Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Boletim do Museu Paraense Emílio Goeldi Ciências Naturais
versão impressa ISSN 1981-8114
Bol. Mus. Para. Emilio Goeldi Cienc. Nat. v.5 n.1 Belém abr. 2010
Notes on the Vertebrates of northern Pará, Brazil: a forgotten part of the Guianan Region, I. Herpetofauna
Notas sobre os vertebrados do norte do Pará, Brasil: uma parte esquecida da Região das Guianas, I. Herpetofauna
Teresa Cristina Sauer Avila-PiresI; Marinus Steven HoogmoedII; Wáldima Alves da RochaIII
IMuseu Paraense Emílio Goeldi. Belém, Pará, Brasil (avilapires@museu-goeldi.br)
IIMuseu Paraense Emílio Goeldi. Belém, Pará, Brasil (marinus@museu-goeldi.br)
IIIUniversidade Aberta do Brasil. Canto do Buriti, Piauí, Brasil (waldima@yahoo.com.br)
ABSTRACT
We discuss the herpetological results of seven expeditions to the Guianan part of Pará, which resulted in a total of 80 species of amphibians (77 frogs and three caecilians) and 95 species of reptiles (36 species of lizards, three species of amphisbaenians, 49 species of snakes, five species of chelonians and two species of caiman). We report six species new to science (three frogs, one caecilian, one lizard, one amphisbaenian), six new records for Brazil (five frogs, one caecilian) and 23 new records for Pará (13 frogs, four lizards, six snakes). For each of the new records we provide comments. Special comment is made about a large population of the toad Atelopus hoogmoedi that seems to be doing well and does not show any signs of population decline as many species of Atelopus at higher elevations do. We provide a complete list of species collected per locality containing data on endemicity, habitat, reproduction and food. For each of the seven collecting sites we provide data on richness and abundance of species. The sites are compared regarding their species composition, even though we can not say how much of the differences are due to specific habitats or geographic variation, seasonal variation or sampling deficiency. We synonymised the Bufonid Rhinella martyi with Bufo margaritifer and selected a lectotype for Rana margaritifera in order to resolve the problems about this name.
Keywords: Amphibia. Reptilia. Guiana Centre of Endemism. Brazil. Species richness. Species list.
RESUMO
Os resultados herpetológicos de sete expedições à parte guianense do estado do Pará são apresentados e discutidos, registrando-se um total de 80 espécies de anfíbios (77 anuros e três Gymnophiona) e 95 espécies de répteis (36 espécies de lagartos, três espécies de anfisbenídeos, 49 espécies de ofídios, cinco espécies de quelônios e duas espécies de jacarés). Dessas espécies, seis são novas para a ciência (três anuros, um Gymnophiona, um lagarto, um anfisbenídeo), seis representam novos registros para o Brasil (cinco anuros, um Gymnophiona) e 23 novos registros para o Pará (13 anuros, quatro lagartos, seis ofídios). Comenta-se cada um dos novos registros. Comentários especiais são feitos sobre uma grande população do sapo Atelopus hoogmoedi, a qual parece estar bem saudável e não mostra sinais de declínio populacional, como muitas espécies de Atelopus em outros lugares de maior altitude. Uma lista completa das espécies coletadas por localidade, incluindo dados sobre endemismo, habitat, reprodução e alimentação, é apresentada. Para cada uma das sete áreas de coleta, apresentamos dados sobre riqueza e abundância de espécies. As áreas são comparadas quanto à similaridade na composição das espécies, ainda que não seja possível indicar quanto das diferenças encontradas deve-se a ambientes específicos ou variação geográfica, variação sazonal ou deficiência na amostragem das espécies. O Bufonidae Rhinella martyi é considerado sinônimo de Bufo margaritifer e um lectótipo para Rana margaritifera é selecionado visando dirimir dúvidas sobre o nome da espécie.
Palavras-chave: Amphibia. Reptilia. Centro de Endemismo Guiana. Brasil. Riqueza de espécies. Lista de espécies.
INTRODUCTION
On December 4, 2006, the State of Pará created five new conservation units in the northern part of Pará, north of the Amazon, in order to establish a large and protected, mostly forested, area that would form a continuous block with similarly protected areas in Amapá (Parque Nacional [PARNA] Montanhas de Tumucumaque), French Guiana, Suriname and Guyana, and with Indian Territories in the region (Figure 1) (Governo do Estado do Pará, 2006). The five conservation units created by the state were: Estação Ecológica (ESEC) Grão-Pará (4.2 million ha), Reserva Biológica (REBIO) Maicuru (1.2 million ha), and the Florestas Estaduais (FLOTA) de Faro (0.6 million ha), Trombetas (3.2 million ha) and Paru (3.6 million ha). Together they cover an area of 13.2 million ha and, with the already existing protected areas - Indian territories (TI) of Trombetas-Mapuera, Tumucumaque, rio Paru d'Este, Nhamundá-Mapuera and Zo'é; two 'Quilombola' (African-Brazilian) territories; the Florestas Nacionais (FLONA) Saracá-Taquera and da Mulata, REBIO do rio Trombetas, and ESEC Jari - they form an enormous block, although with different degrees of protection (besides TI and 'quilombola' sites, that harbour traditional populations, FLOTAs and FLONAs aim at the sustainable use of natural resources). Most of the newly created conservation units are covered by non-flooded tropical rainforest ('terra-firme' forest), but in several places other vegetation types, like flooded forests ('várzea' and 'igapó'), savanna and 'cerradão', are present as well. Only a relatively narrow band of land in 'Calha Norte Paraense' (CNP) along the Amazon is not protected and open to unregulated human occupation. We here consider as CNP that part of Pará that is situated north of the Amazon River.
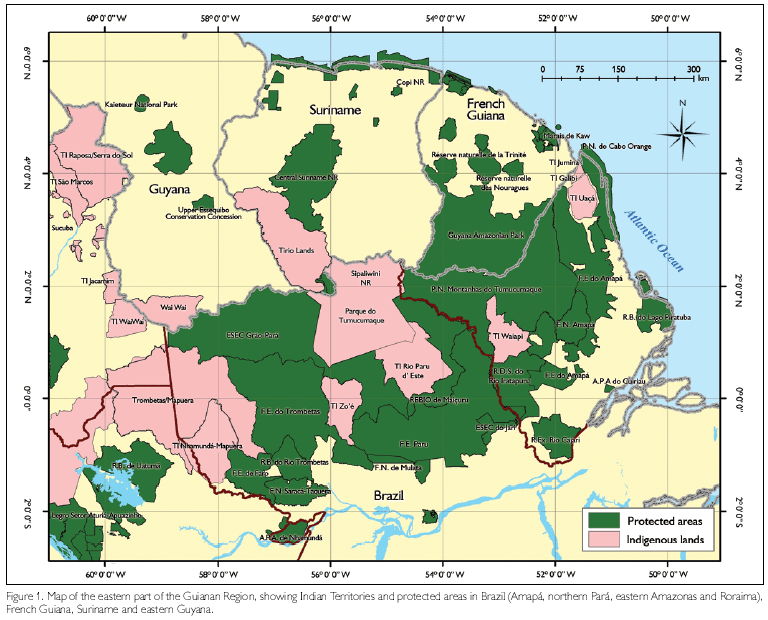
On February 14, 2007 several parties (Government of Pará [SEMA-PA], Conservação Internacional [CI-Brasil], Museu Paraense Emílio Goeldi [MPEG], Instituto do Homem e Meio Ambiente da Amazônia [IMAZON], Instituto de Desenvolvimento Florestal do Estado do Pará [IDEFLOR], Instituto de Manejo e Certificação Florestal e Agrícola [IMAFLORA], and the German Technical Cooperation Agency [GTZ]) signed an agreement to form a consortium to provide data to develop management plans for the five state protected areas ('Projeto Diagnóstico da Biodiversidade das Unidades de Conservação Estaduais do Mosaico Calha Norte, Estado do Pará'). As a result of this agreement seven expeditions to investigate the biodiversity of the recently established protected areas were planned. The localities to be inventoried were chosen by specialists from SEMA-PA, CI-Brasil, Imazon and MPEG, on the basis of satellite photographs, georeferenced databases, and vegetation and altitude data, with the goal to optimize the coverage of the different phytophysionomies and altitudes present in the area during seven three-week long expeditions distributed over a year. An additional overflight helped with the final definition of the areas chosen for sampling. As final transportation to five of the research areas was planned by helicopter, the localities could be chosen independent of road or river transport. Fieldwork started in January 2008 and ended in January 2009. During this period three expeditions to different localities in ESEC Grão-Pará and one each to a locality in the other four newly created conservation units were carried out by 12 - 13 researchers of the MPEG and about ten technical assistants.
Due to its continental size, many Amazonian areas are still poorly known regarding their herpetofauna. The northern part of the state of Pará, in Brazil, is one of these areas, with only a few spots reasonably well surveyed. This area north of the Amazon River forms part of the Guianan Region (or 'Guianas') as defined by Hoogmoed (1979b), mainly delimited by the Orinoco, Negro and Amazonas rivers on the west and south, and the Atlantic Ocean on the north and east. The Guianan Region encompasses the three Guianas (Guyana, Suriname, French Guiana), the southeastern part of Venezuela, and in Brazil the states of Amapá and Roraima, the state of Pará north of the Amazon River, and the relatively small northeastern part of the state of Amazonas, north of the Amazon River and east and north of the rio Negro. Silva et al. (2005) considered the area as the Guianan Area of Endemism, the largest of eight areas of endemism in the Amazon region, with half of its surface in Brazil. According to Silva et al., 2005) in the Brazilian part of this area of endemism, only 4.06% had been deforested, although this percentage may have gone up in the past few years. We do not include part of Colombia in the Guianan Region (and neither do Silva et al., 2005), as was done by the 2002 Paramaribo workshop (Hollowell & Reynolds, 2005 and articles therein), as there are no good zoogeographical reasons for that inclusion. Concerning herpetofauna, the area west of the rio Negro has no Guiana endemics. The Guianan Region has a number of species in common with other areas of Amazonia, but also has a number of endemic species. This is especially true for the 'tepuis', sandstone mountains with elevations above 1,500 m, usually considered a distinct biogeographic region ('Pantepui') within the Guianan Region (Hoogmoed, 1979b; McDiarmid & Donnelly, 2005), in Venezuela, western Guyana and extreme northern Brazil (Roraima and Amazonas States). In Suriname there is one sandstone mountain of 1,200 m altitude, but it has no Pantepui endemics (MSH, pers. obs.), which generally only occur above 1,500 m. In northern Pará, Amapá and French Guiana no tepuis are found and elevations just reach 900 m, thus explaining the absence of any herpetological tepui endemics in the area. A number of lowland species are also endemic to the Guianan region or part of it, although numbers have dropped when new range extensions became available (e.g. Caldwell & Hoogmoed, 1998).
About 350 species of amphibians and a similar number of reptiles are known from the whole of Amazonia, including the Guianas (see Eva & Huber, 2005: 11 for the limits of Amazonia, here considered as the area named Amazonia sensu lato [Ia+IIa+IIb]), of which c. 82% of the amphibians and 62% of the reptiles are endemic (Avila-Pires et al., 2007; Duellman, 1999). Hoogmoed (1979b) estimated that, for the Guianan lowlands, c. 52% of the amphibians and 26% of the reptiles were endemic. However, as more areas were better surveyed, many of the lowland species considered endemic to the Guianas in 1979 were shown to have a wider distribution throughout Amazonia (e.g. Allophryne ruthveni [Caldwell & Hoogmoed, 1998], Bufo guttatus and Lithodytes lineatus [MSH, unpublished data, material in MPEG]). Including also the fauna of the tepuis, Señaris & MacCulloch (2005) found that 54% of the amphibian species from the Guianas were endemic to the region, while Avila-Pires (2005) indicated that 30% of the reptile species were endemic. As there are still large gaps in our knowledge about the herpetofauna of the Guianan Region, both range extensions and new species are expected to be found in northern Pará.
A number of recent herpetofaunal studies focus on (part of) the Guianan Region, among them those on amphibians (Fouquet et al., 2007a, b; Lescure & Marty, 2000; Kok, 2000; Kok et al., 2006a), lizards (Hoogmoed & Lescure, 1975; Hoogmoed & Avila-Pires, 1989; Gasc, 1990), amphisbaenians and snakes (Gasc & Rodrigues, 1980; Chippeaux, 1986; Starace, 1998) from French Guiana; anurans (Hoogmoed, 1969a, b, 1971a, b, 1979a), wormsalamanders (Nussbaum & Hoogmoed, 1979), lizards and amphisbaenians (Hoogmoed, 1973), and some groups of snakes (Hoogmoed, 1977, 1980, 1983, 1985) from Suriname; an increasing number of studies on the herpetofauna of Guyana, especially the western (Pantepui) part of this country (Cole & Kok, 2006; Kok, 2005, 2006a, b, 2008a, b, 2009; Kok et al., 2006b; Kok & Castroviejo-Fisher, 2008; Kok & Kalamandeen, 2008; Lathrop & MacCulloch, 2007; MacCulloch & Lathrop, 2001, 2002, 2004a, b, 2005, 2009; MacCulloch et al., 2006, 2007, 2008a, b), several from the central and southern Guyana lowlands, like the Mabura Hill and Iwokrama region (Donnelly et al., 1998, 2005a, b, 2006; Ernst et al., 2005, 2007; Kok & Ernst, 2007, Kok et al., 2007, Señaris et al., 2008) and from the coastal area (EMC, 2006); the study by Gorzula & Señaris (1999) on the herpetofauna of Venezuelan Guayana, and by Pritchard & Trebbau (1984) on chelonians from Venezuela; and those on amphibians (Lima et al., 2006), snakes (Martins & Oliveira, 1993, 1998), and lizards (Vitt et al., 2008) from the Manaus area, Amazonas, Brazil. More specific papers are those by Hoogmoed & Avila-Pires (1991a), with data on Amphisbaenidae; by Hoogmoed & Avila-Pires (1992) on the lizard genus Arthrosaura, by Cunha et al. (1980), Carvalho (1997, 2002) and Vanzolini & Carvalho (1991), on lizards and snakes from Roraima. Some publications deal with species that occur throughout a large part of the Guianan Region (Campbell & Lamar, 2004; Dixon et al., 1993; Medem, 1983; Noonan & Gaucher, 2005, 2006; Noonan & Wray, 2006; Roze, 1996; Wollenberg et al., 2006, 2008). Conservação Internacional organized a series of expeditions to the Tumucumaque Mountains on the border of French Guiana and Amapá, between 2004 and 2006, but the herpetological results (Lima, 2008) are still under discussion and at the moment only can be used with much care, checking each species record. Hoogmoed (1979b, 1983), Hoogmoed & Avila-Pires (1991b), Señaris & MacCulloch (2005) and Avila-Pires (2005) present lists of Guianan herpetofauna, including data from Amapá, Amazonas, Pará and Roraima. In Avila-Pires (1995), a catalogue of the lizards of Brazilian Amazonia, data on lizards from the Guianan Region can also be found, while data on chelonians may be found in Vogt (2008). Bartlett & Bartlett (2003) is a good general introductory book for the Amazonian herpetofauna, and Rueda-Almonacid et al. (2007) is an excellent fieldguide for chelonians and crocodiles, but both are not complete for the Guianan Region. Avila-Pires et al. (2009) compared the lizard faunas from three sites on the Guiana Shield (Brokopondo and Sipaliwini [both Suriname, the last one on the border with Brazil] and Balbina [Amazonas, Brazil]) with ten sites in other areas of the Amazon Region. They showed that the Guianan sites were most closely related to Belém and Caxiuanã (both in Pará) in the lower Amazon area.
A list of species of amphibians and reptiles present in CNP as a whole does not exist. The areas that have been better studied are the lower Trombetas River, where environmental studies have been done in the context of a large bauxite mining project in the area (U. Galatti, pers. obs.); and the Jari (Monte Dourado) area, at the border with Amapá state, as part of a two-year multidisciplinary study by C. Peres and T. A. Gardner of the University of East Anglia (United Kingdom), and collaborators. Most data on the Trombetas studies, however, are not published. Part of the results of the Jari project, regarding herpetofauna, can be found in Gardner et al. (2007) and Ribeiro-Junior et al. (2008). Hoogmoed and Avila-Pires made collections (material in Museu Paraense Emilio Goeldi, Belém, Pará [MPEG] and in the National Museum of Natural History, Leiden, The Netherlands [RMNH]) in the rio Nhamundá area in 1988, the results of which have only partly (lizards) been published by Avila-Pires (1995). Between 1980 and 2006 personnel of MPEG made several collecting trips to the municipalities Almeirim and Monte Alegre and obtained small, but interesting collections, which are now in MPEG. Besides, occasional expeditions have been made, especially following the large rivers, which account for the sparse data found in the literature and specimens in collections. Avila-Pires (1995) registered a number of lizards from this area, even though only from few localities, showing large gaps of information for the area as a whole. Vogt (1994, 2008) and Haller & Rodrigues (2005, 2006) give data on chelonian species from the Trombetas River.
