Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Boletim do Museu Paraense Emílio Goeldi Ciências Naturais
versão impressa ISSN 1981-8114
Bol. Mus. Para. Emilio Goeldi Cienc. Nat. v.4 n.2 Belém ago. 2009
Morphology and micromorphology of the seed coats of species of Echinodorus (Alismataceae) from Brazilian Northeastern
Morfologia e micromorfologia de sementes de espécies de Echinodorus (Alismataceae) do Nordeste Brasileiro
Ligia Queiroz MatiasI; Geraldo SoaresII
IUniversidade Federal do Ceará. Departamento de Biologia. Centro de Ciências. Fortaleza, Ceará, Brasil (lqmatias@ufc.br)
IIUniversidade Federal do Ro Grande do Sul. Instituto de Biociências. Departamento de Botânica. Porto Alegre, Rio Grande do Sul, Brasil (glgsoares@gmail.com)
ABSTRACT
The morphology and the micromorphological characteristics of the surface of seeds of ten species of Echinodorus Rich. & Engelm. ex. A. Gray which occurs in northeastern Brazil were examined using scanning electron microscopy. The diversity of the observed ornaments allowed to distinguish the seed testa of the studied species as follows: scalariform in E. pubescens (Martius) Seubert, tenuous ribbed in E. tenellus(Martius) Buchenau, tenuous reticulate in E. reticulatus Haynes & Holm-Nielsen, reticulate channeled in E. grandiforus (Chamisso & Schlechtendal) Micheli subsp. aureus (Fassett) Haynes & Holm-Nielsen, reticulated in E. glandulosus Rataj, reticulated-foveate in E. paniculatus Micheli and E. lanceolatus Rataj, and reticulated tenuous foveate in E. palaefolius (Nees & Martius) MacBride, E. subalatus (Martius) Grisebach and E. macrophyllus (Kunth) Micheli subsp. scaber (Rataj) Haynes & Holm-Nielsen. The complete description presents observations of the form of the component cells of the testa of these seeds, their anticlinal boundaries with respective cell junctions and cell wall thicknesses. The results suggest the importance of the seed micromorphology as a synthetic character to infra-generic classification of Echinodorus.
Keywords: Monocotyledons. Aquatic plants. SEM. Seed surface.
RESUMO
As características morfológicas e micromorfológicas da superfície das sementes de dez espécies de Echinodorus Rch. & Engelm. ex. A. Gray que ocorrem no nordeste brasileiro foram analisadas usando microscopia eletrônica de varredura. A diversidade da ornamentação da testa permitiu diferenciar: padrão escalariforme em E. pubescens (Martius) Seubert, tênue-costelada em E. tenellus (Martius) Buchenau, tênue-reticulada em E. reticulatus Haynes & Holm-Nielsen, reticulada-canaliculada em E. grandilforus (Chamisso & Schlechtendal) Micheli subsp. aureus (Fassett) Haynes & Holm-Nielsen, reticulada em E. glandulosus Rataj, reticulada-foveolada em E. paniculatus Micheli e E. lanceolatus Rataj e reticulada tênue-foveolada em E. palaefolius (Nees & Martius) MacBride, E. subalatus (Martius) Grisebach, e E. macrophyllus (Kunth) Micheli subsp. scaber (Rataj) Haynes & Holm-Nielsen. A descrição completa apresenta dados sobre a forma dos componentes celulares da testa, o contorno da fronteira anticlinal com respectivas formas de junções e de espessura das paredes celulares. Os resultados indicam a importância da micromorfologia da semente como caractere sintético para as classificações infragenéricas de Echinodorus.
Palavras-chave: Monocotiledôneas. Plantas aquáticas. MEV. Superfície de sementes.
INTRODUCTION
The Alismataceae have emergent or floating aquatic species. The family is represented by 12 genera and approximately 80 species with sub-cosmopolitan distribution (Haynes & Holm-Nielsen, 1998). Sagittaria Linnaeus and Echinodorus Rich. & Engelm. ex. A. Gray ard are genera with relatively low diversity, but are the only neotropical taxa of this family (Lot & Novelo, 1984; Haynes & Holm-Nielsen, 1989). The genus Echinodorus comprises 26 species (Haynes & Holm-Nielsen, 1994) occurring predominantly in the tropical regions of South America.
