Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Boletim do Museu Paraense Emílio Goeldi Ciências Naturais
versão impressa ISSN 1981-8114
Bol. Mus. Para. Emilio Goeldi Cienc. Nat. v.4 n.2 Belém ago. 2009
Squamata (Reptilia) from four sites in southern Amazonia, with a biogeographic analysis of Amazonian lizards
Squamata (Reptilia) de quatro localidades da Amazônia meridional, com uma análise biogeográfica dos lagartos amazônicos
Teresa Cristina Sauer Avila-PiresI; Laurie Joseph VittII; Shawn Scott SartoriusIII; Peter Andrew ZaniIV
IMuseu Paraense Emílio Goeldi. Coordenação de Zoologia. Belém, Pará, Brasil (avilapires@museu-goeldi.br)
IISam Noble Oklahoma Museum of Natural History. University of Oklahoma. Norman, Oklahoma, U.S.A. (vitt@ou.edu)
IIISam Noble Oklahoma Museum of Natural History. University of Oklahoma. Norman, Oklahoma, U.S.A. (Shawn_Sartorius@fws.gov)
IVGonzaga University. Department of Biology. Spokane, Washington, U.S.A. (zani@gonzaga.edu)
ABSTRACT
We studied the squamate fauna from four sites in southern Amazonia of Brazil. We also summarized data on lizard faunas for nine other well-studied areas in Amazonia to make pairwise comparisons among sites. The Biogeographic Similarity Coefficient for each pair of sites was calculated and plotted against the geographic distance between the sites. A Parsimony Analysis of Endemicity was performed comparing all sites. A total of 114 species has been recorded in the four studied sites, of which 45 are lizards, three amphisbaenians, and 66 snakes. The two sites between the Xingu and Madeira rivers were the poorest in number of species, those in western Amazonia, between the Madeira and Juruá Rivers, were the richest. Biogeographic analyses corroborated the existence of a well-defined separation between a western and an eastern lizard fauna. The western fauna contains two groups, which occupy respectively the areas of endemism known as Napo (west) and Inambari (southwest). Relationships among these western localities varied, except between the two northernmost localities, Iquitos and Santa Cecilia, which grouped together in all five area cladograms obtained. No variation existed in the area cladogram between eastern Amazonia sites. The easternmost localities grouped with Guianan localities, and they all grouped with localities more to the west, south of the Amazon River.
Keywords: Herpetofauna. Amazonia. Species composition. Biodiversity. Biogeography. PAE.
RESUMO
Estuda-se a fauna de répteis Squamata de quatro localidades da Amazônia meridional brasileira, comparando-as entre si e, com relação aos lagartos, com outras nove áreas bem amostradas de toda a Amazônia. O Coeficiente de Similaridade Biogeográfico para cada par de localidades é calculado e analisada sua correlação com a distância entre as localidades. Uma Análise Parcimoniosa de Endemismos (PAE) é realizada, comparando todas as áreas. Um total de 114 espécies foi registrado nas quatro localidades de estudo, representando 45 espécies de lagartos, três de anfisbenas e 66 de ofídios. As duas localidades entre os rios Xingu e Madeira foram as mais pobres em número de espécies; as localidades mais a oeste, entre o Madeira e o Purus, as mais ricas. As análises biogeográficas corroboram a existência de uma separação bem definida entre uma fauna de lagartos ocidental e uma oriental. A fauna ocidental contém dois grupos, os quais ocupam, respectivamente, as áreas de endemismo conhecidas como Napo (a oeste) e Inambari (a sudoeste). A relação entre as localidades estudadas da Amazônia ocidental variou, exceto pelas duas áreas mais ao norte, Iquitos e Santa Cecilia, que se agruparam nos cinco cladogramas de área obtidos. Na parte oriental, a relação entre as áreas se mostrou constante, com as duas localidades mais a leste, ao sul do rio Amazonas, agrupando-se com as localidades da região das Guianas, e, após, às localidades mais a oeste, ao sul do Amazonas.
Palavras-chave: Herpetofauna. Amazônia. Composição de espécies. Biodiversidade. Biogeografia. PAE.
INTRODUCTION
During the last few decades a large effort has been made to understand the high biodiversity observed in the Amazonian region. The idea of a long period of stability has been substituted by one of a dynamic history, with pronounced changes in vegetation cover (Haffer, 1969, 1982), changes in river courses (Salo et al., 1986), tectonic events in upper Amazonia (Räsänen, 1993), and more recently the recognition that tectonic events have been more frequent than previously thought, even in Central and Lower Amazonia (Rossetti et al., 2005; Rossetti & Valeriano, 2007). An important part of this discussion is to understand how the Amazonian fauna is distributed and how areas with different faunal composition relate to each other. For some of the better studied groups, especially birds and primates, the large Amazon tributaries seem to be barriers (primary or secondary). However, a broader pattern has been observed for reptiles, with a western fauna subdivided into a western and a southwestern group, a Guianan fauna with a weaker east-west division, and a few endemic elements in southeast Amazonia (Avila- Pires, 1995; for Guianas also Hoogmoed, 1973, 1979). The strongest biogeographic signal, for both reptiles and primates, seems to be an east-west dichotomy (e.g., Avila-Pires, 1995; Silva Jr. & Sites Jr., 1995; Silva & Oren, 1996; Duellman, 1999; Ron, 2000), with the Madeira and Negro Rivers having the greatest impact in dividing the faunas (Hoogmoed, 1979; Ayres & Clutton-Brock, 1992; Haffer, 1992), as already pointed out by Wallace (1852) while studying the distribution of monkeys in Amazonia. Avila-Pires (1995) has shown that for lizards known distributions south of the Amazon pointed to a transitional zone between the Purus and Tapajós Rivers, rather than a single limit for all species. However, deficiency of sampling in Amazonia hindered better analyses. Between 1995 and 1998 a series of expeditions were made, covering four sites along an approximate east-west transect in southern Amazonia, in the states of Pará, Amazonas, Rondônia and Acre, Brazil. Each of these sites has been extensively surveyed for Squamate reptiles, especially lizards, providing a good opportunity to compare faunas along this east-west transect. These new data are added to a biogeographic analysis comparing different sites in Amazonia, including localities north of the Amazon, and in the eastern and western extremes of Amazonia.