Based on the existing literature, for CNP we may expect approximately 100 species of anurans and up to nine species of Gymnophiona (Lescure & Marty, 2000; Lima et al., 2006; Señaris & MacCulloch, 2005; A.O. Maciel & Hoogmoed, unpublished data). Among reptiles, we could expect about 40 species of lizards, ten species of amphisbaenians, 100 of snakes, 11 of chelonians, and three species of caimans (Hoogmoed, 1973; Chippeaux, 1986; Martins & Oliveira, 1993, 1998; Avila-Pires, 1995, 2005; Starace, 1998; Rueda-Almonacid et al., 2007; Vogt, 2008; MSH, unpublished data). As no tepuis are present in the CNP area, tepui endemics are not expected to be found there, only lowland species (< 750 m). On the other hand, a number of species not present in other Guianan countries (French Guiana, Suriname, Guyana and Venezuela) possibly can be expected to occur in the areas under influence of the Amazon River.
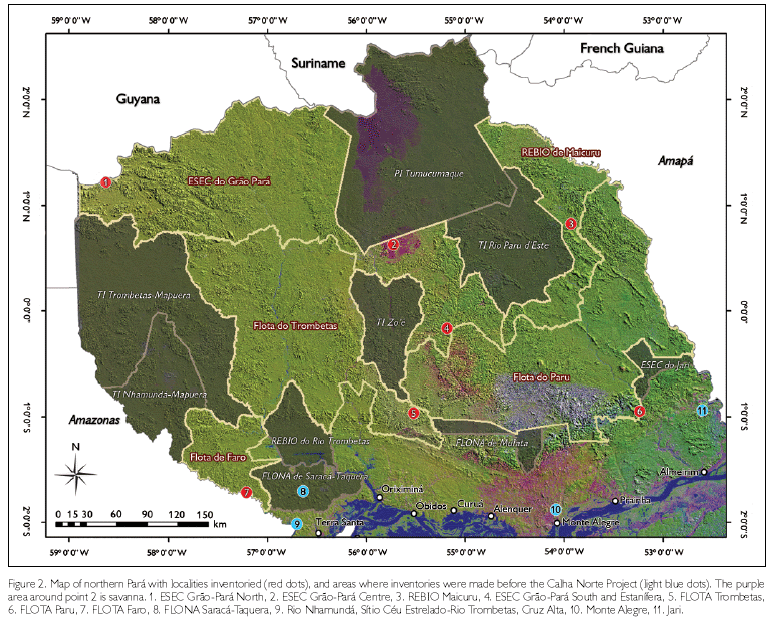
The study here presented as part of the CNP Project intended to inventory the herpetofauna from key localities surveyed during seven expeditions (Figure 2), taking into account the necessity to produce management plans for the five conservation units created by the State of Pará, Brazil, in 2006 - Floresta Estadual de Faro (FLOTA Faro), Floresta Estadual do Paru (FLOTA Paru), Floresta Estadual do Trombetas (FLOTA Trombetas), Reserva Biológica de Maicuru (REBIO Maicuru) and Estação Ecológica do Grão-Pará (ESEC Grão-Pará). Considering the large extension of the area covered by the five conservation units (13.2 million ha), it was impossible, within the period of 13 months, to accomplish intensive studies on the fauna of each of them. Therefore it was decided to select a number of points that together could cover the different phytophysionomies encountered in northern Pará, as explained before, and to perform in each of them a Rapid Assessment Program (RAP). Although the results obtained are not exhaustive, and new studies will be necessary to improve our understanding of the herpetofauna of the area, they represent an important advance in our knowledge, and provide the basis for management plans. We present here an analysis based on all expeditions, because much information is common to all or is complementary, and after that we highlight several species that represent new or interesting zoogeographic data.
MATERIAL AND METHODS
STUDY AREAS
The greater part of CNP is on the Guiana Shield, only a wide band north of the Amazon belongs to the alluvial Amazon valley. The core of the Guiana Shield is made up of pre-Cambrian metamorphic and igneous rocks, especially granites and gneisses. On all sides the core of the Guiana Shield is surrounded by a band of low areas of varying width consisting of alluvial sediments. The higher part of the Guiana Shield is covered with sandstone remnants of the Roraima Formation, which was deposited in Proterozoic time, 1.6-1.8 billion years ago. After uplift, this formation covered the Guiana Shield as an extensive sandstone plateau or tableland. During the Late Cretaceous and in the Tertiary there were new periods of further uplift of the area, at the same time that erosion shaped the present-day table mountains or tepuis, which are concentrated in the NW part of the Guiana Shield, in SE Venezuela and adjacent W Guyana, with some tepuis on the border of Brazil (Roraima and Amazonas States) with these countries (Hoogmoed, 1979b and literature cited therein). In northern Pará no sandstone tepuis are present, and consequently, by definition, no herpetofaunal tepui endemics. The northern and southern part of the Guianan Region are separated by the divide between rivers that are part of the Amazon basin and flow S from the divide to that river, and rivers of the Guianas that flow north directly to the Atlantic Ocean. The mountains of the divide, which is formed by the Acarai Mountains in the West (between Brazil and Guyana) and the Tumucumaque Mountains in the East (between Suriname and French Guiana on one side and Brazil on the other) are relatively low (in some places, like the Sipaliwini area, not higher than 250 m, in one place reaching up to about 900 m, but generally below 800 m). From the Amazon River the area of northern Pará gradually slopes north, up towards the divide. The area is hilly, with rounded hills and elevated plateaus at a level of about 500 m, that at least in part contain bauxite deposits. All rivers in the area run roughly N-S and have numerous rapids and waterfalls from their upper reaches to close to the Amazon, and therefore are difficult to navigate. The Trombetas River in the South and the Suriname River in the north (Hoogmoed, 1973) seem to divide the Guianan Region in an estern and a western part and may form a distribution barrier for some species.
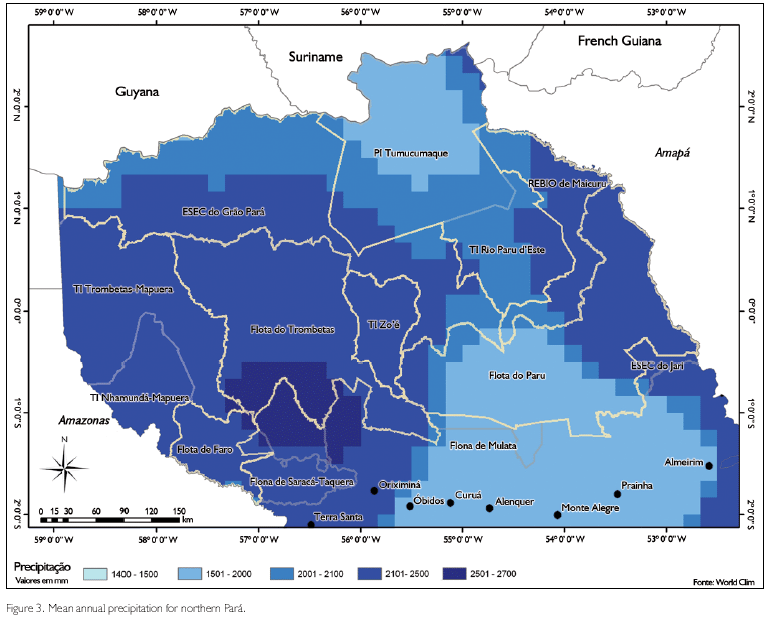
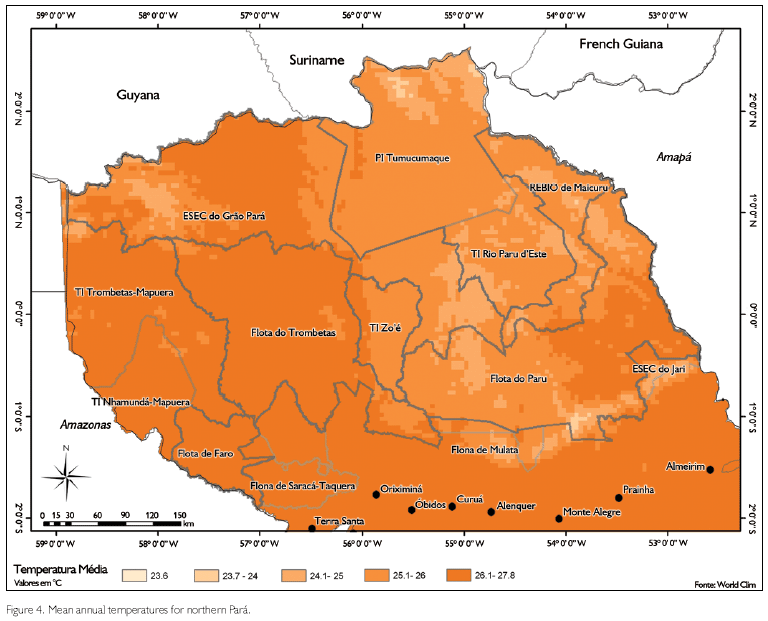
Most areas we inventoried have a wet tropical climate (Am according to the Kõppen classification), but site ESEC Grão-Pará North is in an area that is characterized as Aw (Peel et al., 2007; SUDAM, 1984). The rainy season generally is between December and June, with a short drier break in February/March, the dry season is between July and November. Total mean annual rainfall is around 2,100-2,500 mm per year for most areas inventoried, except for sites ESEC Grão-Pará North and ESEC Grão-Pará Centre, which are in areas with 2,000-2,100 mm, and site FLOTA Paru, which is in an area with a mean annual rainfall of 1,500-2,000 mm (Figure 3). Mean annual temperatures in most of the area are about 25-26 oC, but sites FLOTA's Faro, Trombetas and Paru are in areas where that temperature is about 27 oC (Figure 4) (SUDAM, 1984).
The study areas are located in the Guianan Region of the northern part of Pará, north of the Amazon River, in the five conservation units established in December 2006: one each in the FLOTA's Faro, Trombetas and Paru, one in the REBIO Maicuru and three in the ESEC Grão-Pará (Figure 2). Because of its large size, covering several vegetation types, three expeditions were made to ESEC Grão-Pará: one to the most northwestern part, close to the frontier with Guyana, one to the central part, just south of the Indian Territory of Tumucumaque, and one to the southeastern part, close to the border with FLOTA Paru. A short description of the research areas is provided below. Unfortunately, no details on the vegetation have been provided by the botanists yet, so general terms are used.
FLOTA Faro (0.6 million ha) is situated in the municipalities of Faro and Oriximiná, on the right bank of the rio Nhamundá, which forms the border of Pará with Amazonas. The area belongs to the rio Nhamundá basin and is covered by tropical rain forest (terra-firme forest = 'Floresta Ombrófila Densa das Terras Baixas' according to RADAM-Brasil ["Radar na Amazônia" project] terminology), except in a band of about 700 m along the river and some distance up along the creeks, that is covered by várzea forest ('Floresta Ombrófila Densa Aluvial'), which is inundated during part of the year (Figure 5). Four trails (all in the municipality of Faro) were cut, radiating from a base camp (S 1o 42' 50.44" W 57o 12' 47.88") that was located on the northern (left) bank of the rio Nhamundá, some distance WNW of Faro, where the river runs more or less east-west. Trails 1-3 ran in a northerly direction, parallel to each other and separated by 800 m, for a distance of 3 km. They started out in várzea forest and after about 700 m entered terra-firme forest. Trail 4 ran 1.5 km SW and 1.5 km SE of the camp, following the riverbank through várzea forest. The area studied was between 0 and 30 m above sea level, with low hills. The river is in open contact with the Amazon, no rapids or waterfalls being present downstream from the collecting area. Pitfall traps were installed in each of the trails 1, 2 and 3, with a distance of 250 m between them within each trail. Because of inundation, no poitfalls were installed in trail 4. The first two pitfalls in trail 1 were inundated and did not work. Trail 1 had pitfalls at 50 m, 250 m, 500 m and 750 m. Trail 2 had pitfalls at 600 m, 850 m, 1,100 m and 1,350 m. Trail 3 had pitfalls at 200 m, 450 m, 700 m, 950 m, 1,200 m, 1,450 m, 1,700 m and 1,950 m. FLOTA Faro was sampled during the first expedition, between January 14 and 28, 2008, during the wet season. At that time the level of the river was high.

FLOTA Trombetas (3.2 million ha) is situated in the municipalities of Oriximiná, Óbidos and Alenquer. It forms part of the basin of the Trombetas River and in the north it borders on the western part of ESEC Grão-Pará. Our camp (S 0o 57' 45.97" W 55o 31' 20.28") was in the municipality of Óbidos, in the southeastern corner of the unit, in an area mainly covered by terra-firme forest ('Floresta Ombrófila Aberta Submontana'). The camp was situated in an opening in a forest with many old Cecropia trees, at about 100 m from a creek with rocks and a sandy bottom. It was at the base of a higher area with large rocks and an open forest resembling secondary forest ('capoeira'). Three trails radiated from the camp. Trail 1 ran initially E for 2 km, passing the helicopter landing area at 500 m and than turned N for 7 km, reaching the rio Cuminapanema in a transitional area. Trail 2 ran W for 5 km, first rather level, but after crossing two small creeks steadily uphill, in the last few hundred meters reaching 'Floresta Ombrofila Densa Submontana'. Trail 3 ran SE for 3 km, the first kilometer up a hill (the same one with the helicopter landing area on top) that was strewn with large granite boulders and covered by a low, open type of forest with lianas (Figure 6), and then descending into a lower, flat area with several creeks. The area studied was between 300 and 450 m above sea level, and was hilly, with a number of small, shallow, clear water creeks, sometimes with steep banks, cut about 10 m into the surrounding terrain. The helicopter landing area was at an altitude of 350 m on a rocky hill top with rockslates (lajedos) and an open vegetation of low bushes, cactus (Cereus) and bromeliads (Figure 7). This area formed a distinctive habitat, quite different from the surrounding terra-firme forest. Some large rockslates and boulders also were present in part of the adjacent terra-firme forest (between the helicopter landing area and the camp), which caused some of the open habitat and rockdwelling species to enter the forest. Pitfalls and driftfences were placed in trail 1 at 100 m, 400 m and 950 m from camp. In trail 2 at 200 m, 450 m, 700 m, 1,000 m, 1,250 m, 1,500 m, 1,750 m, 2,100 m, 3,250 m, 3,500 m, 3,750 m, 4,000 m and 4,250 m. The pitfalls at distances over 3,250 m on April 19, 2008 were relocated to 300 m, 1,100 m, 1,350 m, 1,600 m and 1,900 m because of logistical problems with pitfalls beyond 2,100 m. In trail 3 no pitfalls were placed. FLOTA Trombetas was the target of the second expedition, which took place between April 16 and May 1, 2008, in the middle of the rainy season.


REBIO Maicuru (1.2 million ha) is situated in the municipalities of Almeirim and Monte Alegre and is drained by the rivers Maicuru, Paru and Jari. On the north-northeast it reaches the rio Jari (border with Amapá), on the southeast and south it is bordered by FLOTA Paru, and on the west (and partly northwest) it borders on the ESEC Grão-Pará (for a short distance), and the Indian Territories (TI) Rio Paru d'Este and Tumucumaque. Our camp (N 0o 49' 43.03" W 53o 55' 52.32") was located in the municipality of Almeirim, in the middle of the conservation unit, at an altitude of 150 m above sea level, at some distance from the rio Ipitinga and about 15 m above the river plain. The research area was covered with terra-firme forest ('Floresta Ómbrofila Densa Submontana') (Figure 8), but the forest along the river apparently was regularly flooded, as shown by high water marks on the vegetation. This river forest (igapó) differed from terra-firme forest by being denser, with many low-slung lianas and growth of smaller trees and in some places by the presence of large Guadua bamboo stands. The banks of the river were steep, but in several places there were sandy beaches that dropped steeply in the water (Figure 9). From the camp, trail 1 ran NNW for 4.7 km, parallel to, but at some distance from, the river, through terra-firme forest; at about 1 km from camp it crossed a large inundated area along a creek. Trail 2 ran WNW for 5 km through terra-firme forest in terrain with steep hills and ridges. Trail 3 ran SW for 6 km, steadily climbing and near its end reached an altitude of about 550 m. Trail 4 ran S, closely following the river bank through regularly flooded (dry at the time) forest. The area was rather flat, with isolated small hills, but in the SW part there was a large hill-complex reaching an altitude of 550 m. Pitfalls and driftfences were placed in trail 1 at 360 m, 600 m, 800 m and 950 m from camp. In trail 2 they were placed at 350 m, 500 m, 700 m and 900 m from camp. Both trails 1 and 2 ran through terra-firme forest. In trail 3, also through terra-firme forest, no pitfalls were installed. Trail 4 ran through regularly flooded (dry at the time) forest close to the riverbank. In order to get a comparable effort in terra-firme and river bank forest, eight pitfalls were installed in trail 4, at 370 m, 750 m, 900 m, 1,040 m, 1,450 m, 1,600 m, 1,800 m and 2,000 m from camp. The fifth expedition visited REBIO Maicuru between October 21 and November 6, 2008, during the dry season.