The genus Echinodorus was divided by Fassett (1955) into the sub-genera Echinodorus Fasset and Helianthium Fasset based on the insertion of the anthers into the filament and on the number of carpels. Leaf form, the types of translucent marks on the leaves, and the number and arrangement ofthe pericarp glands are other principal taxonomic characteristics used within the family (Micheli, 1881; Lot & Novelo, 1994; Haynes & Holm-Nielsen, 1994). Additionally, Haynes & Holm-Nielsen (1985) indicated that the length ofthe rostrum of the fruit is taxonomically important. Seed characteristics, however, have not yet been employed for taxonomic purposes. They were not considered in a recent revision of Echinodorus (Rataj, 2004) and in phylogenetics analysis (Lehtonen, 2006; Lethonen & Myllys, 2008).
Rogers (1983) and Micheli (1881) described the presence of slightly punctated testa surfaces in species of Echinodorus. The linear and slightly incurvate outline of the embryo lends an uncinate-curvated shape to the seed (Micheli, 1881). In fact, the seed morphology of Echinodorus was described by Haynes & Holm-Nielsen (1985) as U-shaped. The recurvate shape of the seed is an adaptive characteristic, allowing the emergence of the hypocotyls through the micropyle of the seed, breaking through the less rigid basal region of the achene (Kaul, 1978).
The establishment and renewal of Echinodorus populations in semi-arid regions is dependent on the ability of the seeds to resist long periods of desiccation in temporary aquatic habitats and high soil temperatures. Those temporary habitats are resulted of the climate from the Brazilian semi-arid region (Leprun, 1984-1985). In intermittent aquatic ecosystems, seed viability is related to dormancy (Salisbury, 1942), as well as, longevity, allowing germination to be delayed until environmental conditions are favorable (Yeo, 1965; West & Whigham, 1976).
As there is very little information currently available concerning the biology of aquatic plants from intermittent aquatic environments in the semi-arid region of Brazil, a morphological analysis of the seeds of these plants should contribute to our understanding of adaptive strategies encountered in these environments.
The present work examined the morphological and micromorphological characteristics of the seeds of species of Echinodorus from the semi-arid region of Brazil, in a search for taxonomic characters useful in distinguishing these taxa.
MATERIALS AND METHODS
Seeds were obtained from mature fruits collected in the field, with the criteria that these seeds were easily separated from the achenes present on the floral axis, or that the axis was quite dry. Exceptions were made for the species Echinodorus reticulatus Haynes & Holm-Nielsen, E. grandiflorus (Chamisso & Schlechtendal) Micheli subsp. aureus (Fassett) Haynes & Holm-Nielsen, and E. macrophyllus (Kunth) Micheli subsp. scaber (Rataj) Haynes & Holm-Nielsen, whose seeds were obtained from herbarium collections. The examined species are listed below: E. glandulosus Rataj - Ceará: Aiuaba, estrada para Assaré, 09/05/2002, L. Q. Matias 352 (EAC, ICN); Antonina, 18/05/2003, L. Q. Matias 421 (EAC, ICN). E. grandiflorus subsp. aureus, Ceará: Crato, Granjeiro, 11/12/1933, G. D. Luetzelburg s.n. (IPA 22490); E. lanceolatus Rataj - Ceará: Granja, 17/07/2003, L. Q. Matias 482 (EAC, ICN). E. macrophyllus subsp. scaber, Formososa do rio Preto, Arroz, 30/03/2000, Miranda da Silva et al. 385 (HUEFS). E. palaefolius (Nees & Martius) MacBride - Pernambuco: Betânia, Fazenda Cunhãns, 24/04/2002, L. Q. Matias 336 (EAC, ICN). E. paniculatus Micheli - Bahia: Iraquara, 13/05/2003, L. Q. Matias 407 (EAC, ICN). E. pubescens (Martius) Seubert - Ceará: Senador Pompeu, estrada para o Encantado, 07/05/2002, L. Q. Matias 346 (EAC, ICN); Piauí: São Raimundo Nonato, Lagoa do Meio, 06/04/2003, L. Q. Matias 369 (EAC, ICN). E. reticulatus - Bahia (cf.), s.l., 09/1974, D. Andrade-Lima 7880 (IPA). E. subalatus (Martius) Grisebach - Ceará: Caucaia, Lagamar do Cauipe, 18/06/2003, L. Q. Matias 489 (EAC, ICN). E. tenellus (Martius) Buchenau - Bahia: Barra, lagoa marginal do Rio Grande, 11/05/2003, L. Q. Matias 401 (EAC, ICN).