MATERIALS AND METHODS
Four Amazonian sites south of the Amazonas/Solimões (hereafter referred to as 'Amazon') river have been studied, all in Brazil (in bold the names used throughout the text to refer to each one). From east to west they are: (1) Pará: Agropecuária Treviso, c. 101 km S and 18 km E of Santarém, close to Curuá-Una River, 3o 9' S - 54o 50' W; (2) Rondônia: Parque Estadual Guajará-Mirim, 10o 19' S - 64o 33' W; (3) Amazonas: rio Ituxi: Fazenda Scheffer, 8o 20' S - 65o 43' W; (4) Acre: rio Juruá, 5 km N of Porto Walter, 8o 16' S - 72o 47' W (Figure 1).
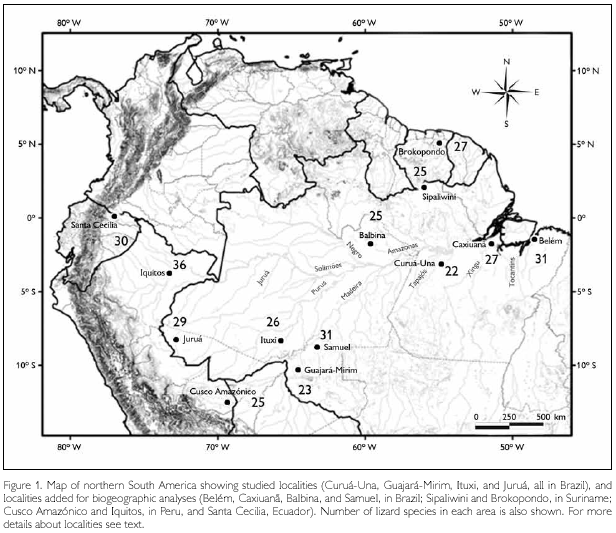
All localities are covered by tropical rain forest. With the exception of Ituxi, terra firme forest dominated; Ituxi consisted of a mix of varzea and terra firme forest. Part of the forest studied in Curuá-Una had been selectively logged eight years before our survey, but forest structure was very close to that of a primary forest. Guajará-Mirim was the least disturbed, including reduced hunting pressure due to its protected status as a state park (Avila-Pires & Vitt, 1998; Caldwell & Araújo, 2005; Vitt & Avila-Pires, 1998; Vitt et al., 1997, 1998). The herpetofauna of all sites was actively surveyed for about three months, always in the rainy season (December to April), by a team of 4–6 persons. Collected specimens have been deposited at the Museu Paraense Emílio Goeldi (MPEG), at the Sam Noble Oklahoma Museum of Natural History (OMNH), and a smaller part at the Instituto Nacional de Pesquisas da Amazônia (INPA).
Lizard faunas from these sites were compared to those of the following localities (use in text indicated in bold font): (1) Belém, Pará, Brazil, based on Avila-Pires (1995) and the collection of Museu Paraense Emílio Goeldi. (2) Estação Científica Ferreira Penna, Floresta Nacional de Caxiuanã, Pará, Brazil (1o 42' S - 51o 31' W). This site has been surveyed intermittently since 1992 (Avila-Pires & Hoogmoed, 1997; Bernardi et al., 2002), and recently more thoroughly, using pitfall traps (M. C. Santos Costa, pers. comm.), with specimens deposited in MPEG. (3) Balbina, c. 90 km northeast of Manaus, in the state of Amazonas, Brazil, and (4) Samuel, c. 36 km east or Porto Velho, state of Rondônia, Brazil. Both localities have been surveyed before and during the flooding of these areas due to the construction of a hydroelectric dam; data from Silva Jr. & Sites Jr. (1995). (5) Forested areas surrounding Sipaliwini savanna, westward to the Kutari River headwaters; and (6) between the Suriname River and Brown's Mountain, Brokopondo District. Both localities in Suriname, based on data from Hoogmoed (1973). (7) Cusco Amazónico, Peru, based on Duellman (2005). (8) Iquitos region, Peru, based on Dixon & Soini (1986). (9) Santa Cecilia, Ecuador, based on Duellman (1978, 1987). Only forest species have been considered in all analyses (therefore no Cnemidophorus spp. or Tropidurus spp. were considered). Hemidactylus mabouia, when present, was not included, because it clearly represents a recent introduction in Amazonia.
