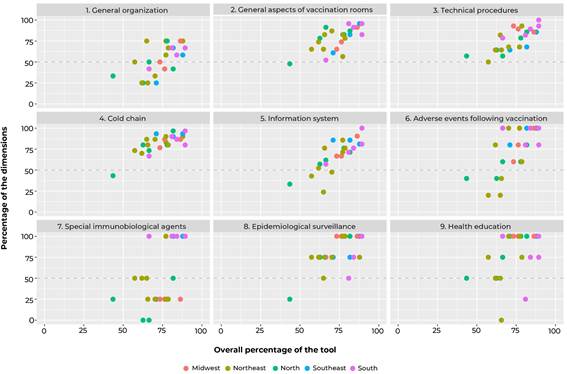
A descriptive analysis of the scores, both overall and by dimension, of the vaccination rooms was performed, presenting median, interquartile range and minimum and maximum values. The items of the dimensions with the lowest median score were described. A scatter plot was used to verify the distributions of the rooms over the Brazilian macro-regions, according to the scores, both overall and by dimension.
Microsoft Excel and R, a statistical software package (v.4.1.0) were used.
Results
A total of 25 rooms were studied, distributed over 19 states: 5 in the North region, 9 in the Northeast region, 3 in the Midwest region, 3 in the Southeast region and 5 in the South region.
The description of the scores, both overall and by dimension, of the checklist is shown in Table 1. The median overall score was 77.1%; ‘health education’ and ‘cold chain’ were the dimensions with the highest medians, while ‘special immunobiological agents’ and ‘general organization’ obtained the lowest medians.
Table 1 Description of the median, interquartile range and minimum and maximum values of scores, both overall score and by dimensions, of selected Brazilian vaccination rooms (n = 25), 2019
| Dimensions of vaccination | Median | Interquartile range | Minimmum value | Maximmum value |
|---|---|---|---|---|
| Overall | 77.1 | 66.4 - 81.7 | 43.5 | 89.3 |
| General organization | 58.3 | 41.7 - 66.7 | 25.0 | 75.0 |
| General aspects of vaccination rooms | 82.6 | 65.2 - 91.3 | 47.8 | 95.7 |
| Technical procedures | 78.6 | 64.3 - 85.7 | 50.0 | 100.0 |
| Cold chain | 86.7 | 80.0 - 90.0 | 43.3 | 96.7 |
| Information system | 71.4 | 57.1 - 80.9 | 23.8 | 100.0 |
| Adverse events following vaccination | 80.0 | 60.0 - 100.0 | 20.0 | 100.0 |
| Special immunobiological agents | 50.0 | 25.0 - 100.0 | 0.0 | 100.0 |
| Epidemiological surveillance | 75.0 | 75.0 - 100.0 | 25.0 | 100.0 |
| Health education | 100.0 | 75.0 - 100.0 | 0.0 | 100.0 |
A detailed analysis of the items revealed that, in the dimension related to ‘general organization’, in 20 vaccination rooms, the implementation or updating of standard operating procedure was not identified, and in 17, it was found that the team responsible for the vaccination room was unaware of such procedures. In addition, the lack of periodic training for professionals working in vaccination rooms was reported by those who were responsible for 15 rooms.
Regarding the dimension ‘special immunobiological agents’, in most vaccination rooms, the professionals were aware of the existing reference centers (n = 22) and the flow to request these immunobiological agents and/or referral to these services (n = 13). However, in 15 rooms, the professionals were unaware of the list of immunobiological agents available; and in 16, they were unaware of their indications.
Figure 1 shows the distribution of scores, both overall and by dimensions, according to the location of the rooms in Brazilian macro-regions. It could be seen inter- and intra-regional heterogeneity, especially regarding the dimensions ‘information system’, ‘adverse events following vaccination’ and ‘special immunobiological agents’. Among the rooms with scores below 50% in the dimensions evaluated, a higher frequency of those located in the North and Northeast regions was found.
Discussion
This study allowed us to observe a positive scenario in the general diagnosis of PHC vaccination rooms in several regions of the country, with emphasis on health education actions, communication of the importance of vaccination, and structural and logistical aspects of the cold chain. It is worth highlighting the need to prioritize the strengthening of actions related to the knowledge of professionals about special immunobiological agents and the general organization of rooms. Furthermore, inter-regional and intra-regional variability in the diagnosis of vaccination rooms was observed, with less favorable results for the North and Northeast regions.
The limitations of this study include the fact that the sample studied is not representative of Brazil and its regions, given that the allocation of the number of centers per region was not proportional (selection bias). Another limitation is related to the variable number of items per dimension of the checklist, which may weaken the comparability of percentage scores of the dimensions.
It is worth mentioning the need to update the tool, in line with the ‘Manual of Rules and Procedures for Vaccination’ of the Ministry of Health,12 and to standardize the evaluation criteria.