As no detailed protocols were encountered for the preparation of Echinodorus seeds for viewing surface ornamentation under scanning electron microscopy (SEM), a number of different techniques were initially tested. Treating seeds with a 50/50 solution of ether/chloroform (Wilkinson, 1983) followed by ultrasonic washing for 15 minutes was found to remove waxes and oily substances (derived from the achene glands) that adhere to the seed surface as the fruit dries. The seeds were subsequently dried at 25 oC for five days, fixed to aluminum supports (stubs), layered with ten to 15 nm of gold by sputtering Balzers SCD 005, and maintained under a vacuum until observation. Samples were examined using a Joel JSM 6060 Scanning Electron Microscopy at 20 K = kv, and digital electro-photomicrographs obtained at 1.440 dpi. Comparisons between the different species were made on the basis of observations of the cells located near the micropyle. Terminology for seed surface characteristics as seen under SEM were based on Murley (1951 apud Stearn, 1973), Barthlott (1981, 1984), and Behnke & Barthlott (1983). Terminology for morphological characters was based on Radford et al. (1974) and the Systematics Association Committee for Descriptive Biological Terminology (1960).
RESULTS
The majority of the seeds of the species of Echinodorus examined were obovate in lateral view (Figures 1, 7, 11, 13, 15, 17, 19). Only E. paniculatus (Figure 3), E. lanceolatus (Figure 5), and E reticulatus (Figure 9) demonstrated oblong seeds. The slightly recurved outline of the embryo lends a U-shaped form to the seed. The cells composing the testa had a distinct longitudinal alignment as seen in a lateral view, and this orientation is determined by the outline of the embryo. As