In order to compare species composition in terms of geographic distribution, we classified species into seven categories – 'Widespread', when occurring in most of Amazonia (endemic or not to the region; species as Pseudogonatodes guianensis and Bachia flavescens, even though missing in some parts, were also included in this category); 'Guianan', if restricted to the Guianan region (all area north of the Amazon and east of the Negro river, as defined by Hoogmoed, 1979) or predominantly in this region (as Arthrosaura kockii and Tretioscincus agilis, that occur south of the Amazon only east of the Xingu river); 'Eastern', when present both north and south of the Amazon, but absent from the western part; 'Southern', if only known south of the Amazon, but throughout a large part of it (Enyalius leechii is the only species in this category); 'Southeastern', referring to two species (Stenocercus dumerilii and Colobosaura modesta), which in Amazonia occur only east of the Tocantins river; 'Western', when present in western Amazonia (Colombia, Ecuador, Peru, and western part of Brazilian Amazonia – but not necessarily in the entire area), but absent from most of the eastern part; and 'Southwestern', with main occurrence in this part of Amazonia (mainly Peru, Bolivia, and southwestern part of Brazilian Amazonia). These definitions were designed to be specifically broad, since the aim is to understand the relationship between areas. Based on this classification, pie graphs showing the proportion of each group per site have been plotted in a map. Distribution data were mainly based on Avila-Pires (1995, 2005), Duellman (1990, 2005), and Hoogmoed (1979), updated to take into consideration the new collections here reported and other material in the herpetological collection of Museu Paraense Emílio Goeldi. Classification of each species is shown in Appendix.
Additionally, species composition has been compared using the Biogeographic Similarity Coefficient, used by Duellman (1990; as a replacement of the name Faunal Resemblance Factor previously in use for the same coefficient), and equivalent to 2C/(N1 + N2), where N1 = number of species in locality 1, N2 = number of species in locality 2, and C = number of species common to both localities. Correlation between these coefficients and the linear geographic distances between localities was calculated to determine the proportion of variation among localities that can be attributed to distance alone.
A Parsimony Analysis of Endemicity (PAE; Rosen, 1985; Rosen & Smith, 1988) was performed to construct an area cladogram on the basis of presence of taxa. By using the same algorithms used for phylogenetic trees and the establishment of an outgroup where absence of species is considered primitive, only presence of species is used to establish area relationships. This is a more meaningful analysis than taking into consideration both presence and absence of species, because it is difficult to ascertain with confidence if the absence is real or represents missing data. PAE was performed with Winclada/Nona (respectively Nixon, 2002 and Goloboff, s/d, choosing heuristic analysis, with 100 replications). For this analysis taxa present in only one area or in all areas have not been considered, because they are uninformative regarding relationships between areas. Subspecies were not considered, except for Anolis nitens, for which strong evidences indicate that they should be considered distinct species (Glor et al., 2001). As a consequence, 46 taxa have been considered informative.
RESULTS
Considering all four sites together, a total of 114 Squamate reptiles were registered, of which 45 (39.5%) were lizards, three (2.6%) amphisbaenians, and 66 (57.9%) snakes (Table 1). When each area is considered separately, Guajará- Mirim is the richest, with 62 species (54.4%) of the total, and Juruá is the poorest, with 50 species (43.9% of the total). Snakes and amphisbaenians, however, are probably undersampled. When only lizards are compared, Juruá is the richest area (29 species) and Curuá-Una the poorest (22 species). Among the other Amazonian sites included in this study for comparison, numbers of snake and amphisbaenian species are available for Caxiuanã, Balbina, Samuel, Cusco Amazônico, Iquitos and Santa Cecilia. These groups together represent between 1.8 to three times the number of lizards in each locality, while in the four studied sites this proportion ranges between 0.7 to 1.7 (Table 2), reinforcing the idea that several species of snakes (and some amphisbaenians) are still to be expected in the four areas studied.