Based on the diagnosis of vaccination rooms, health education actions have stood out, favorable in most of them, a result similar to those of studies conducted in Minas Gerais15 and São Paulo.16 On the other hand, a study carried out in Pernambuco,14 when jointly evaluating the dimensions of health education and epidemiological surveillance, found unfavorable results. Thus, it is worth highlighting the need for the sustainability of educational actions, given that communication with the population make clear the importance of vaccines as individual and collective protective measures, in addition to demystifying rumors, contributing to the link between population and service, greater adherence to vaccination and maintenance of satisfactory vaccination coverage.9,10
Another dimension of great prominence that has been observed in this study and in the literature14-17 was the cold chain, with a satisfactory result and low variability among the services, possibly because they are items that have the power to directly affect the quality and safety of these supplies, despite possible losses due to irregularities in the conditioning and/or logistics of resources.12
It could be seen opportunities for improvement related to the dimension general organization, in the aspects of human resources, process standardization and training. The identification of shortage of human resources can lead to accumulation of tasks and weaknesses in communication and active search for users.20 Taking into consideration the constant updates of the immunization schedule and the variability in the professionals’ understanding of the service process, facts that have already been reported in other studies,13,20 it is worth highlighting the need for permanent education21 and standardization of procedures, aiming at an immediate response to the occurrence of vaccine-preventable diseases, such as the COVID-19 pandemic.
Another opportunity for improvement that should be prioritized, among the dimensions of the checklist, is related to the dimension special immunobiological agents, corroborating other studies.15,17 The fragility of health professionals’ understanding of special immunobiological agents may impair access to this type of input and, consequently, the quality of life of specific populations that need it.22,23 However, a study conducted in a municipality of São Paulo achieved satisfactory results for the dimension special immunobiological agents.16
The inter- and intra-regional inequalities observed in the diagnosis of vaccination rooms, with a less favorable result for most of the dimensions in the rooms in the North and Northeast regions, compared to those in the other regions, reflect the historical inequalities of investment in material and human resources in the Brazilian territory, and trends of a lower vaccination coverage in both regions aforementioned.2-4
This study expands the diagnosis of vaccination rooms in several macro-regions of Brazil, from a contemporary perspective, given that the literature on the subject is scarce and it is not recent. It is noteworthy that vaccination is part of the basic microprocesses of Primary Health Care,24 which should be implemented and monitored regularly in order to ensure conditions for the provision of quality services. Thus, the adopted checklist proved to be easily applicable in different contexts, and can serve as an example for the institutionalization of monitoring culture, evaluation and continuous improvement of immunization services.
Taking these results, it can be concluded that the diagnosis performed enabled the identification of opportunities for improvement in vaccination rooms, and that continuous monitoring and evaluation may support a more assertive action plan in different types of governance
Referências
1. Domingues CMAS, Maranhão AGK, Teixeira AM, Fantinato FFS, Domingues RAS. The Brazilian National Immunization Program: 46 years of achievements and challenges. Cad Saude Publica. 2020;36(Suppl 2):e00222919. doi: 10.1590/0102-311X00222919 [ Links ]
2. Ministério da Saúde (BR). Programa Nacional de Imunizações. Programa Nacional de Imunizações: coberturas vacinais no Brasil (período 2010-2014) [Internet]. Brasília: Ministério da Saúde; 2015 [citado 2020 Maio 13]. Disponível em: https://portalarquivos2.saude.gov.br/images/pdf/2017/agosto/17/AACOBERTURAS-VACINAIS-NO-BRASIL---2010-2014.pdf [ Links ]
3. Arroyo LH, Ramos ACV, Yamamura M, Weiller TH, Crispim JA, Cartagena-Ramos D, et al. Areas with declining vaccination coverage for BCG, poliomyelitis, and MMR in Brazil (2006-2016): maps of regional heterogeneity. Cad Saude Publica. 2020;36(4):e00015619. doi: 10.1590/0102-311X00015619 [ Links ]
4. Césare N, Mota TF, Lopes FFL, Lima ACM, Luzardo R, Quintanilha LF, et al. Longitudinal profiling of the vaccination coverage in Brazil reveals a recent change in the patterns hallmarked by differential reduction across regions. Int J Infect Dis. 2020;98:257-80. doi: 10.1016/j.ijid.2020.06.092 [ Links ]