such, those cells situated near curved regions have distinctly modified isodiametric forms. A well-defined central elliptic depression were observed in the seeds of E. subalatus (Figure 13), but was less evident in E. palaefolius (Figure 17). The seed surface was reasonably smooth in both E. tenellus and E. reticulates, but ornamented in the other species.
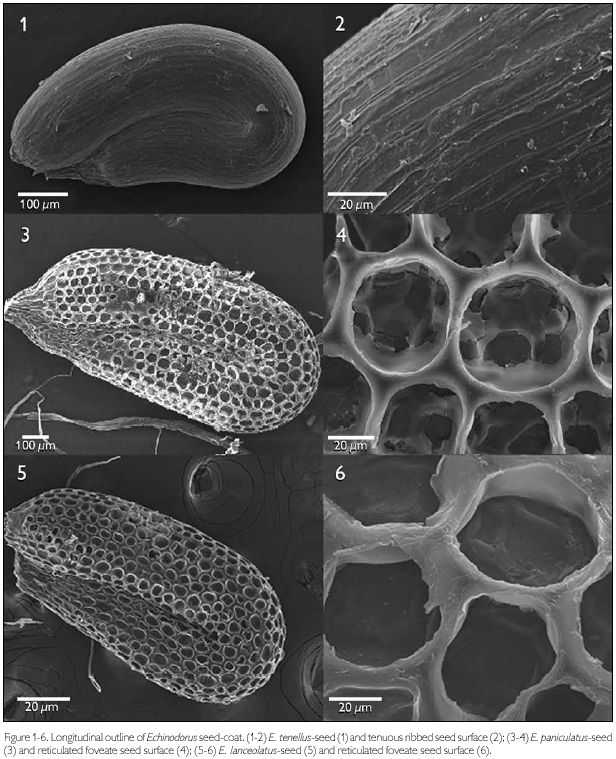
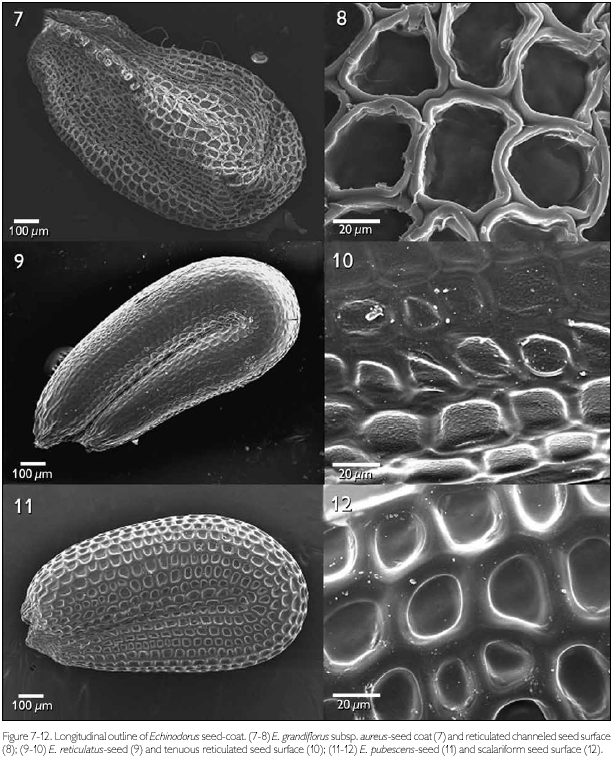
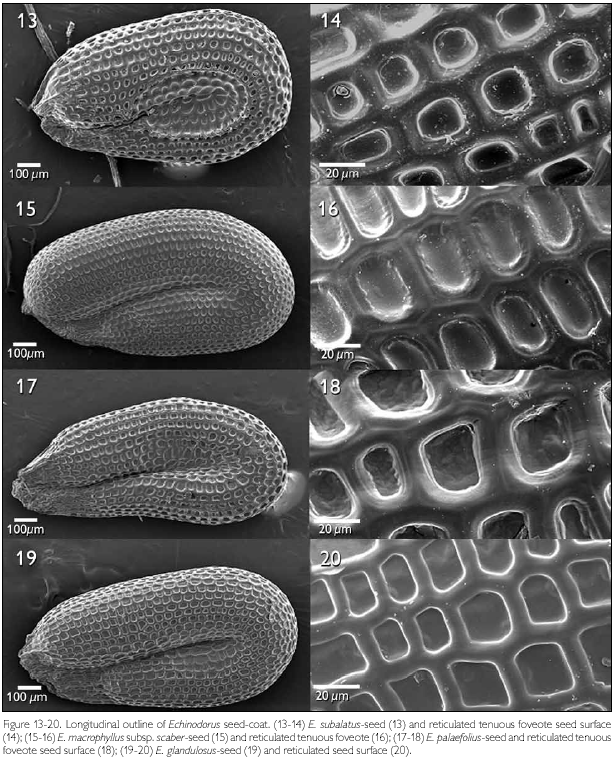
The patterns of micro-texturing or secondary sculpturing observed on the seed surfaces included: tenuous ribbed (Figures 1-2), reticulated-foveate (Figures 3-4), reticulate channeled (Figures 7-8), tenuous reticulate (Figures 9-10), scalariform (Figures 11-12), reticulated tenuous foveate (Figures 13-14), and reticulated (Figures 19-20).
Diagnostic characteristics related to the primary sculpturing of the seed surfaces included: 1) cell forms, 2) the types of cell wall edges and periclinal wall junctions, 3) the thickness of the anticlinal walls.
Echinodorus tenellus showed a tenuous ribbed surface formed by oblong cells with only slightly pre-eminent anticlinal walls (Figures 1-2). The anticlinal walls of this species are thin, not juxtaposed, sub-equal, and rectilinear.
Echinodorus paniculatus and E. lanceolatus showed a reticulate-foveate pattern, with circular cells and slightly thickened, pre-eminent and juxtaposed anticlinal walls (Figures 3-4, 5-6, respectively).
Echinodorus grandiflorus subsp. aureus showed polygonal cells, with four pronounced anticlinal walls that are sub-equal, slightly circular, thickened, and with intercellular spaces (principally in the angular regions), lending a reticulated-canaliculated aspect to the seed surface (Figures 7-8).