FLOTA Paru (3.6 million ha) is situated in the municipalities of Monte Alegre, Alenquer and Óbidos and is drained by the rivers Maicuru, Paru and Jari. On the north it is bordered by the eastern part of ESEC Grão-Pará and REBIO Maicuru, on the east it is bordered by the rio Jari, that forms the border with Amapá. Our camp (S 0o 56' 38.29" W 53o 14' 10.68") was locateded in the municipality of Almeirim, in the SE part of the FLOTA, where its border is formed by the rio Paru (Figure 10). The camp was situated at about 100 m from the W bank of the river, in a large patch of low secondary vegetation, and on the S and E side it was bordered by a belt of dense liana forest of 500 m wide. Along the river there was an irregular band of 'Floresta Ombrófila Aberta de Terras Baixas' with antropogenic influences, in several places caused by the presence of isolated houses on this bank of the river. The vegetation on the riverbank itself was rather open, apparently regularly flooded ('igapó'), with hardly any undergrowth in the forest, although in some places there were large clearings, completely taken over by grass and bamboo. The banks of the river were gently sloping and the water of the river was clear, but not very transparent. Some distance downriver from our camp there was a complex of rapids, separating this part of the river from direct contact with the waters of the Amazon. The vegetation away from the river consisted of terra-firme forest ('Floresta Ombrófila Densa de Terras Baixas'). The area along the riverbank was flat, but at about one kilometer from the river bank became hilly with steep slopes, no plateaus, and traversed by several large and small creeks with clear, transparent water. From the camp, trail 1 ran for 5 km NE through 'Floresta Ombrófila Aberta de Terra Baixa', crossing one creek and ending on the river bank upstream from the camp. There were many signs of human activities in this area, with hunting trails, felled trees, open areas and overgrown agricultural fields. Trail 2 ran NW for 9 km through terra-firme forest, crossing several creeks and with many changes in altitude. Trail 3 ran SW for 4 km, first crossing the liana forest around the camp, after 1 km it reached an open cultivated area and then ran through terra-firme forest, crossing a partly dry creek with isolated pools of water in a rocky bed and reaching a creek with running water. In the creeks there were rock outcrops. Trail 4 branched off from trail 3 at the open cultivated area and then ran S for 4 km, closely following the river bank through open river-bank vegetation ('Floresta Ombrófila Aberta de Terra Baixa'), and crossing some creeks. Altitude in the study area varied from 30 to 100 m. Four pitfalls were placed along each trail at distances of 250 m, 500 m, 750 m and 1,000 m, all in level terrain of about 30 - 90 m. The sixth expedition inventoried FLOTA Paru between December 4 and 19, 2008, at the beginning of the rainy season.

ESEC Grão-Pará occupies 4.2 million ha in the municipalities Oriximiná, Óbidos, Alenquer and Monte Alegre, and runs from the frontier with Guyana in the NW to the TI Tumucumaque, TI Rio Paru d'Este and REBIO Maicuru in the E. A large, more or less triangular western part connects by a narrow neck to a smaller more or less rectangular eastern part. On the south it is bordered by the TI Trombetas-Mapuera, FLOTA Trombetas, TI Zo'é and FLOTA Paru. Because of its great size and different physionomies three localities were sampled in this conservation unit, respectively ESEC Grão-Pará North, Centre and South. Some haphazard collections were made in Camp Curuá (Estanífera) of the mining company rio Tinto. The area is drained, from W to E, by the headwaters of the Mapuera and Trombetas rivers, and by the Paru de Oeste, Cuminapanema, Curuá and Maicuru rivers.
Camp ESEC Grão-Pará North (N 1o 17' 7.51" W 58o 41' 45.24") was situated in the NW part of the unit, in the municipality of Oriximiná, close to the border with Guyana in the Acarai Mountains (Figure 11), at an altitude of 500 m on a hill. The area is covered by terra-firme forest ('Floresta Ombrófila Densa Submontana') and is very hilly, with steep slopes leading down to creeks with clear, transparent water. Relatively small rock outcrops occur sparsely in the area, completely covered by forest. From the camp, trail 1 ran SE for 4.35 km, first descending into a valley at 400 m, than climbing out of the valley up a spur of a hill to 600 m, down again to a second valley at about 460 m and up another hillside to 500 m. Trail 2 ran S for 900 m and then SW, following a spur of the hill on which the camp was situated, at the end slightly going down, and to the west, after 2.8 km joining trail 3 at km 2.5. Trail 3 ran SSW for 4. km, descending into a valley at 400 m altitude, following a creek, at km 2.5 it was joined by trail 2 from the east. Trail 4 started at km 0.9 of trail 2 where it split off to SSE for 2.1 km, down from the camp into a valley at 400 m altitude. Pitfalls were placed in trail 1 at 150 m, 250 m, 450 m, 650 m, 1,800 m, 2,000 m, 2,150 m and 2,260 m from camp; and in trail 3 at 300 m, 550 m, 750 m, 950 m, 1,100 m, 1,250 m, 1,450 m, and 1,650 m from camp. In trails 2 and 4 no pitfalls were placed. Altitudes varied between 350 and 600 m above sea level. ESEC Grão-Pará North was the aim of the fourth expedition, between August 25 and September 11, 2008, during the dry season.

Camp ESEC Grão-Pará Centre (N 0o 37' 49.01" W 55o 43' 42.60") was situated in the municipality of Óbidos, in the northern part of the eastern half of the ESEC, close to the southern border of the TI Tumucumaque. It was at an altitude of 400 m in a transition zone at the W margin of a large island of terra-firme forest ('Floresta Ombrófila Densa Submontana') within a large savanna enclave. The savanna area consisted of hilly terrain (300 to 500 m), with many areas of rock outcrops (from horizonally flat to curved and steep) and with a vegetation of shrubs and low forest (Figure 12), sometimes interrupted by grassy areas with isolated trees (e.g. Curatella) (Figure 13). Creeks in the savanna were rare. One encountered was a deep (3 m) gully with vertical banks and did not contain any water. Another creek arose at the base of a large complex of rock outcrops and contained clear, transparent water. This creek ran at the border between open rock outcrop and savanna forest and formed some deeper pools connected by shallower areas. Open rock outcrops were generally wet and retained water in crevices and under loose rocks, well after rains had stopped (Figure 14). Hillsides were generally steep. The forest was terra-firme forest with large trees and a high canopy at about 30 m. The forest island was traversed by a large creek with (at the time shallow) clear, transparent water, in some places forming deeper pools. The transitional forest between forest and savanna consisted of small, slender-stemmed, low trees with some larger trees interspersed; this vegetation was characterized by the botanists as 'cerradão'. From camp, trail 1 ran N for 5 km through terra-firme forest and slightly undulating terrain, crossing the creek in the forest island at about 1 km from camp. Trail 2 ran NE for 5 km, reaching the E edge of the forest, first dropping into the bed of the aforementioned creek, than steeply climbing up to a plateau at 500 m and than dropping again to a level of 400 m. Trail 3 ran S for 4.4 km, generally through savanna over steep hills, but at 700 m and at 2.5 km entering narrow areas of forest with creeks. Trail 4 ran roughly W for 5 km through open savanna, savanna forest and open rock outcrops, twice crossing narrow areas of terra-firme forest without creeks. The first part of the trail was in an area with steep hills, and after 1450 m descended into a relatively flat area. Pitfalls in trails 1 and 2 were placed at 500 m, 750 m, 1,000 m and 1,250 m from camp, all in forest. In trail 3 (savanna) they were placed at 200 m, 450 m, 750 m and 900 m. In trail 4 (savanna) they were placed at 400 m, 800 m, 1,240 m and 1,440 m. Altitudes varied between 310 and 450 m above sea level. ESEC Grão-Pará Centre was inventoried between January 10 and 31, 2009, during the early rainy season.



Camp ESEC Grão-Pará South (S 0o 9' 55.76" W 55o 11' 11.04") was situated in the municipality of Alenquer, in the SE part of the ESEC, close to its border with FLOTA Paru, and only 6 km NW of the Base Curuá (Estanífera) of Rio Tinto, at an altitude of 300 m. It was situated in a wide creek valley surrounded by hills. The area, according to vegetation maps, was covered by 'Floresta Ombrófila Densa Submontana', but along creeks, around a lake present W of the camp, and on top of a plateau, the forest was clearly different from the terra-firme forest covering the hills. In the creek valleys and near a lake were patches of 'açaizal', açaí forest dominated by Euterpe palms in shallow water. The forest along creeks in low-lying areas generally was open, with a muddy surface and pools, tufts of grasses, few large trees and many thin trees; it was considered as 'igapó forest' by the botanists. From the camp, trail 1 ran NNE for 5 km, first crossing the side of a hill, than dipping into a partly inundated creek valley, before starting a long climb up to a plateau that was covered by a low type of forest (canopy at 10 m) that consisted of closely growing small trees with thin stems (called 'cerradao' by the botanists). Only few larger trees were present. Trail 2 ran E for 5 km, first through a level creek valley, but after 2 km climbing a ridge and going down again to the next creek valley. Trail 3 ran SE for 5 km, through a swampy area along a creek, after 3 km continuing on the lower part of a hill, more or less parallel to a creek valley. Trail 4 ran W for 3.4 km through a creek valley, in the first 50 m crossing a very dense liana forest, than entering igapo forest and crossing a large deep creek, with steep sides and water with much organic particles, several times. Along this trail there were several ponds (1.5 m deep) in the forest. At the end the trail reached a sizeable open lake and ran around it. The lake on most sides was bordered by palms (Attaleiaspinosus, A. maripa, Mautitia flexuosa), but on its north side was bordered by a sloping area with terra-firme forest that reached the water margin. The centre of the lake was covered by a vegetation of Cyperaceae and grasses, along its edges there were waterlilies. The water was very clear and transparent (at least 2 m view). A creek flowed out of the lake on the east and passed the camp about 3 km downstream. On the west a small creek, completely overgrown with vegetation, flowed into the lake. The altitude varied from 300 m in the creek valleys to 450 m on top of the plateaus. Pitfalls were only placed in trails 1 (10 sets) and 3 (6 sets). In trail one they were at 1,550 m, 1,650 m, 1,800 m, 2,000 m, 4,000 m, 4,100 m, 4,200 m, 4,300 m, 4,400 m and 4,500 m. The first four were in terra-firme forest between 320 and 380 m altitude, the last six, from 4,000 m on, were in cerradao forest at 420 m altitude. Those in trail 3 were at distances of 800 m, 1,230 m, 1,280 m, 1,500 m, 1,650 m, and 1,750 m from camp. No pitfalls were placed in trails 2 and 4 because large areas of those trails were inundated creek valley. The third expedition targeted ESEC Grão-Pará South between June 6 and 21, 2008, towards the end of the wet season.
Base Curuá (Estanífera) of mining company Rio Tinto (S 0o 13' 16.5" W 55o 09' 45.0"), in the municipality of Alenquer, although not an area that was systematically collected, like the ones described before, is shortly mentioned because some species were collected here that were not collected elsewhere. It is situated 8 km SE of ESEC Grão-Pará South, on top of a plateau (450 m), with an airstrip and an area where semi-permanent barracks have been mounted for personnel of Rio Tinto working in the area. The area around the campsite and near the airstrip embarking site consists of a low forest (canopy 5 m) with many narrowly spaced thin-stemmed trees (Figure 15). This forest was characterized by the botanists as 'cerradão'. Aong a trail to the rio Curuá there was a small open rock savanna, with large areas of bauxite rock on the surface and a sparse vegetation of herbs, Ananas and shrubs.

LOGISTICS
All expeditions started in Belém and went to Santarém by commercial flight, except the expedition to ESEC Grão-Pará North which flew from Belém to Boa Vista, Roraima. The expedition to FLOTA Faro used a boat as base camp. The expedition to ESEC Grão-Pará North traveled by bus from Boa Vista to Caroebe (still in Roraima) and from there by helicopter to base camp ESEC Grão-Pará North. In the other five expeditions participants were transported from Santarém by one-engine planes to either Camp Curuá (Estanífera) of Rio Tinto, to airstrip '13 de maio' (REBIO Maicuru) or to Monte Dourado (FLOTA Paru). From those places transport to the cam ps was by helicopter, except for the exped ition to FLOTA Paru, which from Monte Dourado went by bus to the rio Paru and crossed the river by canoes with an outboard motor. Base camps, trails and pitfalls were prepared shortly before arrival of the scientific participants. Camp size tended to increase during the year that expeditions were held, from 210 m2 in FLOTA Trombetas, to 350 m2 during the last expedition (ESEC Grão-Pará Centre). Helicopter time amounted to 287:10 h, small airplanes time to 334:53 h.
COLLECTING AND PREPARATION OF HERPETOFAUNA
Reptiles and amphibians were collected by means of two complementary methods, viz. active sampling (AS), which combined two techniques: time constrained searches (TCS) and visual and audio encounter surveys (VES) (no recordings of calls were made); and passive sampling by pitfall traps with drift fences (PD). Systematic collecting took place in all seven expedition areas described above. Camp Curua (Estanifera) is not a major collecting site, no systematic sampling was done there and it has not been included in any calculations, except for total number of species in the whole area, since a few species have been collected only there. For the same reason it appears in the table presented in the Appendix 1.
All trails and most pitfalls were georeferenced, but we here only present the coordinates of the centrally located campsites (mark 0 of the trails of every expedition).
Active sampling (Crump & Scott, 1994; Scott, 1994; Ribeiro-Junior et al., 2008) consisted in actively searching for animals during day and night, along marked trails and in different habitats, noting time spent (unit of collecting = person.hour). AS is important for a general inventory, considering both taxonomic coverage (pitfalls are only adequate for some groups) and coverage of different habitats. This method requires trained personal, and, even with experienced collectors, is subject to personal bias, which causes problems to compare areas or studies done by this method. Another unequal factor, in the present case, was that three expeditions had two herpetologists and four had three, and thus collecting effort in active sampling was not uniform for all expeditions.
Pitfall traps with drift fences (Corn, 1994; Cechin & Martins, 2000; Ribeiro-Junior et al., 2008): each trapping unit consisted of four buckets (pitfalls) of 60 l each, that were dug into the ground with their rim flush with the surface, positioned in a Y with the central bucket connected to the three peripheral ones by eight meters of black plastic sheet with a height of 60 cm. A total of 16 trapping units was used, positioned in two, three or four groups (each group in a different trail), depending on the local conditions (type of substrate, rocks, flooding, logistics). Within each group, trapping units generally were positioned at distances of 250 m from each other, but in FLOTA Trombetas they were partly 125 m apart, and in some cases greater distances were used in order to also sample different vegetations or because of physical problems encountered in the terrain. Pitfalls were checked once a day during the entire sampling period. Trapping units were installed in the week before the start of each expedition and removed at the end of each expedition. Collecting with pitfalls has the advantage to be independent of collector and they collect species (generally (semi) fossorial) that are only rarely caught during active collecting. On the other hand, pitfalls are directed to leaf litter and terrestrial species, although some arboreal species that come to the ground are also collected with certain regularity; other groups, especially arboreal/climbing and aquatic species, large terrestrial species and medium-sized to large snakes, and a number of amphibians that are able to climb or jump out of the buckets, are rarely collected. Besides, it is not always possible to use pitfalls; flooded or rocky areas, or areas far from basecamp, can not be sampled by this method, because of the impossibility to install the buckets in the first areas, and the impossibility to check the pitfalls every day in the last case.
Specimens collected by the fieldworkers or by other researchers were considered as occasional encounters.
For each specimen observed by the herpetologists and/or collected the following data (if applicable) were annotated: identification, locality (GPS or distance in trail), habitat, microhabitat, day, time, and name of collector/ observer. Collected animals' standard measurements and weight were taken, and when possible the sex was determined. In many cases notes of life colouration were made, and digital photographs and tissue samples (liver or muscle) for molecular studies were taken.