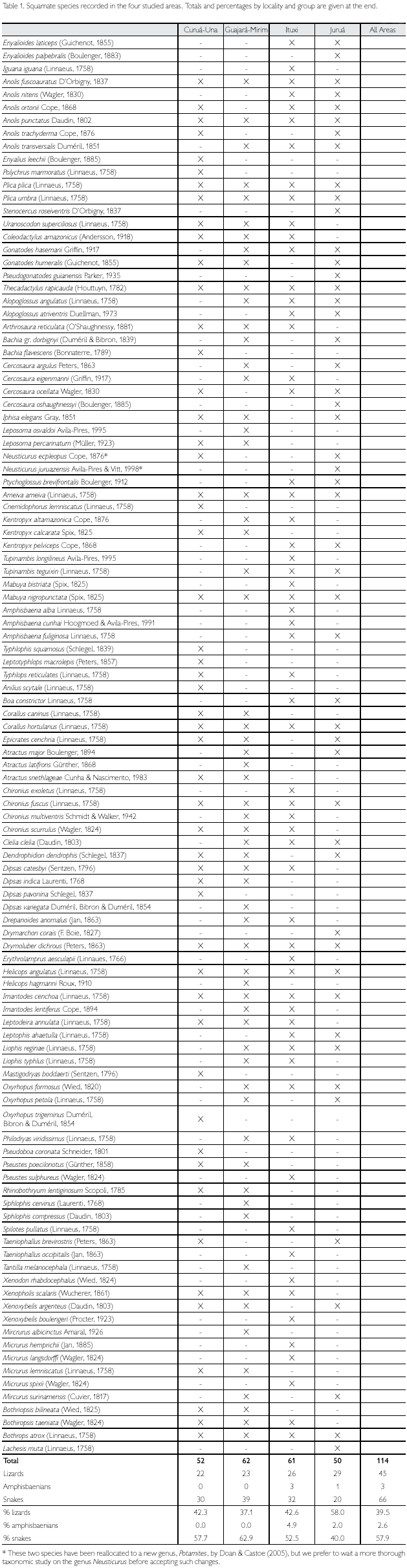
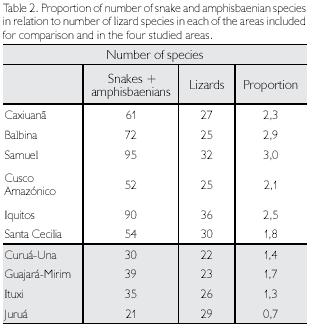
Considering only lizards, two areas, Curuá-Una and Guajará-Mirim, have a lower number of species (respectively 22 and 23), with the other two, Ituxi and Juruá, containing respectively 26 and 29 species, a number closer to that also found in other areas (Table 2). Since Curuá-Una had been subjected to selective logging before our studies in the area, we cannot rule out that this low number is a consequence of disturbance. Guajará-Mirim, however, is a protected area, thus disturbance is less likely an explanation for its low number of species, even though the history of the area previous to protection is not known (see also below). When the additional Amazonian sites are included in the comparison, lowest values in lizard species are found in the more central Amazonian sites, except for Samuel, and in Cusco Amazónico, in the extreme southwest corner of Amazonia (Figure 1). Although part of these differences may be due to deficiency of collecting, central Amazonian sites may well have less species than peripheral ones, since species restricted to the west do not reach sites progressively further east, those from the extreme east are similarly absent from more central sites and, for the localities south of the Amazon, Guianan species are absent (Figure 2). The larger number of species in Samuel, when compared to Ituxi and Guajará-Mirim, either could be because of sampling a more heterogeneous area, or due to better sampling as a result of the complete flooding of a large area – a question that at present remains open.
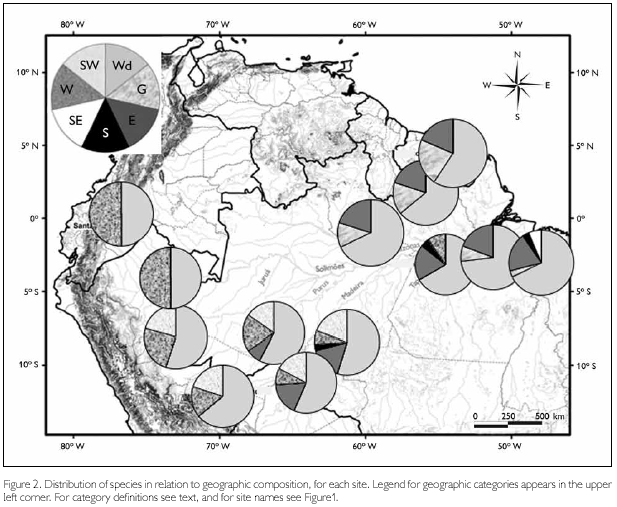
Regarding species composition in each site (Figure 2), Ituxi, Guarajá-Mirim, Samuel, Curuá-Una and Belém are more mixed than remaining sites, where species from two or three categories are present. In the case of Belém, this is due to a mainly Guianan species (Arthrosaura kockii) and two species (Stenocercus dumerilii and Colobosaura modesta) restricted to this easternmost part of Amazonia. Curuá-Una contains two species, Anolis trachyderma and Neusticurus ecpleopus, which are distributed mainly in the western part of Amazonia. Guajará-Mirim and Samuel, east of the Madeira River, and Ituxi, west of the Madeira River, present a mixture of eastern, western, and southwestern elements. They represent therefore composite areas.