Echinodorus reticulatus showed polygonal, with three to five, slightly pre-eminent, thick anticlinal cell walls, constituting a tenuous-reticulate pattern (Figures 9-10). The periclinal walls were rugose and differ in this aspect from all the other species examined.
Echinodorus subalatus (Figures 13-14), E. macrophyllus subsp. scaber (Figures 15-16), and E. palaefolius (Figures 17-18) showed a reticular delineation of the seed surface, although their fovea are deeper, constituting a reticulate-foveate pattern. The cells were polygonal, with 4-5 sides, and have thick juxtaposed anticlinal walls of different sizes.
Echinodorus pubescens showed regularly aligned cells, with a predominance of oblong cells with four juxtaposed anticlinal walls that are thick and rectilinear, giving the testa a scalariform pattern (Figures 11-12).
The testa of E. glandulosus showed polygonal cells, with 4-5 anticlinal walls, pronounced, rectilinear, and juxtaposed cell walls, constituting a reticulate ornamentation pattern (Figures 19-20).
DISCUSSION
The seed morphology ofthe examined species of Echinodorus showed similar patterns to the description presented by Kaul (1978) and Haynes & Holm-Nielsen (1985) for others species ofthe genus. As explained by Kaul (1978), the recurvate shape of the seed is thus an adaptive character and very important to the establishment of seedlings.
Micromorphological examinations revealed that the testa of all analyzed species were similar in terms of cell distribution in lateral view, all of them forming distinct longitudinal alignments. As these cells vary in form, depending on the outline of the embryo, they do not furnish any taxonomically useful information. But the patterns of seed coat ornamentation, cell form, anticlinal cell wall thickness, anticlinal cell wall junctions, and the form of the anticlinal wall junctions yield important taxonomic criteria for the studied species. Those epidermal characters were applied to others aquatic plants in systematic treatments (Chance & Bacon, 1984; Shaffer-Fehre, 1991; Chuang & Ornduff, 1992; Chuang & Constance, 1992; Suseela et al., 1998), recognizing their importance as diagnostic characters as well (sensu Stuessy, 1990).
The dwarf plants passing as E. tenellus are the basis of the subgenus Helianthium (Fassett, 1955) and that species presented the more distinctive seed coat sculpture: tenuous ribbed surface. The others species are representatives of the subgenus Echinodorus (Fassett, 1955) and they had an evident reticulated pattern, excepted E. pubescens, due to regularly aligned cells, with a predominance of oblong cells with four juxtaposed anticlinal walls. In that way, the section Palaefolii proposed by Rataj (2004) seems not to be an uniform group, because of the differences of the micromorphology of the seed coats of E. pubescens, E. subalatus, and E. palaefolius.
Among the species with a reticulate seed surface pattern, E. glandulosus demonstrated the most defined and compact delineation, with rectilinear anticlinal walls with predominantly right angles that lend a geometric aspect to the seed surface. This pattern contrasts greatly with that of E. grandiflorus subsp. aureus, where the non-juxtaposed sinuous walls ofthe anticlinal cells generate a reticulate-cannulate pattern.
Echinodorus macrophyllus (Kunth) Micheli and E. scaber Rataj are representative species of the section Macrophylii Rataj (Rataj, 2004). The recognition at infraspecific level of E. macrophyllus subsp. scaber by Haynes & Holm-Nielsen (1986) was justified by the not distinctive fruit morphology. Then, a future analysis of the seed micromorphology of both species may be important to resolve this taxonomic problem.
Echinodorus subalatus, E. palaefolius, and E. macrophyllus subsp. scaber demonstrated similar micromorphological characteristics, having polygonal cells with juxtaposed and rectilinear anticlinal walls around the foveas that generated a reticulate tenuous-foveate pattern. However, E. subalatus, E. palaefolius (Palaefolii section sensu Rataj, 2004), and E. macrophyllus subsp. scaber (Macrophylii section) belong to different infraspecific categories. Therefore, those sections seem an artificial taxonomic arrangement. In spite of the similarities among the seeds of these species, it is possible to distinguish E. subalatus by the presence of an elliptical and well-defined central depression.
Seed coat ornamentation allows the taxonomic separation of E. tenellus, E. pubescens, E. reticulates, and E. grandiflorus subsp. aureus; while E. paniculatus and E. lanceolatus demonstrate great similarity in terms of the micromorphological characteristics of their seeds.