Collected specimens were euthanized by an overdose of veterinary anesthetic, fixed in formaldehyde 4% (one part of commercial formaldehyde 37% and nine parts of water) for maximally 24 hours, labeled with fieldnumbers (a field series 'CN' [Calha Norte] was created for all specimens collected during the expeditions), and preserved in alcohol 70%, except tadpoles that were preserved in 4% formaldehyde (Franco et al., 2002). Tissue samples were preserved in absolute alcohol, maintained at environmental temperature in the field and transferred to a freezer after arrival in the museum. Even though we refer here to number of specimens per site, it is important to keep in mind that each site covers a few square kilometers and different environments. Thus specimens dealt with as coming from one site may have been collected up to 8,000 m apart. All material was deposited in the herpetological collection of Museu Paraense Emílio Goeldi (MPEG) in Belém, Pará, Brazil (Appendix 2). Collections were made under licence 001/2008 of the Secretaria Estadual do Meio Ambiente (SEMA-PA).
Collecting effort, number of specimens collected per group, and rarefaction curves for amphibians and reptiles separately, are presented for each major collecting site (thus, not for Estanífera). Species rarefaction curves were constructed with the help of the program EstimateS version 7.5 (Colwell, 2005), on the basis of grouped AS and PD collections, considering as sampling unit each collecting day - they represent the cumulative number of species against the increase of collecting effort, obtained after 50 randomizations. Species composition in the seven sites is compared, using the Biogeographic Similarity Coefficient (Duellman, 1990), equivalent to 2C/(N1 + N2), where N1 = number of species in locality 1, N2 = number of species in locality 2, and C = number of species common to both localities. We present a gross estimation of relative abundance of amphibians and reptiles, for each site and for each group as a whole, based on numbers of specimens caught by pitfall traps and all those collected by active searches. Species observed but not collected are added to the graphs as presenting '0' (zero) specimens.
Species were considered endemic for the Guianan Region mainly based on Hoogmoed (1979b) (reptiles and amphibians), Señaris & MacCulloch (2005) and Duellman (1999) for amphibians, and Avila-Pires (2005) for reptiles.
For familial and generic nomenclature we adhere to the nomenclature used before the Faivovitch et al. (2005), Frost et al. (2006), and Grant et al. (2006) papers, because we are not convinced that the wholesale changes in nomenclature proposed by these authors are really necessary and correct. We prefer to await further independent studies that corroborate the alterations those publications proposed. We have made an exception for the former species of Colostethus in the Guianan Region with a mid-lingual papilla, which are clearly recognizable morphologically and now are named Anomaloglossus (Grant et al., 2006). Also, we have not yet taken into account the changes proposed for the genus Eleutherodactylus by Heinicke et al. (2007) and by Hedges et al. (2008). Discussion about these issues is still going on, and by maintaining the pre-2005 names we keep the relation with older (= pre-2005) literature, thus facilitating the work of conservationists and managers of natural areas.
Two recent publications, Zaher et al. (2009) and Vidal et al. (2010), proposed changes in the classification of South American Colubrid snakes. Because of the short time to properly evaluate them (when they appeared this paper already had been completed), we preferred not to incorporate their changes here.
We tried to identify material of the Bufo granulosus complex with Narvaes & Rodrigues (2009), but had serious problems trying to separate the species recognized by these autors in the area north of the Amazon River (Gorzula & Señaris (1999) who also doubted the validity of different taxa in Venezuelan Guiana). We doubt whether all taxa recognized by Narvaes & Rodrigues (2009) are real entities and for the time being we have adhered to the use of Bufo granulosus for the medium-sized granular toad with dorsally directed nostrils, occurring north of the Amazon, realising that e.g. Bufo mirandaribeiroi Gallardo, 1965 is a good taxon.
COLOUR PHOTOS
We present colour photos of some of the species found during the expeditions (Figures 16-75). They generally appear in the same sequence as used in Appendix 1.

RESULTS AND DISCUSSION
COLLECTING EFFORT AND SPECIES RICHNESS
Collecting effort per expedition for each method is shown in Table 1, which also refers to the main habitats found in each site. Taking all areas together we registered 80 species of amphibians (77 frogs and three caecilians) and 95 species of reptiles (36 species of lizards, three of amphisbaenians, 49 species of snakes, five species of chelonians and two species of caiman). The large lizard Tupinambis teguixin (Linnaeus, 1758) (jacuraru or teiu) seems to have been seen in some areas, but not by herpetologists. Because of doubts remaining about the identification, we have not included this species in our list, although it is expected to occur throughout the area (see below). Comparing these numbers with what is expected to occur in the area based on the literature (see introduction), we see that lizards are well represented, followed by frogs, while caecilians, amphisbaenians, snakes and chelonians are well below the expected richness, being clearly underrepresented in the samples. Chelonians, living mainly in aquatic environments, need special collecting techniques, not used in the surveys. Caecilians, partially aquatic and partially fossorial, and amphisbaenians, which are mainly fossorial, are only sporadically found, even when digging extensively for them. Snakes have usually secretive habits and are known to need long periods for inventorying them satisfactorily in tropical rainforests.
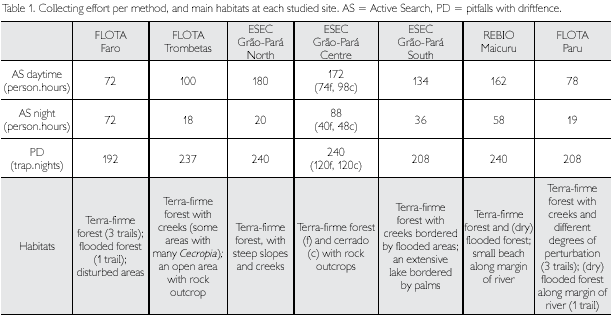
Table 2 shows the number of species per expedition. The highest number of amphibians (36 species) was registered for ESEC Grao-Para South and the lowest number (21 species) for the FLOTA Faro. The highest number of reptiles (42 species) was collected in ESEC Grao-Para North, and the lowest number (27 species) in ESEC Grão-Pará South. However, none of the rarefaction curves, calculated for each site, separately for amphibians and reptiles, reached the asymptote (Figure 76), indicating that more species are expected to occur in each area. The need of prolonged effort to adequately survey the herpetofauna is well documented in the literature (e.g., Duellman, 1978; Myers & Rand, 1969). Duellman (2005) reported that, in Cusco Amazónico, it took 442 person-days to record 89% of the species of anurans, 81% of the lizard species, and 79% of the snake species present in the area. Ribeiro-Junior etal. (2008) also showed the necessity of a large collecting effort, using multiple techniques, for an adequate representation of the herpetofauna. Results of collections vary due to several factors, among others time of the year, meteorological circumstances during the expedition, micro- and meso-habitats sampled, and in the case of active collecting the collectors and the number of collectors. For example, in ESEC Grão-Pará South, the larger number of amphibian species was partly due to the presence of ponds and a lake that provided good conditions for amphibians, especially Hylidae. Although similar habitats have not been encountered in the other sampled areas, it is very unlikely that they do not occur in all conservation units. Besides, it should be remembered that some amphibians use for reproduction temporary pools that only form during the rainy season, and some species have explosive reproduction which only lasts a few days. All these factors influence the number of species found during the limited period of an expedition.
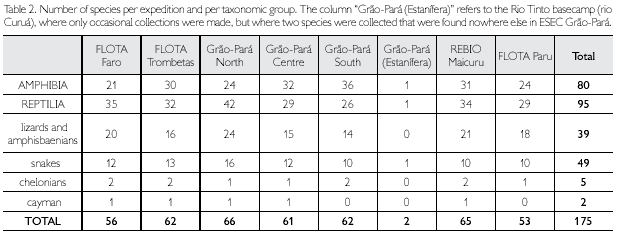
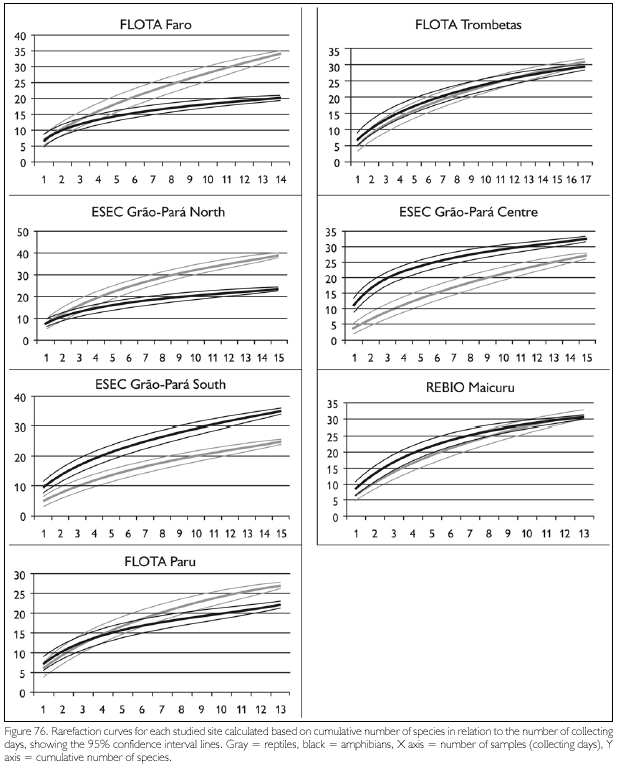
Comparing species richness obtained for each area with number of specimens collected (Figure 77), the studied sites in FLOTA Trombetas, REBIO Maicuru and ESEC Grão-Pará show an increasing number of species with increasing number of specimens collected. Richness in FLOTA Faro and FLOTA Paru, on the other hand, is proportionally less in relation to the number of specimens collected. These two areas are situated along large rivers bordered by flooded forest, a habitat that usually has less species than terra-firme forest (also present in these sites). However, the savanna vegetation that covered part of ESEC Grao-Para Centre also harbors a lower number of species than terra-firme forest, but in spite ofthat, relative richness was proportional to that in other sites. The two FLOTA, Faro and Paru, also had in common areas of disturbed and secondary forests, and one possibility is that the lower relative richness of species in these two areas is a result of environmental disturbance. Habitat alteration may have caused the disappearance, or a population reduction (making them more difficult to be captured), of a number of species.
SPECIES COMPOSITION
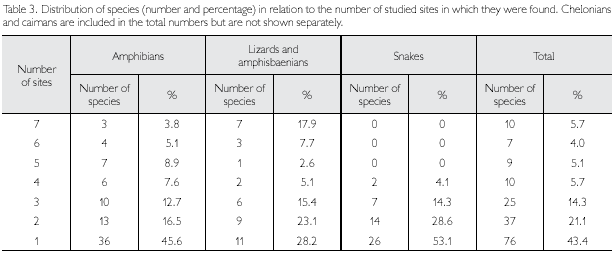
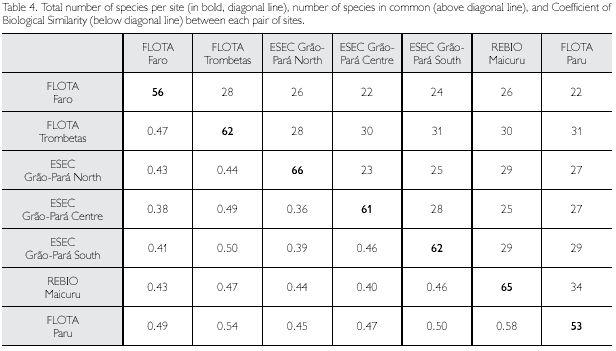
A complete list of species (including authors and years), expeditions during which they were collected and basic biological data are presented in the Appendix 1. Looking at the herpetofauna as a whole, only 5.7% of the species were found in all seven sites, while 43.4% were found in only one of the sites (Table 3). Lizards and amphisbaenians, as a group, showed the most even distribution in the samples, with almost 18% of the species captured in all sites, and only 28% in only one site. Of amphibians, about 46% were found in only one site, and of snakes 53%. The maximum number of sites a species of snake occurred in was four, once more showing the haphazardness of finding these animals. The low number of amphibians common to all sites is partially linked to differences in habitats available and surveyed, as well as in period of the year. But certainly for all groups part of the differences is due to chance and should decrease as collecting effort increases. The same applies when we look at the Biogeographic Similarity Coefficient (BSC) (Table 4). This coefficient, that represents the proportion of species common to two areas, in relation to the total number of species in both areas, is lowest (0.36) between ESEC Grão-Pará Centre and ESEC Grão-Pará North. While the northern part of this ESEC lies in the Acarai Mountains, covered by rainforest in an extremely hilly area, the site in the central part of this conservation unit presents a large isolated area of open vegetation (savanna) (with its specific fauna), with a patch of more or less isolated forest in its interior, which explains part of the difference found between these two areas. In addition, the northern sector was sampled in August-September, thus during the dry season, while sampling in the central sector occurred in January, in the early rainy season, which probably also accounts for part of the differences found. Going to the other extreme, FLOTA Paru and REBIO Maicuru were the most similar areas (BSC 0.58). These two areas were sampled within a period of three months and both have seasonally flooded areas (igapó) influenced by a river, which may explain their larger similarity. But again, the expectation is that these coefficients, between all these areas, will become larger (indicating more similar herpetofaunas) as a better representation of the herpetofauna, from all around the year, is obtained.
Some species however are restricted to special habitats, which may account for real differences between sites. Thus, for example, Hyla wavrini, a várzea and igapó species, was only collected in FLOTA Faro, whereas in other localities Hyla boans, a very similar species that occurs along creeks and rivers in terra-firme rainforest or in gallery forest (Hoogmoed, 1990a), was collected. It is possible that these two species are mutually exclusive. Atelopus hoogmoedi and the two species of Centrolenidae collected depend on the presence of (relatively) clear, running water with rapids in terra-firme forest, and only were recorded for ESEC Grão-Pará Centre (with unidentified Centrolenid tadpoles collected in ESEC Grão-Pará North and South). Although they are without doubt present in other areas of CNP their distribution is limited to specific habitats. The same is true for Leptodactylus myersi, restricted to large, open rocky slabs, either surrounded by terra-firme forest (FLOTA Trombetas) or in savanna areas (ESEC Grao-Para Centre). Savanna enclaves, as encountered in ESEC Grao-Para Centre, have a herpetofauna that is largely different from that of forested areas, among which Adenomera hylaedactyla, Leptodactylus longirostris, Anolis auratus, Kentropyxstriatus, Gymnophthalmus cf. underwoodi and Pseudoboa neuwiedii. These species are restricted to these enclaves and, some of them, to river beaches along the Amazon River.
The caimans Paleosuchus trigonatus and P palpebrosus (Cuvier, 1807) are small and live in creeks in the forest, while Caiman crocodilus occurs in rivers and larger creeks which are not completely roofed over by forest canopy. Paleosuchus trigonatus was found in creeks in FLOTA Trombetas and ESEC Grao-Para North and Centre. Paleosuchus palpebrosus was not encountered during the expeditions, but it is known from Oriximina, Trombetas River (Medem, 1983), and is probably present in other areas of CNP as well. Caiman crocodilus was found in FLOTA Faro and in REBIO Maicuru, and it is possible that it also occurs in the Jari, Paru and Trombetas rivers, but not far from the main course of these larger rivers.
Dendrobates tinctorius (blue variant) only was encountered in the Acarai Mountains near the border with Guyana, in the northern part of ESEC Grao-Para, where it was quite numerous. However, there are also records of this species from Porto Trombetas (blue variant) (material in MPEG) and in Monte Dourado (variant with large dorsal yellow patch and lines) (material in MPEG), where they occur in some forest localities, but not in others. In this case, the distribution of the species seems to be patchy, but it is not clear which environmental parameters are important to decide in which parts of an area it occurs.
The southeastern portion of ESEC Grao-Para, including the studied points in the centre and south of the reserve, presents patches of forest consisting of small, slender-stemmed, low trees with some larger trees interspersed, known as 'cerradao'. No amphibian or reptile species was found only in this type of vegetation, where at least some of the forest species are present.