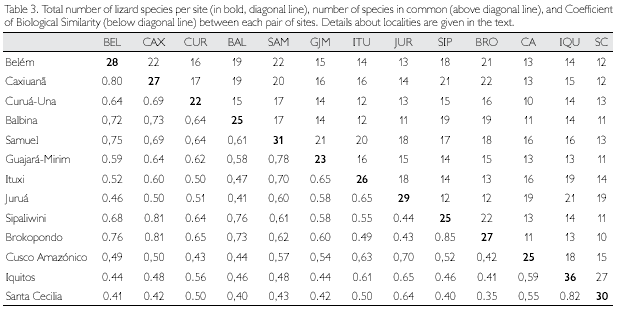
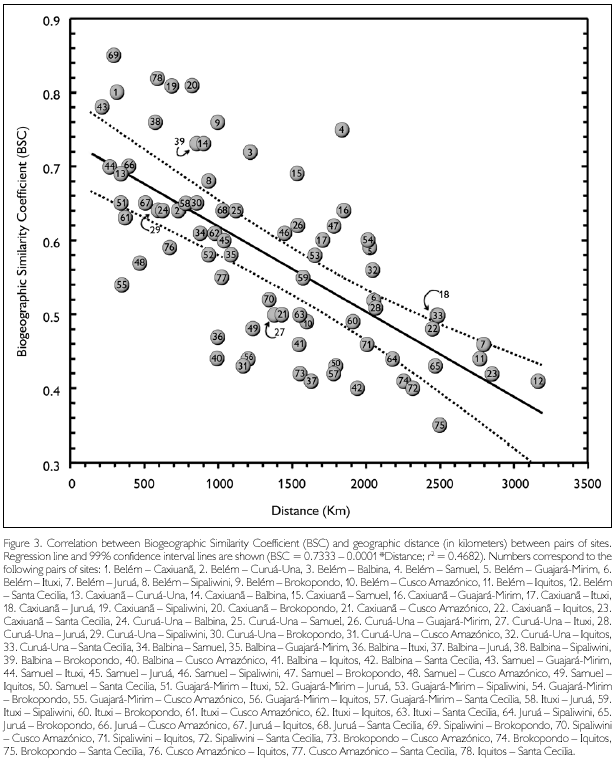
Biogeographic Similarity Coefficients (BSC) between the studied sites, based solely on lizards, are presented in Table 3, while correlation between BSC and geographic distance is shown in Figure 3. Distance explains about 47% of the variation observed among sites. Rondonian localities (Guajará-Mirim and Samuel) are less similar than expected by distance alone (BSC distinctly below the 99% confidence interval of the regression line) in relation to western localities in Peru (Cusco Amazónico, Iquitos) and Ecuador (Santa Cecilia), but not in Brazil (Ituxi, Juruá). Balbina, in Brazilian Guiana, is also more different than expected by distance alone in relation to all western localities (Ituxi and Juruá included), while differences between these localities and Sipaliwini and Brokopondo, in Suriname, could be in large part explained by distance. Curuá-Una, in central Amazonia south of the Amazon, and Cusco Amazónico, in southern Peru, are also less similar than expected when distance is taken into consideration. Conversely, eastern Amazonian localities (Belém, Caxiuanã) are more similar than expected by distance alone in relation to Guianan localities (Balbina, Sipaliwini, Brokopondo), as well as among Guianan localities. These localities (eastern Amazonia and Guianan) are also more similar than expected in relation to Rondonian localities.
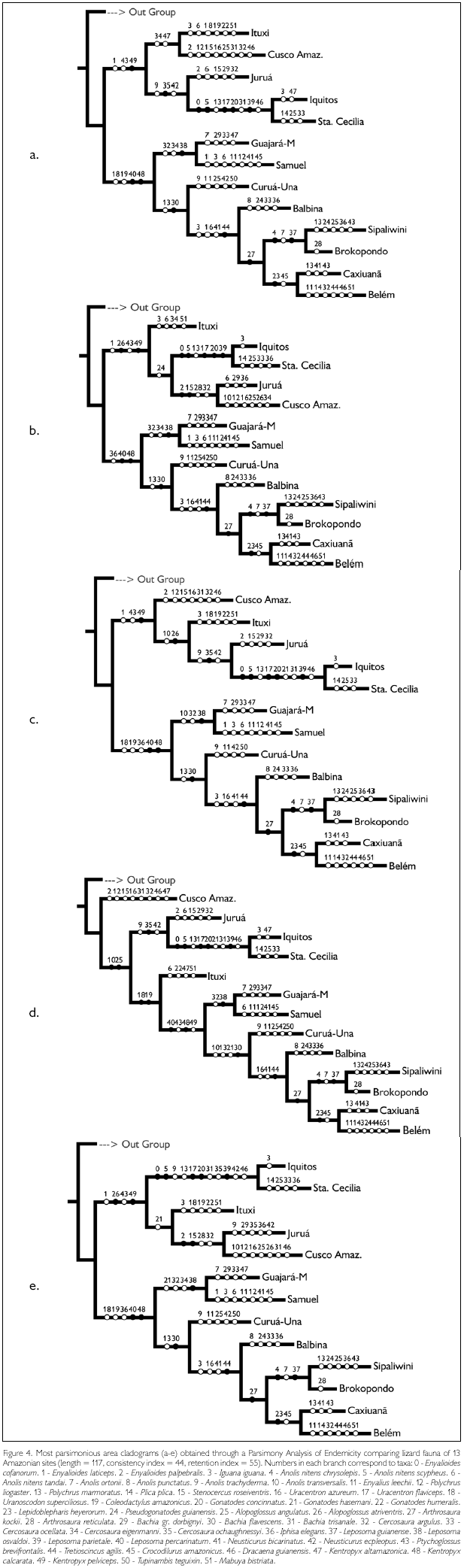
Parsimony Analysis of Endemicity resulted in five most parsimonious trees, with 117 steps and consistency index of 44 (Figure 4 and Appendix). In all five trees an east-west dichotomy is evident, with an eastern group formed by ((Guajará-Mirim – Samuel) (Curuá-Una (Balbina (Sipaliwini – Brokopondo) (Caxiuanã-Belém)))). This whole group is linked by the presence of Leposoma percarinatum and Kentropyx calcarata. The relationship between the western sites varies, except for a sister relationship between Iquitos and Santa Cecilia, but Kentropyx pelviceps is common to all of them. Ituxi groups in one case with the eastern sites (linked to them by the presence of Uranoscodon superciliosus and Coleodactylus amazonicus), in all other cases with the western sites. Even though Guajará-Mirim and Samuel, both in Rondônia, appear always linked to the eastern group, they share a number of species with the western sites (which are absent from eastern sites), e.g. Enyalioides laticeps, Anolis transversalis, Bachia gr. dorbignyi. Cusco Amazónico and Samuel have in common Polychrus liogaster; Cusco Amazónico, Ituxi, Guajará-Mirim and Samuel Cercosaura eigenmanni; and these sites plus Juruá have in common Gonatodes hasemani.