Echinodorus paniculatus and E. lanceolatus differ from others studied species by having circular cells and an ample central fovea. However, the similarities between the seed surfaces of these two species inhibit the use of their micromorphological characteristics for taxonomic purposes. In the same way, the vegetative and floral morphology of those species is quite similar (Matias, 2007, Matias et al., 2007). But, the species could be identified only by the presence of glands in fruit (Rataj, 1968) and the anatomical characters of the scapes (Matias et al., 2008).
Although the micromorphological characteristics of the seed surfaces did not permit the distinction of all the species examined, the observed patterns allow the identification of groups of similar species, which may be important for future infra-generic classification. As such, the ornamentation patterns and the characteristics related to the primary sculpturing seen in E. lanceolatus and E. paniculatus, and in E. subalatus, E. palaefolius, and E. macrophyllus subsp. scaber may represent two similar groups. Additionally, these characteristics should be taken into consideration in the infra-generic classification of Echinodorus as synthetic character.
ACKNOWLEDGEMENTS
We thank the Fundação Boticário de Proteção à Natureza, the Programa Institucional de Capacitação Docente e Técnica/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PIDCT/CAPES), and the Centro de Microscopia Eletrônica da Universidade Federal do Rio Grande do Sul.
REFERENCES
BARTHLOTT, W., 1981. Epidermal and seed surface characters of plants: systematic applicability and some evolutionary aspects. Nordic Journal of Botany 1: 345-355.
BARTHLOTT, W., 1984. Microstructural features of seed surfaces. In: V. H. HEYWOOD & D. M. MOORE (Eds.): Current concepts in plant taxonomy: 95-105. Academic Press, London.
BEHNKE, H. D. & W. BARTHLOTT, 1983. New evidence from the uttrastructural and micromorphological fields in angiosperm classification. Nordic Journal of Botany 3: 43-66.
CHANCE, G. D. & J. D. BACON, 1984. Systematic implications of seed coat morphology in Nama (Hydrophyllaceae). American Journal of Botany 7(6): 829-842.
CHUANG, T. I. & L. CONSTANCE, 1992. Seeds and systematic in Hydrophyllaceae: Tribe Hydrophylleae. American Journal of Botany 79(3): 257-264.
CHUANG, T. J. & R. ORNDUFF, 1992. Seed morphology and systematic of Menyanthaceae. American Journal of Botany 79(12): 1396-1406.
FASSETT, N. C., 1955. Echinodorus in the American tropics. Rhodora 57: 133-156, 174-188, 202-212.
HAYNES, R. R. & L. B. HOLM-NIELSEN, 1985. A generic treatment of Alismatidae in the Neotropics with special reference to Brazil. Acta Amazonica 15(1-2): 153-193.
HAYNES, R. R. & L. B. HOLM-NIELSEN, 1986. Notes on Echinodorus (Alismataceae). Brittonia 38(4): 325-332.
HAYNES, R. R. & L. B. HOLM-NIELSEN, 1989. Speciation of Alismatidae in the Neotropics. In: L. B. HOLM-NIELSEN, I. C. NIELSEN & H. BALSLEV (Eds.): Tropical Forests. Botanical dynamics, speciation and diversity: 211-219. Academic Press, London.
HAYNES, R. R. & L. B. HOLM-NIELSEN, 1994. The Alismataceae. Flora Neotropica 64: 1-112.
HAYNES, R. R., D. H. LES & L. B. HOLM-NIELSEN, 1998. Alismataceae. In: K. KUBITZKI (Ed.): The families and genera of vascular plants - Flowering plants monocotyledons Alismatanae and Commelinanae (except Graminae): 4: 11-16. Springer, Berlin.
KAUL, R. B., 1978. Morphology of germination and establishment of aquatic seedlings in Alismataceae and Hydrocharitaceae. Aquatic Botany 5: 139-147.
LEHTONEN, S., 2006. Phylogenetics of Echinodorus (Alismataceae) based on morphological data. Botanical Journal of the Linnean Society 150: 291-305.