COMPARISON WITH A SITE IN SOUTHERN GUYANA
As pointed out above, publications dealing with the fauna of northern Pará are scarce. However, there is one paper (Senaris et al., 2008) that deals with a site in southern Guyana that is only about 50 airline kilometers southwest of our collecting site ESEC Grão-Pará North. This is much nearer than ESEC Grão-Pará North is to any of the other six studied localities in CNP It could be expected that the herpetofaunas of these two areas would be very similar. Although the methodology of Senaris et al. (2008) differs considerably from ours (they did not use pitfalls and driftfences, only opportunistic surveys and Visual Encounter Survey) it seems worthwhile to make some comparisons. Senaris et al. (2008) reported 26 species of amphibians (25 frogs, one Gymnophiona) and 34 species of reptiles (12 lizards, one amphisbaenian, 16 snakes, three chelonians, two caiman). The respective numbers for ESEC Grão-Pará North were 24 species of amphibians (23 frogs, one Gymnophiona) and 42 species of reptiles (24 lizards, 16 snakes, one chelonian, one caiman). Only five species of frogs, nine species of lizards, five species of snakes, one species of chelonian and one species of caiman were common to both localities. Especially the number of frogs in common was low. Another remarkable fact was that Senãris et al. (2008) only collected one Gymnophthalmid lizard (an aquatic one) and 13 species of Hylidae, whereas in contrast to these numbers, in ESEC Grão-Pará North we collected 11 species of Gymnophthalmids and only two species of Hylidae. Both expeditions took place in the dry season, so climate does not explain the differences found. Even though differences in habitats found in each area (e.g. presence of large rivers and Indian villages in the Guyana site, both absent in the Brazilian site) can explain part of the differences observed, they are arguably also due to the use of pitfalls in only one of the sites (Brazil) and to collector bias (collectors in the Guyana team predominantly work with frogs, those in the Brazilian Acarai team predominantly with reptiles). In our opinion this comparison reinforces the results of the Biogeographic Similarity Coefficient, and shows the weakness of RAP's that usually only obtain a relatively small proportion of the herpetofauna available. However, combining the results of both expeditions we come to a total of 45 amphibians and 60 reptiles for this cross border area, a result closer to what could be expected than that obtained by either of the expeditions.
DATA FROM OTHER LOCALITIES IN CNP
As mentioned above several other studies took place in CNP most of them not published. These studies yielded collections (in MPEG and RMNH) which provide further data for an inventory of CNP.
The studies in Jari (Monte Dourado) conducted by the University of East Anglia (Gardner et al., 2007; Ribeiro-Junior et al., 2008) were undertaken with a special purpose and focused on leaf litter frogs and lizards (Stokstad, 2008). Consequently, in those collections (deposited in MPEG) hardly any Hylids are present. From Jari a number of species (20) were reported that were not collected during our recent work in the CNP localities [Bufo granulosus Spix, 1824; Hyla raniceps Cope, 1862; Scinax sp. n. 2 (to be described by MSH shortly) [slides Enrico Bernard]; Leptodactylus macrosternum Miranda-Ribeiro, 1926; Gonatodes sp. n. (to be described shortly by Sturaro & Avila-Pires) [MPEG 23822-27; MPEG 27719]; Cnemidophorus cryptus Cole & Dessauer, 1993 (all female population); Tupinambis teguixin (Linnaeus, 1758); Anilius scytale (Linnaeus, 1758); Epicrates cenchria (Linnaeus, 1759); Typhlops reticulata (Linnaeus, 1766); Atractus snethlagae Cunha & Nascimento, 1983; Oxybelis aeneus (Wagler, 1824); O. fulgidus (Daudin, 1803); Oxyrhopus melanogenys (Tschudi, 1845); Philodryas viridissimus (Linnaeus, 1758); Siphlophis cervinus (Laurenti, 1768); Spilotes pullatus (Linnaeus, 1758); Micrurus psyches (Daudin, 1803); Bothrops brazili Hoge, 1953; Rhinoclemmys punctularia (Daudin, 1801)]. These species therefore can be added to the total list of species known from CNP.
Personnel of MPEG collected a further 12 species of snakes in Jari that were not collected during the project of the University of East Anglia and of which the following seven have not been collected elsewhere in CNP: Eunectes deschauenseei Dunn & Conant, 1936; Helicops leopardinus (Schlegel, 1837); H. polylepis Günther, 1861; Hydrodynastes gigas (Herrmann, 1804); Liophis cobellus (Linnaeus, 1758); Liophis lineatus (Linnaeus, 1758); Mastigodryas bifossatus (Raddi, 1820). Moreover they collected material of the caecilian Microcaecila sp. n. (to be described shortly by Maciel & Hoogmoed) [MPEG 14596-97].
Also some small collections were made in Monte Alegre that yielded some species of squamates not yet known from elsewhere in CNP These are Tropidurus hispidus Spix 1825 [MPEG 24119-22, 24170-71]; Leptotyphlopsseptemstriatus (Schneider, 1801) [MPEG 21514-15]; Epicrates maurus Gray, 1849 [MPEG 21507-08] .
Ecological herpetological work on plateaus in Floresta Nacional Saracá-Taquera (J.FM. Sarmento & U. Galatti, unpublished data) provided additional species (ten) that were not collected during the Calha Norte expeditions, in Jari or in Almeirim: Hyla marmorata (Laurenti, 1768); Phrynohyas resinifictrix (Goeldi, 1907); Leptodactylus fuscus (Schneider, 1799); Hemidactylus mabouia (Moreau de Jonnés, 1818); Amphisbaena alba (Linnaeus, 17858); Chironius carinatus (Linnaeus, 1758); Dipsas variegata (Duméril, Bibron & Duméril, 1854); Drymarchon corais (Boie, 1827); Umbrivaga pygmaea (Cope, 1868) [MPEG 20996] (this is the first record of this species for Pará); Micrurus spixii Wagler, 1824. The lizard Hemidactylus mabouia, however, is an introduced species, present only in human altered habitats and it will not be counted as part of the local herpetofauna.
Hoogmoed and Avila-Pires in 1988 collected reptiles and amphibians on the banks of rio Nhamundá (Sitio Céu Estrelado) and on the right bank of rio Trombetas (Cruz Alta). During this work they obtained several species not obtained elsewhere in CNP: Typhlonectes compressicauda (Duméril & Bibron, 1841); Cnemidophorus lemniscatus (Linnaeus, 1758) (with males and females); Uracentron azureum (Linnaeus, 1758); Helicops hagmanni Roux, 1910; Peltocephalus dumerilianus (Schweigger, 1812); Podocnemis expansa (Schweigger, 1812); Podocnemis unifilis Troschel, 1848.
Morales (2002) described Colostethus sumtuosus from the Trombetas river. This species was not collected during our recent or any of the other expeditions.
Avila-Pires (1995) described Tretioscincus oriximinensis from the village of Oriximiná on the bank of the Amazon. This species was not collected during our recent or any of the other expeditions.
França et al. (2006) reported two species of snakes from Monte Alegre that had not been reported from CNP before: Mastigodryas pleei (Duméril, Bibron & Duméril, 1854) (also collected by MSH on the frontier between Brazil and Suriname (Sipaliwini savanna) [MSH 1970-68, material in RMNH] and Phimophis guianensis (Troschel, 1848).
A.O. Maciel & M.S. Hoogmoed, in a paper that has been submitted to Zootaxa, report Potamotyphlus kaupii (Berthold, 1859) from Cachoeira Porteira, Oriximiná, Pará.
Summarizing, at the moment we are aware of three species of caecilians, eight species of frogs, seven species of lizards, one species of amphisbaenid, 29 species of snakes, four species of turtles and one caiman (Medem, 1983) that have been collected in CNP, but were not collected or observed during our recent expeditions. Adding those species to our totals for CNP we get the following totals per group: caecilians six species, anurans 85 species, lizards 43 species, amphisbaenians four species, snakes 78 species, chelonians nine species, and caiman three species. This gives a total of 90 amphibians and 137 reptiles. These numbers, which refer to all habitats present in the entire area, are getting close to what may be expected (see Introduction). However, our knowledge about the distribution of species within CNP is still very limited and mainly based on 11 localities (Figure 2) with different intensity of collecting effort.
DATA FROM SURINAME NEAR THE BRAZILIAN BORDER
Parker (1940) described Ninia hudsoni from the border between Suriname and Brazil.
Hoogmoed (1969b) described Dendrobates azureus from a locality in Suriname close to the Brazilian border. This species was synonimised with D. tinctorius by Wollenberg et al. (2006), but we are ofthe opinion that this synonymisation is not correct, and that possibly the authors have been confused by using terrarium animals with unreliable locality data and by the fact that there also is a blue variant of D. tinctorius (collected by us in ESEC Grão-Pará North) that is similar to, but different from D. azureus in pattern. The pattern of D. azureus, as described by Hoogmoed (1969b; Letters et al., 2007: figure 708), consists of black spots haphazardly distributed on a blue background, without any reminiscence of the basic light linear pattern on the back of D. tinctorius (light head spot and two dorsolateral lines converging on the sacral area and continuing as a sacral line to the cloaca, [Letters et al., 2007: figures 691-700, 701706). This basic light pattern is still distinctly recognisable in the blue form of D. tinctorius, present in the western part of northern Pará (Letters et al., 2007: figure 707).
Hoogmoed & Gorzula (1979) described Ololygon trilineata [= Scinax trilineatus (Hoogmoed & Gorzula, 1979)] based on material from Venezuela, Guyana and Suriname. The Suriname paratype (RMNH 18260) from the Sipaliwini savanna was collected within 7 km of the Brazilian border.
Heyer (1994) reported a specimen of Leptodactylus pallidirostris Lutz, 1930 from the Sipaliwini savanna, Suriname. This name now has been synonymised with L. validus Garman, 1887 by Yanek et al. (2009).
Nussbaum & Hoogmoed (1979) described Microcaecilia taylori Nussbaum & Hoogmoed, 1979, from a locality in Suriname (Sipaliwini) that is less than 7 km from the border with Brazil.
Hoogmoed (1977) reported Leptotyphlops septemstriatus (Schneider, 1801) in Suriname from two localities close to the Brazilian border.
During fieldwork in the Sipaliwini savanna in Suriname in 1968 and 1970, MSH collected Hydrodynastes bicinctus (Herrmann, 1804) from within 7 km ofthe border with Brazil [RMNH 15965 and fieldnumber MSH 1970127, material in RMNH].
R. A. Mittermeier in 1976 collected a series of Pseudopaludicola boliviana Parker, 1927 on the Sipaliwini savanna in Suriname [Museum Comparative Zoology (MCZ 92418-25)]
The species mentioned above might be expected to occur in northern Para as well, but to our knowledge have not yet been collected there. None of these species has been included in the species counts for northern Para.
SPECIES ABUNDANCE
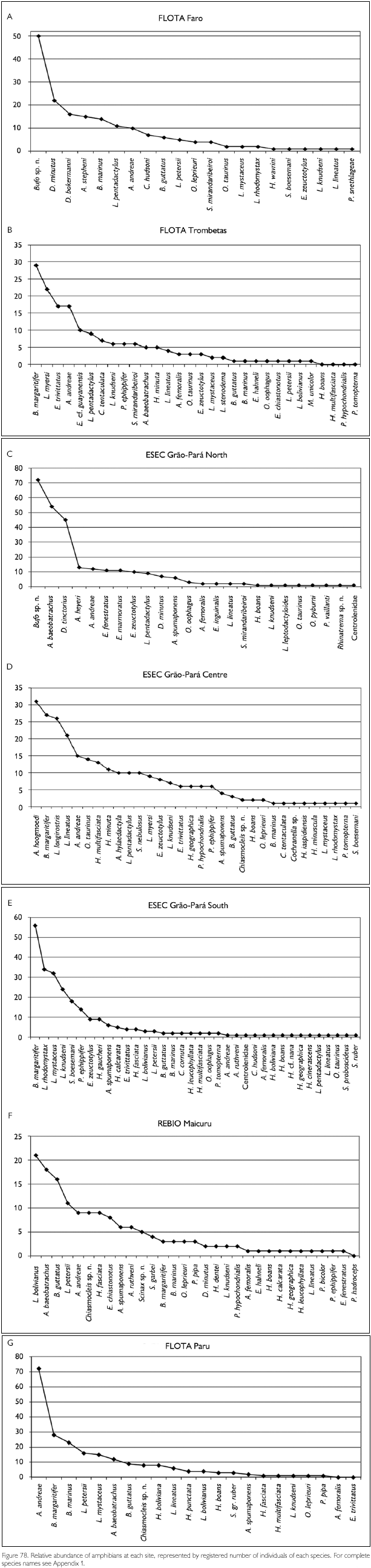
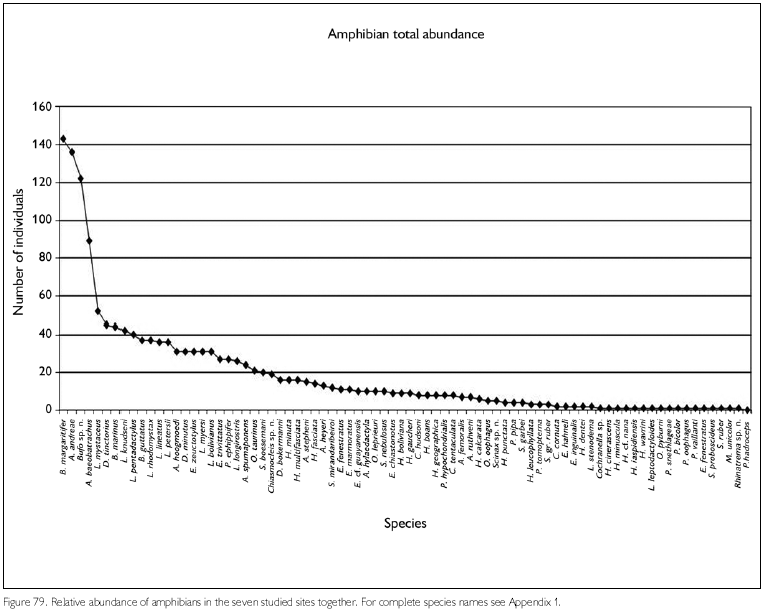
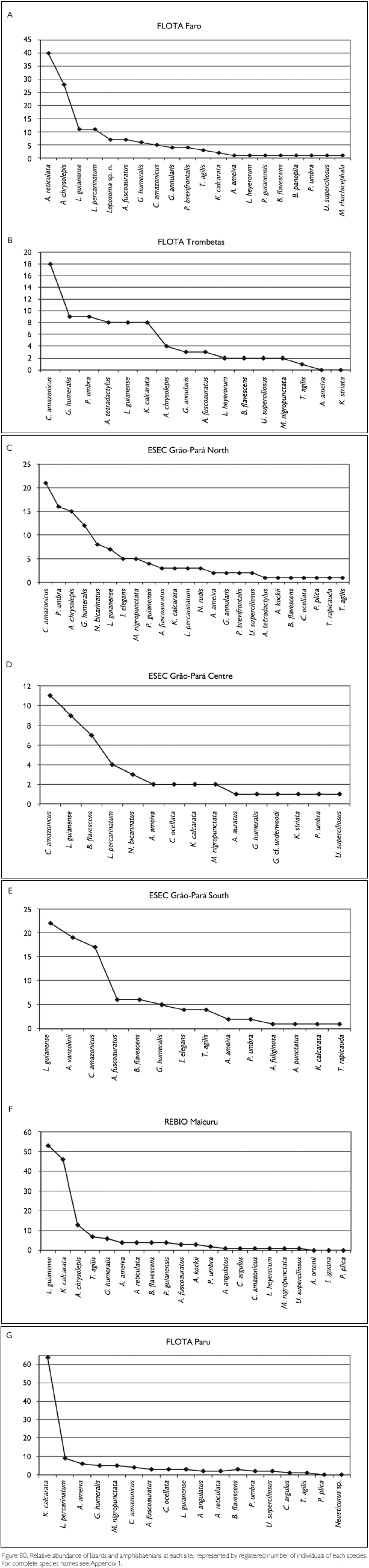
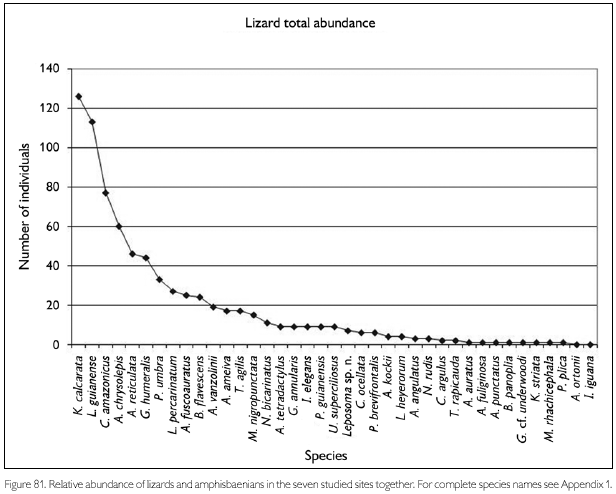
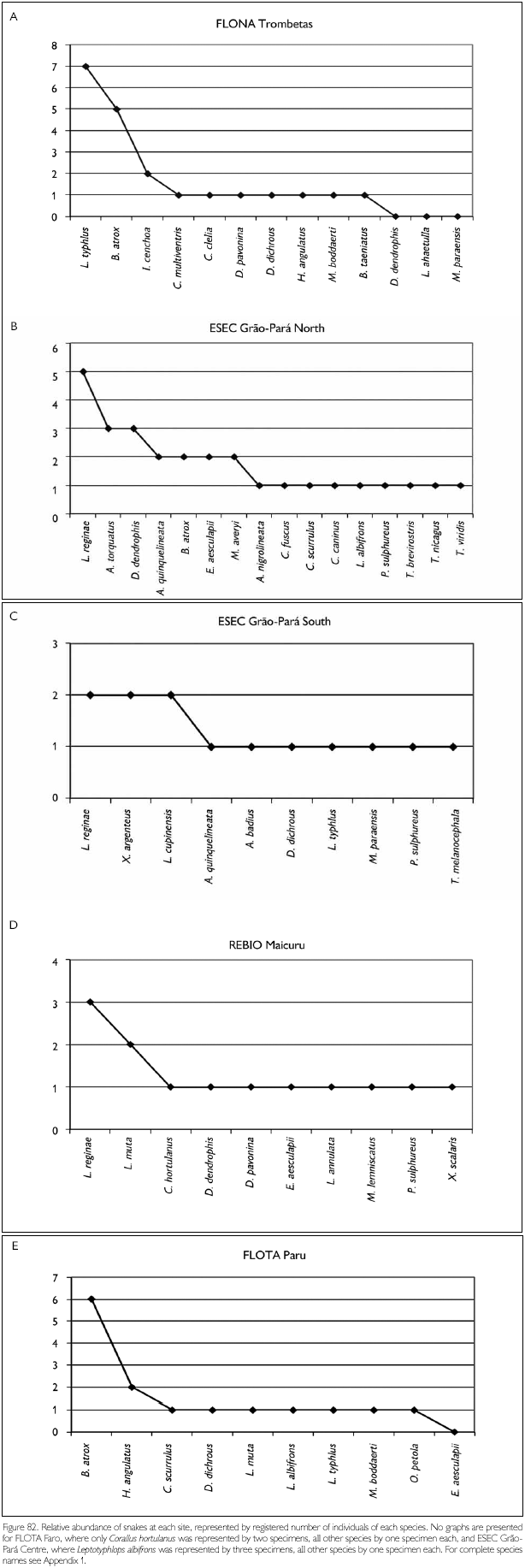
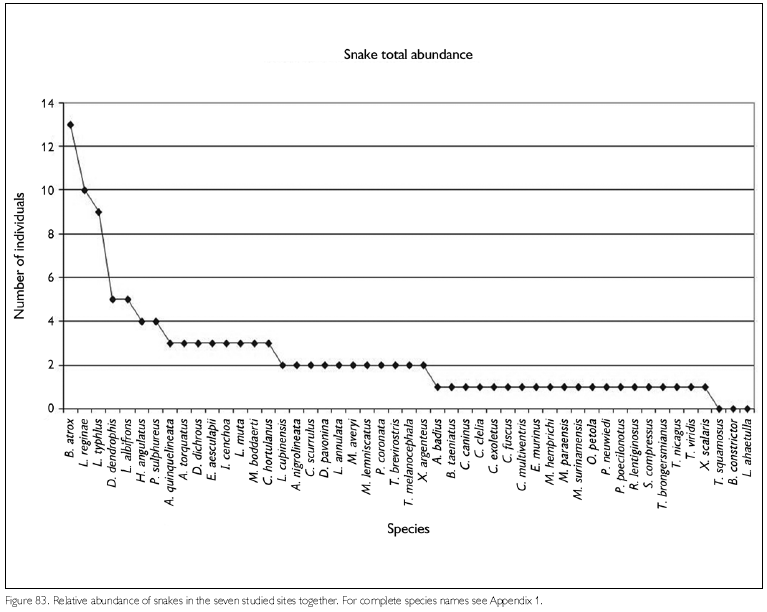
Faunal assemblages in different places may have similar composition but differ in species abundance. Comparison between areas and definition of conservation measurements therefore also should take into consideration this parameter. To obtain data on abundance, however, is quite difficult, especially in tropical rainforests where many species appear to have low densities. A coarse measure of relative abundance may be obtained by looking at numbers of registered individuals, even though these numbers tend to underrepresent the most common species (where not all individuals seen are registered) and species with seclusive habits or which occupy habitats not well surveyed. Amphibians that form breeding aggregations, some only during short times, present another difficulty in such comparisons, since their numbers are not correlated with total collecting effort. Anyway, comparing numbers of registered specimens gives an idea of the most observable species in each area, during the period of the expedition. Figures 78-79, 80-81 and 82-83 show the number of specimens per species collected at each site and for all sites together, for respectively amphibians, lizards and snakes (except for snakes from FLOTA Faro and ESEC Grao-Para Centre, where but for one species only one specimen per species was registered). Even when only the five most common species in each area are compared, no two sites were the same. No species, in any of the groups, appear between the five most abundant in all sites. Among amphibians, Bufo margaritifer, Adenomera andreae, Bufo sp. n., Anomaloglossus baeobatrachus, and Leptodactylus mystaceus are the most numerous in all areas together. Considering these five species, A. andreae was among the five most abundant species in five of the seven sites, B. margaritifer in four of the sites, and Bufo sp. n., Anomaloglossus baeobatrachus and L. mystaceus in only two sites. Some of the most common species in one site, like Atelopus hoogmoedi and Dendrobates tinctorius, were found in no other site at all. The five most abundant lizards were Kentropyx calcarata, Leposoma guianense, Coleodactylus amazonicus, Anolis chrysolepis and Arthrosaura reticulata. Leposoma guianense was among the five most abundant species in five sites, and was the most abundant species in two of them. Coleodactylus amazonicus was the most abundant lizard species in three sites, and the third most abundant in a fourth site. Kentropyx calcarata and Anolis chrysolepis appeared each in three sites among the most abundant species, while Arthrosaura reticulata was abundant in only one site, FLOTA Faro. The five most abundant species of snakes were Bothrops atrox, Liophis reginae, Liophis typhlus, Dendrophidion dendrophis and Leptotyphlops albifrons. Bothrops atrox, the most abundant species, was represented by 13 specimens, while of the last two species of this list only five specimens were found. As pointed out before, no species was found in more than four of the sites, and only D. dendrophis was sampled in four sites; the other four species were registered in three sites. Differences in the most abundant species are less likely to result from sampling bias or by chance, and are more likely to reflect real differences. However, especially for amphibians part of the differences may be due to different periods of the year, but another part reflects probably differences in the available habitats - even though for most species we do not know exactly which conditions favor them.