DISCUSSION
Our results point to a deficiency in snake data for our four studied sites, which was to be expected. Duellman (1978, 2005) already showed that long-term surveys are needed for representative sampling of snakes. Sampling of lizards, on the contrary, seems to be reasonably complete as a result of our three-month surveys.
Regarding the distribution of lizards, all analyses point to a main east-west dichotomy in Amazonia, while relationships within each of these groups are less clearly defined. For most vertebrates a distinct western fauna is evident, usually also with a distinction between a western and a southwestern group, while eastern Amazonian areas may appear either as a distinct group (e.g., in lizards – Ron, 2000; Silva Jr. & Sites Jr., 1995 –, and partially in snakes – Silva Jr. & Sites Jr., 1995), or as progressively more basal areas (e.g., amphibians and primates – Ron, 2000). Avila-Pires (1995), analyzing the lizard fauna of Rondônia as a whole, found a dubious relationship of this region with eastern or western faunas. In the present study, the two sites in Rondônia grouped with the eastern sites, even though they also contain species that have a western or southwestern distribution.
Relationships between eastern areas are more variable, with eastern areas south of the Amazon more related to each other than to Guiana (e.g., in snakes and lizards according to Silva Jr. & Sites Jr., 1995), or easternmost areas south of the Amazon more similar to Guiana than to areas further west, especially Rondônia (e.g., lizards according to Ron, 2000, and this study). Ayres & Clutton-Brock (1992) found that primate faunas on opposite sides of the Amazon became more similar closer to the river's mouth. Silva (1995), analyzing patterns of distribution of the avifauna associated with cerrado vegetation, concluded that one of the main corridors linking savannas north and south of the Amazon could have been along the Atlantic coast, a possibility also raised by Avila-Pires (1995) in relation to Tropidurus hispidus. Part of the similarity between Guiana and the easternmost fauna south of the Amazon could be therefore a result of migration across the lower Amazon River.
A number of areas of endemism have been recognized in Amazonia, initially for birds (e.g., Haffer, 1978, 1985; Cracraft, 1985), later on also for primates (e.g., Silva & Oren, 1996), and partially for other groups (Ron, 2000; Silva et al., 2005). When we look at lizard distribution, western and southwestern groups correspond to the Napo and Inambari areas of endemism. Guiana as a whole is considered an area of endemism, also recognizable for lizards. For the other areas of endemism (Imeri, in northwestern Amazonia, and Rondônia, Tapajós, Xingu and Belém, in southeastern Amazonia – Silva et al., 2005), there is little or no evidence for lizards. A single species, Gonatodes tapajonicus, is known from only one locality in the Tapajós area of endemism, and another species, Stenocercus dumerilii, is restricted to the Belém area of endemism. Moreover, the Tocantins River forms the eastern limit of the distribution of Plica plica, and the Xingu River the western limit (south of the Amazon) of Arthrosaura kockii, Colobosaura modesta, and Tretioscincus agilis, indicating a barrier effect of these rivers for at least a few species.
The Madeira River is considered the main barrier between the eastern and western faunas. Its importance as a faunal divisor, together with the Negro and Amazon Rivers, was already recognized by Wallace (1852), and has been since confirmed by several other studies (Silva et al., 2005). Data on lizards by Avila-Pires (1995) corroborated the importance of the Madeira River in this aspect, even though she observed that species limits occurred variably between the Purus and Tapajós Rivers. Of the five area cladograms we obtained in the PAE analysis, four showed a division between the sites west and east of the Madeira River, while one showed the Ituxi site, in the interfluvium Madeira-Purus, as basal to the group containing all eastern sites. Looking at the Biogeographic Similarity Coefficient, differences in the lizard fauna in Guajará-Mirim and Samuel, east of the Madeira River, and Ituxi and Juruá, west of it, could be explained by distance, with no evidence of a faunal barrier between these sites (while between Rondonian sites and those in Peru and Ecuador distance alone could not explain the low BSC). As Rondônia lies in the upper Madeira River basin, one may suppose that, even if the river forms a barrier, this would not be as effective upstream as downstream. However, Aleixo (2004) found that for birds of the Xiphorhynchus elegans group, populations of X. e. elegans (Pelzeln, 1868) from the western bank of the lower Madeira River were more similar to other populations to the east, while upriver the Madeira River separated two clades of this group, X. e. elegans and X. e. juruanus (Ihering, 1904). Undoubtedly this is a complex region, where distinct faunas coalesce. It is plausible that a barrier existed formerly around the area of the Madeira River, the river acting as a secondary, but partial barrier. The Madeira River is a "white-water" river, thus relatively slow flowing and meandering, which facilitates river crossing, even if it is a large river (by far the largest southern tributary of the Amazon). Thus both historical and present conditions would explain the large dissimilarity of faunas on the two sides of the river, while at the same time several species are presently found that have clearly crossed the river.