5. Sato APS. What is the importance of vaccine hesitancy in the drop of vaccination coverage in Brazil?. Rev Saude Publica. 2018;52:96. doi: 10.11606/S1518-8787.2018052001199 [ Links ]
6. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de normas e procedimentos para vacinação [Internet]. Brasília: Ministério da Saúde; 2014 [citado 2021 Mar 30]. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/manual_procedimentos_vacinacao.pdf [ Links ]
7. Silveira MF, Buffarini R, Bertoldi AD, Santos IS, Barros AJD, Matijaseviche A, et al. The emergence of vaccine hesitancy among upper-class Brazilians: Results from four birth cohorts, 1982-2015. Vaccine. 2020;38(3):482-8. doi: 10.1016/j.vaccine.2019.10.070 [ Links ]
8. Barello S, Nania T, Dellafiore F, Graffigna G, Caruso R. 'Vaccine hesitancy' among university students in Italy during the COVID-19 pandemic. Eur J Epidemiol. 2020;35(8):781-3. doi: 10.1007/s10654-020-00670-z [ Links ]
9. Jaca A, Mathebula L, Iweze A, Pienaar E, Wiysonge CS. A systematic review of strategies for reducing missed opportunities for vaccination. Vaccine. 2018;36(21):2921-7. doi: 10.1016/j.vaccine.2018.04.028 [ Links ]
10. Crocker-Buque T, Mounier-Jack S. Vaccination in England: A review of why business as usual is not enough to maintain coverage. BMC Public Health. 2018;18(1):1351. doi: 10.1186/s12889-018-6228-5 [ Links ]
11. Evangelista MJO, Guimarães AMDN, Dourado EMR, Vale FLB, Lins MZS, Matos MAB de, et al. Planning and building health care networks in brazil's federal district. Cien Saude Colet. 2019;24(6):2115-24. doi: 10.1590/1413-81232018246.08882019 [ Links ]
12. Mendes EV, Matos MAB, Evangelista MJO, Barra RP. A Construção Social da Atenção Primária à Saúde [Internet]. 2. ed. Brasília: CONASS; 2019 [citado 2021 Ago 30]. 192 p. Disponível em: https://www.conass.org.br/biblioteca/a-construcao-social-da-atencao-primaria-a-saude-2a-edicao [ Links ]
13. Galvão MFPS, Almeida PC, Lopes MSV, Coutinho JFV, Martins MC, Barbosa LP. Avaliação das salas de vacinação de unidades de Atenção Primária à Saúde. Rev Rene. 2019;20:e39648. doi: 10.15253/2175-6783.20192039648 [ Links ]
14. Araújo ACM, Guimarães MJB, Frias PG, Correira JB. Evaluation of vaccination rooms of the state of Pernambuco in 2011. Epidemiol Serv Saude. 2013;22(2):255-64. doi: 10.5123/s1679-49742013000200007 [ Links ]
15. Siqueira LG, Martins AMEBL, Versiani CMC, Almeida LAV, Oliveira CS, Nascimento JE, et al. Avaliação da organização e funcionamento das salas de vacina na Atenção Primária à Saúde em Montes Claros, Minas Gerais, 2015. Epidemiol Serv Saude. 2017;26(3):557-68. doi: 10.5123/S1679-49742017000300013 [ Links ]
16. Vasconcelos KCE, Rocha SA, Ayres JA. Evaluation of vaccination rooms in the primary health care network of the Municipality of Marília, State of São Paulo, Brazil, 2008-2009. Epidemiol Serv Saude. 2012;21(1):167-76. doi: 10.5123/s1679-49742012000100017 [ Links ]
17. Cunha JO, Oliveira IMB, Santos AD, Cunha MWN, Santos FJ, Santos JMJ. Avaliação da padronização dos procedimentos nas salas públicas de vacinas do município de Itabaiana, Sergipe, Brasil. Rev Bras Pesq Saúde. 2018;20(1):70-8. doi: 10.21722/rbps.v20i1.20610 [ Links ]
18. Instituto Israelita de Responsabilidade Social Albert Einstein. e-Planifica [Internet]. São Paulo: Instituto Israelita de Responsabilidade Social Albert Einstein; 2021 [citado 2021 Ago 30]. Disponível em: https://planificasus.com.br/index.php [ Links ]
19. Ministério da Saúde (BR). Programa Nacional de Imunização. Programa de avaliação do instrumento de supervisão sala de vacinação - PAISSV [Internet]. Brasília: Ministério da Saúde; 2004 [citado 2021 Abr 19]. Disponível em: http://pni.datasus.gov.br/apresentacao.asp [ Links ]
20. Barros MGM, Santos MCS, Bertolini RPT, Pontes Netto VB, Andrade MS. Perda de oportunidade de vacinação: aspectos relacionados à atuação da atenção primária em Recife, Pernambuco, 2012. Epidemiol Serv Saude. 2015;24(4):701-10. doi: 10.5123/S1679-49742015000400012 [ Links ]
21. Martins JRT, Alexandre BGP, Oliveira VC, Viegas SMF. Permanent education in the vaccination room: what is the reality?. Rev Bras Enferm. 2017;71(Suppl 1):668-76. doi: 10.1590/0034-7167-2017-0560 [ Links ]
22. Nóbrega LAL, Novaes HMD, Sartori AMC. Avaliação da implantação dos Centros de Referência para Imunobiológicos Especiais. Rev Saude Publica. 2016;50:58. doi: 10.1590/S1518-8787.2016050006183 [ Links ]
23. Wolkers PCB, Yakuwa MS, Pancieri L, Mendes-Rodrigues C, Furtado MCC, Mello DF. Crianças com diabetes mellitus tipo 1: acesso aos imunobiológicos especiais e à puericultura. Rev Esc Enferm USP. 2017;51:e03249. doi: 10.1590/S1980-220X2016049103249 [ Links ]
24. Mendes EV. A construção social da Atenção Primária à Saúde. Brasília: Conselho Nacional de Secretários de Saúde; 2015. 193 p. [ Links ]
Received: February 20, 2022; Accepted: May 24, 2022










 texto en
texto en