OBSERVATIONS ABOUT SOME SPECIES
Amphibians
Atelopus hoogmoedi is a colourfull small toad that until recently was known as Atelopus spumarius or A. spumarius hoogmoedi (Frost, 2009). The distribution of A. spumarius was supposed to reach from the Andes to the Guianas, with a gap in between those two extremes in western Amazonia. Letters et al. (2002) were of the opinion that this was a species complex and that for the Guiana population the name A. hoogmoedi would be available. Letters et al. (2005) used the name A. hoogmoedi for the Guiana population. Atelopus hoogmoedi was described from French Guiana and is known to occur th roughout the three Guianas and adjace nt Brazil (Noonan & Gaucher, 2005). In Pará the species was known from one small area in Monte Dourado and from a rather undefined locality "Brazil, 30 km S of the Suriname border" (material in RMNH), with outlying populations in Tucuruí, Serra de Carajás, Itaituba and near Santarém, all four localities in Pará south of the Amazon River. The species is also known from several localities in Amapá (Lima, 2008) and from the surroundings of Manaus, Amazonas (Lima et al., 2006). The Brazilian populations mentioned here are rather uniform in dorsal pattern (dark brown with vermiculate yellow to pale greenish lines on the back), but in Amapá the colour may become dark purple, with lighter purple vermiculations in some populations. The ventral colour is variable, from bright yellow everywhere, to bright yellow with bright or faint red palms, soles and seat patch, or entirely bright purple in Amapá in some populations. The genus Atelopus is known for the fact that many species (all from the high Andes) are threatened by extinction (a few already are extinct) probably as a result of infection with the chytrid fungus Batrachochytrium dendrobatidis (=Bd), which can cause the populations to collapse in a short time. Luger et al. (2008) checked on the viability of populations of A. hoogmoedi in Suriname (Brownsberg) and in Guyana (Mabura Hill Forest Reserve) and found healthy populations in numbers that are comparable with "those recorded for other Atelopus species before catastrophic declines". During our stay in ESEC Gráo-Pará Centre we encountered a large population of A. hoogmoedi. We collected 31 adult individuals, both males (most) and females (no juveniles) in 15 days, all during active searching, none were found in pitfalls. In daytime males were regularly heard calling. During this field period we spent 74 person-hours collecting in forest in daytime and 40 person-hours at night, a total of 114 person-hours. Atelopus hoogmoedi were collected both at day (most) and at night in two relatively small areas where trails crossed a creek in terra-firme forest. The species does not occur in savanna areas. Thus our collecting rate was 31/114 = 0.272 A. hoogmoedi per person-hour spent in the field, but it should be noted that only a small part of our field time was spent in A. hoogmoedi habitat near creeks, although this cannot be quantified. Our research was not exclusively directed at A. hoogmoedi, but to an inventory of the herpetofauna in general. Thus, the value of 0.272 calculated above should be considered as a minimum value and the real population size may be considerably larger than suggested by this number. Although not directly comparable to the data from Suriname (57 specimens in 37 days) and Guyana (202 specimens, during 393.5 transect hours [0.513 individuals per transect hour]) it is our impression that the population we encountered was comparable to, or larger than, those in Suriname and Guyana and thus appears healthy. At ESEC Gráo-Pará Centre we have the special situation of a large forest island in the middle of a savanna area that probably to a large extent is isolated from the terra-firme forest surrounding the savanna. Apparently there is some gallery forest along creeks that connects it in some places to the surrounding terra-firme forest. Atelopus hoogmoedi is restricted to that terra-firme forest island and does not occur in savanna. Whether the species occurs in the terra-firme forest surrounding our research area or in the gallery forests is not known as we were not able to collect there, but it seems likely. The large forest island in the savanna may form a natural refuge for A. hoogmoedi, isolating it to a certain degree from surrounding populations in continuous terra-firme forest. In the case of a Bd infection in the terra-firme forest surrounding the savanna, the population of A. hoogmoedi at collecting station ESEC Grão-Pará Centre might be naturally protected against infection, just by this isolation. Considering the increasing spread of Bd (fortunately not yet found in Amazonia) this area merits special attention for conservation purposes. In contrast to most other species of Atelopus (except those in Suriname, French Guiana and Amapá that have the same altitudinal range as A. hoogmoedi), A. hoogmoedi occurs only at low elevations, from 20 m at Monte Dourado, Pará, to at the most 600 m in Carajás, Pará and 700 m on the Lely Mountains in Suriname (MSH, unpublished data). According to Ron (2005) the presence of Bd at lower elevations at the moment is not very likely because medium temperatures seem to be too high for Bd infections. Thus, we might conclude that the fact that this species occurs at low altitudes provides a certain protection against Bd infection. However, this can not be considered a safeguard against Bd infection, as the way of infection and transport of the pathogen is not yet completely known.
Bufo margaritifer is a large species of toad (SVL females 87 mm, SVL males 66 mm [Hoogmoed, 1986], but in our CNP material resp. 95 mm [CN515] and 77.5 mm [CN496]) with well developed bony cranial crests, especially in the supratympanic region, which may extend vertically or horizontally, and with neural vertebral spines protruding through the dorsal skin in females, whereas males have low cranial crests and no protruding vertebral spines. In our material from FLOTA Paru, however, there is one male (CN 1726, SVL 67 mm) that has a testis, vocal slits and copulatory warts, but externally very much resembles a female by having high cranial crests, a large jaw knob and protruding neural spines. Both sexes have a distict bony knob on the corner of the mouth (generally smaller in males) and an oblique row of tubercles from the parotoid gland to the groin (Hoogmoed, 1986). Fouquet et al. (2007a), thinking they were describing a new species, actually redescribed B. margaritifera under the name Rhinella martyi, as a result of not being acquainted with the species of the B. margaritifer group, putting too much emphasis on molecular data, working with few specimens from a restricted area and not knowing how exactly B. margaritifer should be defined. Neither did they bother to check whether one of the many synonyms available could be used for their "new" species. Laurenti (1768, p. 30) in his description of Rana margaritifera refers to two drawings in Seba (1734; pl. 71, figures 6 and 7) and for his "Var. β" he refers to Seba (1734: pl. 71 figure 8, which shows an aberrant specimen with five fingers on the left hand, the right hand not being visible). Thus, the two specimens represented in the drawings (Seba, 1734 pl. 71 figures 6 and 7 being a dorsal and ventral view of one specimen) thus can be considered the type series of Rana margaritifera Laurenti, 1768. The type locality is given as Brazil ("Habitat in Brasilia"), probably based on the names used in the text in Seba (1734). Because of the problematic situation concerning this group of toads, as demonstrated by Fouquet et al. (2007a) and by Haas (2004) who considered a female of B. margaritifer with well developed crests as a male, and a male of B. margaritifer with low crests as a female of another species, it seems useful to indicate the specimen depicted in Seba (1734 pl. 71, figures 6 and 7) as the lectotype of Ranamargaritifera Laurenti, 1768, in order to avoid further confusion. The specimen depicted in Seba (1734 pl. 71, figure 8) becomes the paralectotype of Rana margaritifera Laurenti, 1768. This species, mentioned by Hoogmoed (1979b) as B. typhonius, has a distribution that at least covers Guyana, Suriname, French Guiana and, in Brazil, Amapá and Pará north and south of the Amazon. Thus, this is not a Guiana endemic. The description of Rhinella martyi completely agrees with the data available for the lectotype of B. margaritifer and the artifical distribution provided for it, due to lack of material, completely falls within the known distribution of B. margaritifer. Therefore, we here synonymise R. martyi with B. margaritifer. This species generally inhabits rainforest and is an explosive breeder in temporary pools and inundated areas, where large numbers may assemble. Although this species is active in daytime on the forest floor, males form choruses and call at night sitting in shallow water or on objects floating in the water (Hoogmoed, 1990b). The other species described by Fouquet etal. (2007a), Rhinella lescurei, is a good species that was already recognised by Hoogmoed (1979 b) as Bufo sp. "B", with an altitudinal distribution of 0-300 m, and occurring in Guyana, Suriname and French Guiana (many localities [MSH, unpublished data]). Thus it is not restricted to French Guiana as suggested by Fouquet et al., 2007a). During our recent surveys in CNP we did not collect this species. This small species is easily distinguished from B. margaritifer by its greenish iris (golden in B. margaritifer), a character not mentioned by Fouquet et al. (2007a), by several morphological characters described by Fouquet et al. (2007a), and by its ecology. This is a species that is active in daytime, and of which the males call singly, separated several meters from each other, from elevated posts 50 - 300 cm above the ground (rocks, leaves, palm-fronds, lianas), always near creeks in the rainforest, during daytime. They lay their eggs in small bodies of standing water near creeks (Hoogmoed, 1990b).
Bufo sp. n., collected in FLOTA Faro and ESEC Grão-Pará North, is a small species of the Bufo margaritifer group, with green iris and no cranial crests. The species is also known from French Guiana (Hoogmoed & Avila-Pires, 1991b), Jari (Monte Dourado, Pará) and Amapá, and wil be described as a new species in a forthcoming paper by MSH. It was mentioned by Hoogmoed (1979b) as Bufo sp. "A".
Cochranella sp. It has not yet been possible to identify this species with certainty. The taxonomy of Centrolenidae in the Guianan Region is still in flux and several species from other areas in eastern Amazonia, both in the Guianan Region and south of the Amazon river, still await identification. The presence of this taxon constitutes a new record for Pará. Generally Centrolenidae are considered absent from south of the Amazon River in eastern Amazonia, but several species (still to be identified) have been collected in localities in Pará like Caxiuaná, Gunma, lower rio Xingu etc., thus changing the general idea of the distribution of this family (Señaris & Ayarzagüena, 2005).
Hyalinobatrachium iaspidiense was collected in ESEC Gráo-Pará Centre, together with Cochranella sp., above a creek in a large area of terra-firme forest surrounded by savanna. Yánez-Muñoz et al. (2009) synonymised H. nouraguensis with H. iaspidiense and discussed some new localities for the species in Peru and Ecuador. Moreover, they mentioned localities in Venezuela, Guyana, Suriname (collected by MSH) and French Guiana and two localities in Brazil: Presidente Figueiredo, in the Guianan part of the state of Amazonas, and the lower Cristalino River in northern Mato Grosso. The present record is the first for the state of Pará.