ACKNOWLEDGEMENT
We first thank Janalee P. Caldwell, M. Carmosina Araújo, Robson A. Souza, and Verônica R. L. Oliveira for help during field work. We thank W. E. Magnusson for help in Manaus, Antonio and Dark Leite, Gilson L. Favorato (CEMEX timber company), Marcelo Scheffer, Neuzari Pinheiro (Prefeito of Porto Walter, Acre), Secretaria de Estado de Desenvolvimento Ambiental, Rondônia, SOS Amazônia, and Acre Union of Seringueiros for providing housing and logistic support at the study sites. M. S. Hoogmoed gave valuable suggestions to the manuscript. Paulo F. P. Souza Jr., MPEG/LSR, and Marcelo J. Sturaro helped with the maps. Brazilian agencies contributing to logistics or research and collecting permits include Instituto Nacional de Pesquisas da Amazônia (INPA), CNPq (Portaria MCT no. 170, de 28/09/94), Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA, permit no. 073/94-DIFAS). This research was conducted under a research agreement between the Sam Noble Oklahoma Museum of Natural History and the Museu Paraense Emílio Goeldi. National Science Foundation grants DEB-9200779 and DEB-9505518 to L. J. V. and J. P. Caldwell, and CNPq (Auxílio Pesquisa 475295/01-3) to TCSAP, supported part of the work.
REFERENCES
ALEIXO, A., 2004. Historical diversification of a terra-firme forest bird superspecies: a phylogeographic perspective on the role of different hypotheses of Amazonian diversification. Evolution 58: 1303-1317.
AVILA-PIRES, T. C. S., 1995. Lizards of Brazilian Amazônia (Reptilia: Squamata). Zoologische Verhandelingen 299: 1-706.
AVILA-PIRES, T. C. S., 2005. Reptiles. In: T. HOLLOWELL & R. E. REYNOLDS (Eds.): Checklist ofthe terrestrial vertebrates ofthe Guiana Shield. Bulletin of the Biological Society of Washington 13: 25-40.
AVILA-PIRES, T. C. S. & M. S. HOOGMOED, 1997. The Herpetofauna. In: P. L. B. Lisboa (Org.): Caxiuanã: 389-401. Museu Paraense Emílio Goeldi, Belém.
AVILA-PIRES, T. C. S. & L. J. VITT, 1998. A new species of Neusticurus (Reptilia: Gymnophthalmidae) from the Rio Juruá, Acre, Brazil. Herpetologica 54: 235-245.
AYRES, J. M. & T. H. CLUTTON-BROCK, 1992. River boundaries and species range size in Amazonian primates. American Naturalist 140: 531-537.
BERNARDI, J. A. R., N. RUFINO, R. G. N. COSTA & A. T. ROCHA, 2002. Répteis. In: P. L. B. LISBOA (Org.): Caxiuanã: populações tradiconais, meio físico e diversidade biológica: 533-540. Museu Paraense Emílio Goeldi, Belém.
CALDWELL, J. P. & M. C. ARAÚJO, 2005. Amphibian faunas of two eastern Amazonian rainforest sites in Pará, Brazil. Occasional Papers of the Sam Noble Oklahoma Museum of Natural History 16: 1-41.
CRACRAFT, J., 1985. Historical biogeography and patterns of differentiation within the South American avifauna: areas of endemism. Ornithological Monographs 36: 49-84.
DIXON, J. R. & P. SOINI, 1986. The reptiles of the upper Amazon basin, Iquitos region, Peru: 1-154. Milwaukee Public Museum, Milwaukee.
DOAN, T. M. & T. A. CASTOE, 2005. Phylogenetic taxonomy of the Cercosaurini (Squamata: Gymnophthalimidae), with new genera for species of Neusticurus and Proctoporus. Zoological Journal of the Linnean Society 143: 405-416.
DUELLMAN, W. E., 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publications of the University of Kansas Museum of Natural History 65: 1-352.
DUELLMAN, W. E., 1987. Lizards in an Amazonian rain forest community: resource utilization and abundance. National Geographic Society Research 3: 489-500.
DUELLMAN, W. E., 1990. Herpetofaunas in Neotropical rainforests: comparative composition, history, and resource use. In: A. H. GENTRY (Ed.): Four neotropical rainforests: 455-505. Yale University Press, New Haven.
DUELLMAN, W. E., 1999. Distribution patterns of amphibians in South America. In: W. E. DUELLMAN (Ed.): Patterns of distribution of amphibians: a global perspective: 255-328. John Hopkins University Press, Baltimore.
DUELLMAN, W. E., 2005. Cusco Amazónico. The lives of amphibians and reptiles in an Amazonian rainforest: 1-433. Comstock Publishing Associates, Cornell University Press, Ithaca.
GLOR, R. E., L. J. VITT & A. LARSON, 2001. A molecular phylogenetic analysis of diversification in Amazonian Anolis lizards. Molecular Ecology 10: 2661-2668.