LEHTONEN, S. & L. MYLLYS, 2008. Cladistic analysis of Echinodorus (Alismataceae): simultaneous analysis of molecular and morphological data. Cladistics 24: 218-239.
LEPRUN, J. C., 1984-1985. La conservation et la gestion des sols dans le Nordeste brésilien. Particularités, bilan et perspectives. Cahier ORSTOM. Pédologie 21: 257-284.
LOT, A. & A. NOVELO, 1984. Afinidades floristicas de las monocotiledoneas acuáticas mesoamericanas. In: S. P. DARWIN & A. L. WELDEN (Eds.): Biogeography of Mesoamerica. Proceedings of a symposium: 147-153. Tulane University, New Orleans.
LOT, A. & A. NOVELO, 1994. Alismataceae. In: G. DAVIDSE, M. SOUSA & A. O. CHARTER (Eds.): Flora Mesoamerica: Alismataceae a Cyperaceae: 6: 3-8. Universidad Nacional Autônoma de México, México.
MATIAS, L. Q., 2007. O gênero Echinodorus (Alismataceae) no domínio da caatinga brasileira. Rodriguésia 58(4): 743-774.
MATIAS, L. Q., A. SOARES & V. L. SCATENA, 2007. Systematic consideration of the petiole anatomy of species of Echinodorus Richard (Alismataceae) from north-eastern Brazil. Flora 202: 395-402.
MATIAS, L. Q., A. SOARES & V. L. SCATENA, 2008. Anatomy of Echinodorus (Alismataceae) capes from northeastern Brazil. Edinburg Journal of Botany 65(1): 1-11.
MICHELI, M., 1881. Alismaceae. In: A. DECANDOLLE (Ed.): Monographie phanerogamarum: 29-83. Masson, Paris.
RADFORD, A. E., W. C. DICKSON, J. R. MASSEY & C. R. BEL, 1974. Vascular plant systematics. Harper & Row, New York.
RATAJ, K., 1968. Echinodorus paniculatus Michelli and its ally E. lanceolatus Rataj, sp. Nov. (American Alismataceae). Bulletin du Jardin Botanique National de Belgique 38: 401-408.
RATAJ, K., 2004. A new revision of the swordplant genus Echinodorus Richard, 1848 (Alismataceae). Aqua 1: 1-142.
ROGERS, G. K., 1983. The genera of Alismataceae in the southeastern United States. Journal of Arnold Arborretum 64: 383-420.
SALISBURY, E. J., 1942. The reproductive capacity of plants. Bell, London.
SUSEELA, M. R., U. N. RAI, P. GUPTA & S. DEVI, 1998. Cell wall ultrastructure of the testa of Nymphoides hydrophylla (Lour.) Kuntze. Phytomorphology 48(3): 289-293.
SHAFFER-FEHRE, M., 1991. The position of Najas within the subclass Alismatidae (Monocotyledones) in the light of new evidence from seed coat structures in the Hydrocharitoideae (Hydrocharitales). Botanical Journal of the Linnean Society 107: 189-209.
STEARN, W. T., 1973. Botanical latin. David & Charles, London.
STUESSY, T. F., 1990. Plant taxonomy. The systematic evaluation of comparative data. Columbia University Press, New York.
SYSTEMATICS ASSOCIATION COMMITTEE FOR DESCRIPTIVE BIOLOGICAL TERMINOLOGY, 1960. II - Terminology of simple symmetrical plane shapes (Chart 1). Taxon 9(8): 245-257.
WEST, D. & D. F. WHIGHAM, 1976. Seed germination of arrow arum (Peltandra virginica L.). Bartonia 44: 44-49.
WILKINSON, H. P., 1983. Leaf anatomy of Gluta (L.) Ding Hou (Anacardiaceae). Botanical Journal of Linnean Society 86: 44-49.
YEO, R. R., 1965. Life history of sago pondweed. Weeds 13: 314-321.
 Mailing address:
Mailing address:
Museu Paraense Emílio Goeldi
Editor do Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais
Av. Magalhães Barata, 376
São Braz – CEP 66040-170
Caixa Postal 399
Telefone/fax: 55-91 - 3249 -1141
E-mail:boletim@museu-goeldi.br
Recebido: 19/05/2009
Aprovado: 28/08/2009