Centrolenid larvae were collected in all three localities in ESEC Gráo-Pará, but no connection could be established between them and the two species of which adults were collected in ESEC Gráo-Pará Centre. In this last area an egg mass was discoverd hanging from the tip of a leaf of a bush over a creek, close to the calling stations of the males of H. iaspidiense and Cochranella sp. collected there. From MSH's experience in Suriname it is clear that the eggmass does not belong to H. iaspidiense, which lays its clutches on the upper surface of leaves, not at the tips. Thus, the eggmass probably belongs to Cochranella sp. found closeby, but there is no certainty for that. We were not succesful in raising the tadpoles much beyond hatching from the eggs.
Allobates spumaponens was described from Guyana by Kok & Ernst (2007) and is here reported for the first time from Brazil. It was collected in all three localities in the ESEC Grao-Para, in REBIO Maicuru and FLOTA Paru. Thus its distribution is much wider than was known up till now and it may turn out to occur throughout the western part of the Guianan Region. Identification was made by comparison with the original description (Kok & Ernst, 2007).
Dendrobates tinctorius (blue variant, not to be confused with D. azureus from Suriname) only was collected by us in ESEC Grao-Para North, where it was very abundant throughout the area. It is a blue and black, or a black and blue, frog in which the basic tinctorius pattern of a light head patch and two dorsolateral stripes converging on the sacrum and continued from there as a sacral line to the cloaca, is reflected in the blue elements. The blue may be restricted to some narrow lines, often interconnected, on a black background, or it may expand to form the background color with the black reduced to a number of larger and smaller spots, still leaving the basic pattern visible. During our visit there was much courtship activity, with specimens following each other and pressing each other to the ground. D. tinctorius (blue variant) at present is known from ESEC Grao-Para North, FLONA Saraca-Taquera in CNP and from the Konashen area in southern Guyana (Seharis et al., 2008). All these localities are west of the Trombetas River, and this distribution is similar to that of Leposoma sp. n. and Bachia panoplia. D. tinctorius from eastern CNP (Jari) are black with the basic yellow pattern known for this species.
Epipedobates cf. guayanensis and E. hahneli. Haddad & Martins (1994) reviewed the species of the E. pictus group, concentrating on Brazil, and came to the conclusion that only one species, E. hahneli, occurred along the Amazon and its main southern tributaries. At FLOTA Trombetas we collected syntopically two small species of Epipedobates of similar size (25 mm svl, both with larvae and calling males - apparently in full breeding season in mid-May, like all other Dendrobatids in the area). They differed in call, in body shape, and in pattern and colour of lipstripe, lateral stripe, dorsolateral stripes, belly, colour and shape of spots in the axilla, in the inguinal region, on the thighs and on the tibia. We have identified the specimens with yellow axil, inguinal and tibia spots, a white lipstripe that starts a short distance in front of the eye, no lateral white stripe and narrow white dorsolateral stripes as E. hahneli, a species that also occurs in Amapá (Mazagão [MPEG 810, 6936], Oiapoque [MPEG 20381-82]), Amazonas (Balbina [MPEG5966-67], Mamirauá [MPEG 7281-83, 7286-7289, 7310-7318], Urucu [MPEG 5155], Benjamin Constant [MPEG 539495, 5489-90, 5505-5507, 561817668]), Pará (Paraupebas [MPEG 25076-83], Belo Monte [MPEG 22008], São Felix do Xingu [MPEG 9347-48]), southern Suriname (Sipaliwini airstrip, south bank of Coeroeni River [Posts Tigrie and Gonini]) (Hoogmoed, 1971a, b as Dendrobates pictus) and French Guiana (Azevedo-Ramos et al., 2004; Lescure & Marty, 2000; Lima, 2008; Silverstone, 1976 [part of his Phyllobates pictus]). The other taxon, with red spots in axil, inguina and back of thigh (the last two spots connected by a narrow orangish stripe across the dorsal surface of the thigh), a large red spot on the back of the tibia, a white lip stripe that commences close to the tip of the snout, an irregular white lateral stripe and relatively broad golden to orange dorsolateral stripes, we provisionally have identified as E. cf. guayanensis, a taxon that was described as Dendrobates pictus guayanensis from northern Venezuelan Guiana (Heatwole et al., 1965), and with whose description our specimens agree well. This taxon was not considered by Haddad & Martins (1994) in their revision of the pictus-group, although their description of E. pictus from Bolivia and SW Brazil resembles our material of E. cf. guayanensis very much. But the distance between the localities of E. pictus and E. cf. guayanensis seems too large to consider them as one species. Letters et al. (2007) reporting E. cf. guayanensis (as Ameerega pictus guayanensis) from Venezuela and Guyana, reached the same conclusions as we do, and suggested that this may be a good species. We can not yet eliminate the possibility we are dealing with a new species here, and further study is needed to provide a decisive answer. We have located more material of Epipedobates cf.guayanensis in the collection of MPEG: from Mazagão, Amapá [MPEG 6935], from Jari (Monte Dourado), Pará [MPEG 17495-505], and from Monte Alegre, Pará [MPEG 19747-52 , 20192, 20197-20203]. Only in Mazagão, Amapá, both species were also registered syntopically. E. cf. guayanensis was much more abundant (ten specimens) in FLOTA Trombetas than E. hahneli, of which we only collected a single specimen in a rather open area of terra-firme forest. In REBIO Maicuru we only collected one specimen of E. hahneli and no E. cf. guayanensis. Unfortunately no calls could be recorded. Based on the material we examined, E. cf. guayanensis does not occur south of the Amazon River, and E. hahneli seems to be rare north of the Amazon, although present from Amapá west through Pará to Balbina, in Amazonas. E. hahneli is known from south of the Amazon, from Belo Monte (Pará) in the east to Benjamin Constant (Amazonas) in the west. The eastern end of its distribution area in Brazil falls largely outside the "predicted niche" for this species computed by Twomey & Brown (2008), showing that such models should be considered with much care.
Hyla dentei, described from Amapá, was reported from French Guiana by Lescure & Marty (2000) and from additional localities in Amapá by Lima (2008). The species was only collected at REBIO Maicuru and is a new record for Pará.
Hyla gaucheri was described from coastal French Guiana and also is known from coastal and isolated savanna areas in interior Suriname (MSH, unpublished data). The species was collected near some pools and a lake in ESEC Grão-Pará South and is here reported for the first time from Brazil. The presence of pools and the lake in this locality caused the number of Hylid frog species to be considerably higher here than in any of the other localities sampled, despite the relatively advanced season (end of rainy season).
Phrynohyas hadroceps was described from southern Guyana (Duellman & Hoogmoed, 1992) and since has been reported from French Guiana (Lescure & Marty, 2000). Its characteristic call is a loud, regular, "woody" sound, like a metronome, that is repeated hours at an end. This call was heard (but not recorded) in REBIO Maicuru, but no specimen could be observed or collected. On the basis of the call, we report this species as new for Brazil.
Scinax garbei is known from Ecuador, western Brazil, adjacent Peru, Bolivia, Colombia and Venezuela (Duellman, 1972; Frost, 2009; La Marca et al., 2004). Its easternmost known locality in Brazil was Manaus, Amazonas (Lima et al., 2006). The record from REBIO Maicuru extends the eastern border of distribution well into Pará, for which this is a new record.
Scinax proboscidea was described from Suriname and since has been reported from several other localities in the Guianas (Duellman, 1972; Lescure & Marty, 2000; MSH, unpublished data) and Amapá (Lima, 2008). The record from ESEC Grão-Pará South is the first record for Pará.
Scinax sp. n. is a small species of Scinax that was only collected in REBIO Maicuru. It does not agree with any of the known species from the area and will be described as new in a separate paper.
Scinax gr. ruber is a large species related to S. ruber, but certainly different from it and from S. x-signatus (Spix, 1824). Its correct identification still has to be checked.
Adenomera heyeri was recently described from French Guiana (Boistel et al., 2006; Angulo et al., 2006) and has not yet been reported from outside that country, although MSH (unpublished data, material in RMNH) has collected it in several places in Suriname (Lely Mountains, Patamaca, Brownsberg, Kabalebo, Mozes Creek, Van Ams Creek, 20 km N. Lucie River, Oelemari, Loè Creek, Airstrip Tafelberg) and French Guiana (Mont Mahury, Mont La Gabrielle, Dégrad des Cannes) as well. The record for ESEC Grão-Pará North is the first record for Brazil.
Eleutherodactylus chiastonotus was known from Suriname, French Guiana and Amapá, and its presence in northern Pará was to be expected. The records from FLOTA Trombetas and REBIO Maicuru are the first for Pará.
Eleutherodactylus fenestratus is generally considered a species from south of the Amazon River, but reaching Manaus and southern Guyana (Lima et al., 2006).We have collected it in ESEC Grão-Pará North, which is the first record from Pará north of the Amazon.
Eleutherodactylus inguinalis was described from the border of Suriname and Brazil, but had not yet been reported from northern Pará, where it was to be expected, as it was already known from several localities in Suriname (MSH unpublished data, material in RMNH), French Guiana (Lescure & Marty, 2000) and Amapá (Lima, 2008). Our records from ESEC Grão-Pará North and REBIO Maicuru are the first for Pará.
Eleutherodactylus marmoratus is known from the three Guianas and Amapá (MacCulloch et al., 2004; Lima, 2008) and its presence in northern Pará was to be expected. The record from ESEC Grão-Pará North is the first for Pará.
Leptodactylus bolivianus has a d istribution from southern Central America to Bolivia and the Guianas, but had not yet been reported from northern Pará where it was expected to occur. It is here reported from FLOTA Trombetas, ESEC Grão-Pará South, REBIO Maicuru and FLOTA Paru. The species also has been collected in Jari (Monte Dourado), Pará.
Leptodactylus myersi was described from Roraima (Brazil), Suriname and French Guiana, from isolated rock outcrops and granitic inselbergs in savannas and in rainforest (Heyer, 1995). The species was collected in FLOTA Trombetas in an isolated open area with flat granitic rock outcrops and boulders and a low open vegetation, surrounded by terra-firme forest, and in ESEC Grão-Pará Centre on rock outcrops (lajedos) in a savanna and in cerradão (transitional) forest bordering the savanna. However, it should be noted that in FLOTA Trombetas two specimens of this species were collected well inside the forest, in areas with large rocks, about 100 m and 300 m respectively from the open rock area. This indicates that the species does enter forest, apparently in association with rocks, at least for some distance. Heyer (2005) reported the species from the campos de Ariramba, near Monte Alegre. The present records nicely fill the gap between the Suriname/French Guiana localities, the southern CNP locality and the Roraima localities.
Chiasmocleishudsoni, a minute fossorial species, was described from southern Guyana, close to the border with Brazil. It was reported from the neighbourhood of Manaus, Amazonas, by Lima et al. (2006) and is also known from southern Suriname close to the Brazilian border (MSH, unpublished data). It could be expected to occur in Pará, but had not yet been reported from there and our records are the first for the state.
Chiasmocleis sp. n. is a small fossorial species of a genus from which recently a number of new species have been described, but all from south of the Amazon (Caramaschi & Cruz, 2001; Peloso & Sturaro, 2008) or from the Atlantic forest. The species here referred to does not agree with any of those newly described species (C.jimi Caramaschi & Cruz, 2001; C. avilapiresae Peloso & Sturaro, 2008), or with C. shudikarensis Dunn, 1949, known from Guyana, Suriname, French Guiana and Amazonas, Brazil (Dunn, 1949; Lescure & Marty, 2000; Lima et al. 2006; MSH, unpublished data). The distribution given by Rodrigues et al. (2004) for C. shudikarensis seems to be too extensive and to include distribution areas of other species as well.
Otophryne pyburni was known from eastern Colombia close to the Brazilian border, southern Venezuela, Suriname (Campbell & Clarke, 1998 [no locality]; MSH, unpublished data: Tepoe [RMNH MSH fieldnumber 4017] and Kwamalasemoetoe [Slide J. de Bruin]), French Guiana and Amapá (Campbell & Clarke, 1998; Lescure & Marty, 2000). Carvalho et al. (2007) reported it from Parque Nacional Pico de Neblina, Amazonas State, Brazil, and MacCulloch et al. (2008b) mentioned it from Pará, without further specification, and without indicating it in their map. We recorded it from ESEC Grão-Pará North and we also collected it at Monte Dourado, Pará [MPEG 17605]. These are the first specific localities from Pará. Senaris & Acosta-Galvis (2004), in their distribution map (which differs from the distribution as given in the text), show a narrow band through northern Pará connecting the known distribution areas of French Guiana/Amapá with the localities in Venezuela and E. Colombia. We interprete this map as not being based on material from Pará, but as just an interpretation of a possible distribution. In fact, based on our collecting data the species most likely occurs throughout much of northern Pará and in southern Suriname (Kwamalasemoetoe and Tepoe, MSH, unpublished data). In Guyana it is known from Kartabo, in the northern part of the country (Campbell & Clarke, 1998). A distinctive character of the species is the presence of a solid, black, heart-shaped spot around the cloaca. It is visible in Figure 4 of the description by Campbell & Clarke (1998), but they did not specifically mention it. In the ESEC Grão-Pará North a large series of tadpoles was collected, including metamorphosing specimens, which will be described elsewhere.
Synapturanus mirandaribeiroi is a medium-sized fossorial species living just under the mat of superficial roots in rain forest, which makes it difficult to collect without using pitfalls. It was described from southern Guyana and is known from Guyana, Suriname, French Guiana, southern Venezuela, and eastern Colombia; it was reported moreover from the neighbourhood of Manaus, Amazonas (Lima et al., 2006) and from Amapá (Lima, 2008), in Brazil. The records from FLOTA Faro, FLOTA Trombetas and ESEC Grão-Pará North are the first ones for Pará.
Pipa snethlageae was described from Belém, Pará, in 1914 and since has only rarely been collected. Trueb & Cannatella (1986) reported 13 specimens from six localities in the Amazon basin: four in Brazil, and one each in Colombia and Peru. Recently the species was reported from French Guiana by Massemin et al. (2003, 2007). This species is neither new for the fauna of Brazil nor for that of Pará, but it never has been reported from northern Pará. Our specimen [CN 319] was collected with a fishing net in inundated igapó forest on the bank of rio Nhamundá, in FLOTA Faro. MSH in 2006 collected a juvenile in the rio Mutum, Amazonas (Reserva de Desenvolvimento Sustentável Cujubim) [MSH 10111 in MPEG], also in inundated forest. Another specimen [MPEG 16939] was collected in Juruti, Pará, on the south bank of the Amazon River. This seems to be a species restricted to large rivers and lakes of the Amazonian lowlands, that just enters the Guianan Region in its southern part along large rivers and in the east (French Guiana) via the coastal marshes of Amapá, as happens with other amphibians and reptiles. Its distribution area completely falls within that of Pipa pipa. The species is easily separated from the other species of Pipa by not having a skin appendage under the snout, like Pipa pipa, by having only a simple tubercle-like appendage at the corners of the mouth, by having a wide and short head and by having the tips of the fingers forming a square, flat surface perpendicular to the longitudinal axis of the fingers, with a pointed tubercle sticking out of each of the tips of the square.
Rana palmipes has a wide distribution in Amazonia and also is present in a small isolated area in northeastern coastal Brazil (northern end of the Atlantic forest). Generally the species is associated with large bodies of water like ponds and creeks. In the study area the species strangely enough only was encountered on top of the plateau where Rio Tinto's basecamp Rio Curuá (Estanífera) is established, near small pools on the road, in 'cerradão' forest (consisting of very thin small trees, standing very close together) close to the airstrip, with the nearest larger water body (rio Curuá) being hundreds of meters away at a lower elevation as well. This is the first record of this species in Pará north of the Amazon.
Microcaecilia unicolor was only known with certainty from French Guiana (A. O. Maciel & M.S. Hoogmoed, unpublished data), but its occurrence in this part of the Guianan Region (FLOTA Trombetas) does not come as a surprise because, like many other Gymnophiona, this species is difficult to collect and only known from relatively few specimens and localities. Earlier reports of this species from Brazil were based on mis-identified material.
Rhinatrema sp. n. was found in ESEC Grão-Pará North and in Porto Trombetas (also in northern Pará). It differs from R. bivittatum (Guérin de Méneville, 1838) in several morphological and colour characters and shortly will be described by A.O. Maciel & M.S. Hoogmoed [type-material in MPEG].
Reptiles
Amapasaurus tetradactylus was collected in FLOTA Trombetas and in ESEC Grão-Pará North (Acarai Mountains). Since its description in 1970 it had not been found again until it was collected during the 2004-2006 Tumucumaque Expeditions of Conservation International, in northwestern Amapá (Lima, 2008), close to the border with Pará. The new localities here reported for Pará suggest that the species is widely distributed in northern Pará, but it is not yet possible to say whether this is a continuous distribution, or which environmental parameters define its occurrence. In the Acarai Mountains the species was collected at a short distance from the border with Guyana, so this species may turn up in southern Guyana as well.