GOLOBOFF, P. A., s/d. Nona ver. 2.0. Published by the author. Fundación Miguel Lillo, Tucumán.
HAFFER, J., 1969. Speciation in Amazonian forest birds. Science 165: 131-137.
HAFFER, J., 1978. Distribution of Amazon birds. Bonner Zoologischen Beiträge 29: 38-78.
HAFFER, J., 1982. General aspects of the refuge theory. In: G. T. PRANCE (Ed.): Biological diversification in the tropics. Proceedings of the Fifth Symposium for Tropical Biology 6-24.
HAFFER, J., 1985. Avian zoogeography of the Neotropical lowlands. Neotropical Ornithology 36: 113-146.
HAFFER, J., 1992. On the 'river effect' in some forest birds of southern Amazonia. Boletim do Museu Paraense Emílio Goeldi, série Zoologia 8: 217-245.
HOOGMOED, M. S., 1973. Notes on the herpetofauna of Suriname IV. The lizards and amphisbaenians of Suriname: 1-419. Biogeographica IV. Dr. W. Junk Publishers, The Hague.
HOOGMOED, M. S., 1979. The herpetofauna ofthe Guianan Region. In: W. E. DUELLMAN (Ed.): The South American herpetofauna: its origin, evolution, and dispersal: 7: 241-279. Monograph of the Museum of Natural History, University of Kansas, Lawrence.
NIXON, K. C., 2002. WinClada ver. 1.00.08. Published by the author, Ithaca, NY.
RÄSÄNEN, M., 1993. La geohistoria y geologia de la Amazonia Peruana. In: R. KALLIOLA, K. PUHAKKA & W. DANJOY (Eds.): Amazonia Peruana - vegetación húmeda tropical en el llano subandino: 43-67. Paut & Onern, Jyväskylä.
RON, S. R., 2000. Biogeographic area relationships of lowland Neotropical rainforest on raw distributions of vertebrate groups. Biological Journal of the Linnean Society 71: 379-402.
ROSEN, B. R., 1985. Long-term geographical controls on regional diversity. The Open University Geological Society Journal 6: 25-30.
ROSEN, B. R. & A. B. SMITH, 1988. Tectonics from fossils? Analysis of reef coral and sea urchin distribution from late Cretaceous to Recent, using a new method. In: M. G. AUDLEY-CHARLES & A. HALLAM (Eds.): Gondwana and Tethys: 275-306. Geological Society of London Special Publication, London.
ROSSETTI, D. F., P. M. TOLEDO & A. M. GOES, 2005. New geological framework for Western Amazonia (Brazil) and implications for biogeography and evolution. Quaternary Research 63: 78-89.
ROSSETTI, D. F. & M. M. VALERIANO, 2007. Evolution of the lowest Amazon basin modeled from the integration of geological and SRTM topographic data. Science Direct 70: 253-265.
SALO, J., R. KALLIOLA, I. HÄKKINEN, Y. MÄKINEN, P. NIEMELÄ, M. PUHAKKA & P. COLEY 1986. River dynamics and the diversity of Amazon lowland forest. Nature 322: 254-258.
SILVA, J. M. C., 1995. Biogeographic analysis ofthe South American Cerrado avifauna. Steenstrupia 21: 48-67.
SILVA JR., N. J. & J. W. SITES JR., 1995. Patterns of diversity of Neotropical Squamate reptile species with emphasis on the Brazilian Amazon and the conservation potential of indigenous reserves. Conservation Biology 9: 873-901.
SILVA, J. M. C. & D. C. OREN, 1996. Application of the parsimony analysis of endemicity in Amazonian biogeography: an example with primates. Biological Journal of the Linnean Society 59: 427-437.
SILVA, J. M. C., A. B. RYLANDS & G. A. B. FONSECA, 2005. O destino das áreas de endemismo da Amazónia. Megadiversidade 1(1): 124-131.
VITT L. J., P A. ZANI & A. C. MARINHO-LIMA, 1997. Heliotherms in tropical rain forest: the ecology of Kentropyx calcarata (Teiidae) and Mabuya nigropunctata (Scincidae) in the Curua-Una of Brazil. Journal of Tropical Ecology 13: 199-220.
VITT L. J. & T. C. S. AVILA-PIRES, 1998. Ecology of two sympatric species of Neusticurus (Gymnophthalmidae) in the western Amazon of Brazil. Copeia 1998: 570-582.
VITT, L. J., S. S. SARTORIUS, T. C. S. AVILA-PIRES & M. C. ESPOSITO, 1998. Use of time, space, and food in the gymnophthalmid lizard Prionodactylus eigenmanni from the western Amazon of Brazil. Canadian Journal of Zoology 76: 1681-1688.
WALLACE, A. R., 1852. On the monkeys ofthe Amazon. Proceedings of the Zoological Society of London 20: 107-110.
 Mailing address:
Mailing address:
Museu Paraense Emílio Goeldi
Editor do Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais
Av. Magalhães Barata, 376
São Braz – CEP 66040-170
Caixa Postal 399
Telefone/fax: 55-91- 3249 -1141
E-mail:boletim@museu-goeldi.br
Recebido: 20/05/2009
Aprovado: 13/08/2009