Bachia panoplia, which was only known from the surroundings of Manaus, Amazonas, and from Oriximiná, Pará, was collected in FLOTA Faro, which may indicate its distribution is limited to the southwestern part of the Guianan Region, west of the Trombetas River.
Leposoma sp. n. still has to be described, but already was known from the surroundings of Manaus, Amazonas (Vitt et al., 2008), and seems to extend its distribution at least to the western part of northern Pará, like B. panoplia. It was only collected in FLOTA Faro, whereas Leposoma guianense occurred in all studied sites and Leposoma percarinatum, a parthenogenetic species, in five of the seven sites. In FLOTA Faro all three species were collected, and it would be interesting to know if and how they interact.
Gymnophthalmus cf. underwoodi is a savanna inhabitant probably with a relatively large distribution in its specific habitat in southern Guiana and possibly beyond, in Alter-do-Chão, Santarém, south of the Amazon River. Our only specimen is a female and was captured in ESEC Gráo-Pará Centre under a rock on a rock slab in savanna. Whether males are present in this taxon is unknown. Most likely this is the species that was reported by Carvalho (1997) from Campos de Ariramba and Alter-do-Cháo as "Gymnophthalmus com cauda vermelha" [= Gymnophthalmus with red tail], and, fleetingly, by Vanzolini & Carvalho (1991) from "northern Pará". It differs from Gymnophthalmus vanzoi Carvalho, 1997 by lacking a light upper lip, having two white bands bordered by black on the mentals, indistinct dorsolateral stripes, black flanks, and by having a reddish tail. It is similar to G. underwoodi in pattern, but differs from it by its reddish tail and by having all scales of the tail, from base to tip smooth, whereas in G. underwoodi the scales towards the tip of the tail have low keels, forming ten longitudinal ridges. We need to compare this specimen more extensively with material from other species/populations.
In FLOTA Paru a single specimen of Neusticurus was observed swimming in a creek in rain forest close to a waterfall. The specimen could not be captured, and specific identification was not possible because of light conditions. Considering what is known about the distribution of this genus in Guiana (Avila-Pires, 1995; Hoogmoed, 1973) it probably was either N. bicarinatus or N. rudis. Both species were collected in the same creek in ESEC Gráo-Pará North.
Ptychoglossus brevifrontalis was considered a western Amazonian species, until one specimen was reported from the border of Suriname and Brazil by Hoogmoed (1973). In recent years however the species has been found in many localities in eastern and central Amazonia (Pinto & Quatman, 2005; Peloso & Avila-Pires, in press). The material from FLOTA Faro and ESEC Gráo-Pará North has been incorporated in the paper by Peloso & Avila-Pires (in press) and is the first reported from Pará, but we are aware of material from several other localities in Pará south of the Amazon as well.
The small amphisbaenian Mesobaena rhachicephalus Hoogmoed, Pinto Rocha & Pereira, 2009, with conical, pointed snout, belongs to a genus that was only known from southwestern Venezuela and adjacent eastern Colombia, at the edge of the Guianan Region (Gans, 1971). Its occurrence in FLOTA Faro (with two additinal specimens from Porto Trombetas, Pará) (Hoogmoed et al., 2009) came as a surprise. Being a fossorial animal its collecting is highly dependent on chance, but it is quite possible that its distribution is restricted to part of the Guianan Region. A number of small amphisbaenians in Amazonia show relatively small distributions (Hoogmoed & Avila-Pires, 1991a), although a closer study of them may show some of them to be synonymous with others (e.g. Hoogmoed & Mott, 2003). We can state here already that the large sample of small amphisbaenids in ESEC Grão-Pará South (19 specimens) has enabled us to establish that Amphisbaena tragorrhectes Vanzolini, 1971, described from this area, is a junior synonym of Amphisbaena vanzolinii. Further arguments for this synonymisation will be provided in a forthcoming paper.
We use the name Leptotyphlops albifrons (Wagler, 1824) for the species that by some authors is still named L. tenella or tenellus Klauber, 1939. We consider this species to occur from Trinidad to the Guianas, but not south of the Amazon.We do not agree with the reasoning of Franco & Pinto (2009) that the name Stenostoma albifrons Wagler, 1824 would be a nomen dubium because of a lot of wrong identifications (which is true). In our opinion the drawing presented by Wagler (1824) clearly shows what later was described as L. tenella (large eyes, dark body with light zigzag lines and yellow spots on snout and tip of tail), in which we also concur with Franco & Pinto (2009). However, we think these authors are too much fixed on the type-locality given by Wagler (1824) (environs of Belém), where the species never again has been found in nearly 200 years. Spix's localities are not always reliable, and the type specimen may have been collected on the Guiana side of the Amazon. Thus there is no reason to declare S. albifrons Wagler a nomen dubium, but instead it becomes a senior synonym of L. tenella Klauber. Other material from south of the Amazon apparently has been erroneously identified.
Leptotyphlops cupinensis CN902A, from Grao-Para South, was regurgitated by a half-grown Apostolepis quinquelineatus after it had been collected. It only concerned the posterior part of the body and tail. CN767 was collected while digging in black earth at the edge of a lake. It was inadvertently cut in several pieces, one of which was the posterior part of the body and tail, but the head could not be recovered. When dug up it was patternless bright orange. Comparison of both specimens shows them to belong to the same species - same number of scales under the tail (16), 14 scales around the middle of the tail and around the posterior part of the body, the spine at end of tail not very distinct, but present, and same body colour (no pattern), although that of CN902A was largely faded. We compared the remains of our specimens with specimens of several species of Leptotyphlops, Typhlophis squamosus and Liotyphlops ternetzii in the MPEG collection. T. squamosus has a blackish dorsal region and for that reason does not qualify. Lioptyphlops ternetzii has a high number of scales around the posterior end ofthe body (Dixon & Kofron, 1983), and therefore does not fit our specimens. The species of Leptotyphlops examined or known to us from previous studies (Hoogmoed, 1977), either have a blackish body with light zigzag stripes and a white spot on tip of tail (L. albifrons), a brown body with a white spot on the postanal scales (L. collaris Hoogmoed, 1977), a brown back and white belly (L. dimidiatus (Jan, 1861)), dorsal scales with brown spots (L. macrolepis (Peters, 1857)) or a distinct pattern of longitudinal lines (L. septemstriatus). Moreover, they all have either ten or 12 scales around the middle ofthe tail. Leptotyphlops cupinensis is the only Leptotyphlops known from the Guianan Region that is patternless and light and has 14 scales around the middle of the tail. The number of subcaudals of our two specimens falls well within the variation known for L. cupinensis (14-17) (Bailey & Carvalho, 1946; Orejas Miranda, 1967; Hoogmoed, 1977). We therefore came to the conclusion that the remains we have fit well with the same parts of Leptotyphlops cupinensis of which the colour was described by Bailey & Carvalho (1946) as "pale flesh, in alcohol creamy white, with no trace of a pigmented pattern" and by Hoogmoed (1977), from a specimen in alcohol, as "pale yellowish brown without apparent pattern". Two slides of a live specimen of L. cupinensis from the rio Teles Pires, Mato Grosso, Brazil, show a similar colour as CN767 when it was dug up. It seems that Leptotyphlops cupinensis is the only species of Leptotyphlops known from the Guiana Shield that in life might be patternless bright orange. Thus, by a process of elimination and on the basis of colour, scales around posterior body, scales around the middle of the tail and number of subcaudals, we deduce that the remains we have belong to L. cupinensis. We realize there is margin for error, but nevertheless we are rather confident about this identification. This is the first mention of this species from Pará. From the Guianan Region it has been reported from Serra do Navio, Amapá, Brasil, and Lely Mountains, Suriname (Hoogmoed, 1977). It is not known from French Guiana.
Typhlophis squamosus was known from Venezuela, Guyana, Suriname and French Guiana (Kok & Rivas Fuenmayor, 2008). Cunha & Nascimento (1978) reported the species from eastern Pará, but not from north of the Amazon River. We collected a specimen in ESEC Grão-Pará Centre, but unfortunately it escaped before it could be photographed or preserved. Its identification does not pose a problem, it had the typically dark body and the light pink head known for this species (see Starace, 1998 for a picture). It is a new record for northern Pará.
Corallus caninus was considered a species with a wide Amazonian distribution. Recently Henderson et al. (2009) demonstrated that two species occurred in the Amazon area, viz. Coralluscaninus in the Guianan region and C. batesii(Gray, 1860) in the rest of Amazonia. According to these authors C. batesii also could be a species complex. In Appendix 1 we indicate C. caninus therefore as a Guianan endemic.
Apostolepis nigrolineata generally is considered a species from south of the Amazon, where it is widely distributed (Lema & Renner, 1998; Lema, 2001). We collected it in ESEC Grão-Pará North and Centre. In the ESEC Grão-Pará North it was sympatric with A. quinquelineata.
Atractus badius. This species has been cited for a large area in Amazonia, but Hoogmoed (1980) pointed out that it was restricted to the Guianan Region and that specimens examined from outside that region belonged to different species. He reported a specimen from Serrra do Navio, Amapá. One specimen was collected in ESEC Grão-Pará South, which constitutes the first record for Pará.
Taeniophallus nicagus was resurrected as a valid species by Myers & Cadle (1994) and at that time was only known from Suriname. Martins & Oliveira (1998) reported it from the surroundings of Manaus. Hoogmoed and M. A. Ribeiro-Junior in 2006 collected a specimen in southern Amapá, near Mazagão, in terra-firme forest [MPEG 23312]. We collected it in ESEC Grão-Pará North and this constitutes the first record for Pará. In this locality the species was collected sympatrically with T. brevirostris.
Thalesius viridis was known only from Suriname and French Guiana (Hoogmoed, 1985; Ferreira-Yuki, 1993) until it was reported (as Xenodon werneri Eiselt, 1963) by Lima (2008) from the Tumucumaque Mountains, in Amapá. One specimen was collected in ESEC Grão-Pará North, and this constitutes the first record for Pará. Again, as it was collected close to the border with Guyana, it may turn up in the southern part of that country as well.
Micrurus averyi was described from the border of Suriname and Brazil and was already known from Manaus, Amazonas, but the record from ESEC Grão-Pará North is the first record of this species from Pará.
CONCLUSIONS
The goal of the expeditions to northern Pará was to obtain a good impression of the herpetofauna present in the area. With a total of 80 amphibians and 95 reptiles collected or observed out of an expected total of 109 amphibians and 164 reptiles, we may conclude that the results of the expeditions were satisfactory. Taking into account material collected in other areas of northern Pará, reported in literature or present in the collection of MPEG, we even get a better result: 89 amphibians and 138 reptiles. We collected six species new to science (three frogs [Bufo sp. n., Scinax sp. n., Chiasmocleis sp. n.], one caecilian [Rhinatrema sp. n.], one lizard [Leposoma sp. n., already reported in the literature], and one amphisbaenian [Mesobaena rhachicephala]); one species of lizard possibly new to science [Gymnophthalmus cf. underwoodi, possibly already reported in the literature]; six new records for Brazil (five frogs [Allobates spumaponens, Epipedobates cf. guayanensis, Hyla gaucheri, Phrynohyas hadroceps, Adenomera heyeri], one caecilian [Microcaecilia unicolor]) and 23 new records for (northern) Pará (13 frogs [Cochranella sp., Hyalinobatrachium iaspidiense, Hyla dentei, Scinax garbei, Scinax proboscideus, Eleutherodactylus chiastonotus, E. fenestratus, E. inguinalis, E. marmoratus, Leptodactylus bolivianus, Chiasmocleis hudsoni, Synapturanus mirandaribeiroi, Rana palmipes], four lizards [Amapasaurus tetradactylus, Bachia panoplia, Leposoma sp. n., Ptychoglossus brevifrontalis], six snakes [Leptotyphlops cupinensis, Apostolepis nigrolineatus, Atractus badius, Taeniophallus nicagus, Thalesius viridis, Micrurus averyi]). These data show that our knowledge of the herpetofauna of northern Pará has increased considerably, and has come to a level comparable to our knowledge about the herpetofauna of the two neighbouring countries Suriname and French Guiana.
It will be clear that these results are just a first step towards a better knowledge of the herpetofauna of northern Pará. We still have to learn a lot about geographic distribution within the area, and about ecological and topographic factors determining that distribution. It is hoped that the establishment of the protected areas in northern Pará will lead to a further intensification of research in that area, in order to be able to better protect the herpetofauna that still has many novelties to offer.
FINAL REMARKS
This study of seven sites in the state of Pará, Brazil, north of the Amazon, as part of a large project aiming to establish management plans for a number of state conservation units, has allowed us to greatly improve our knowledge of this region, until now only poorly studied. These data will certainly give a better basis for establishing conservation policies for the area. However, it is important to keep in mind that for such a large area the results obtained are just partial, and that faunistic studies in conservation units should not be limited to those that are necessary for elaborating initial management plans. It is important that long term inventories are planned and executed in conservation units, in order to effectively know their biodiversity and monitor it, and if necessary to adapt management plans to new data. Even though the use of some statistical tests, as estimators of richness (e.g., Chao 1, 2, Jackknife 1, 2, Bootstrap) may help in some analyses, it should be realized that these statistical tests are just that and they only can provide an estimate based on the data assembled, frequently over a short period. These tests do not take into account important biological (and other) factors that are of utmost importance to the organisms studied and that directly influence any estimation based on numbers collected. At best these tests can give some estimate based on the data available, just for the short period and for the speciefic area when and where they were obtained, and they should not be used to extrapolate data for larger areas. In the case of the herpetofauna, future studies should consider the effects of seasonality, since not all species are active throughout the year, or their apparent abundance in different periods of the year may vary. Moreover, our study indicates that many species are not evenly distributed in the whole region, but in most cases we do not know which environmental parameters are important for their distribution. Finally, not all microhabitats can be sampled adequately, and thus a number of habitat specialists (canopy, fossorial, aquatic) usually remains underrepresented. Only long term, careful studies can effectively lead to a thorough knowledge of the environment, giving better support for their conservation.
A positive development is that the northern part of Pará, together with Amapá and the neighbouring Guianan countries (Figure 1), at present form a carefully planned corridor of protected areas, following a landscape-scale approach to conservation - a core of Indian territories and more restrictive conservation units ('Estação Ecológica', 'Reserva Biológica', 'Parque Nacional', according to the Brazilian system of conservation units), surrounded by areas where the use of biodiversity is regulated to guarantee its sustainable use ('Floresta Estadual', 'Floresta Nacional', 'Reserva de Desenvolvimento Sustentável'). This gives hope that the fauna and flora of this region may escape from the threat of extinction, if indeed economic greed does not override our efforts of truly searching for a sustainable world.
ACKNOWLEDGEMENTS
We thank all students that participated in the fieldwork: Paula Carolina de Almeida, Annelise d'Angiolella, Adriano Oliveira Maciel, Pedro Luiz Vieira del Peloso, and also M.Sc. Marco Antonio Ribeiro Junior, all of whom had to work hard, often under adverse conditions, but at the same time learned the pleasant and not-so-pleasant aspects of fieldwork in isolated places. Weverton Souza Bandeira Mota was part of the ichthyological team, but in his spare moments at night joined the herpetological team as much as possible, thus effectively increasing its efficiency. Adauto Lima Cardoso tried to compensate the poor collecting results of mammal traps by joining the herpetological team at night and provided us with some interesting specimens. A special word of thanks is due to the field assistants that installed the pitfalls and drift fences and also participated in checking the pitfalls, at times installed at places that required long walks in hilly terrain: Zailson Santos da Silva, Francisco Alves Martins and Ronei de Sousa Oliveira. Also many other participants in the expeditions contributed to the final inventory by contributing specimens, or photos that served as records for the presence of certain species, even if not collected. Ariane Araujo provided slides of live Leptotyphlops cupinensis that were made by Robson W Ávila. Luis Barbosa of Conservação Internacional (Belém) provided the maps.
Conservação Internacional (CI-Brasil) financed travel from Belém to Santarém and Boa Vista, the costs of the boat for the first expedition, subsistence costs of all expeditions, as well as grants to some of the researchers. Waldima Alves da Rocha wants to express her gratitude to CI-Brasil for the grant that enabled her to participate in the project and in publishing the results. As conditioned by the mining research licence given by SEMA-PA, mining company Rio Tinto, exploring for bauxite in the area, covered all costs regarding logistics from Santarém and Boa Vista to the campsites, and took care of opening up heliports, mounting and disassembling camps, flying in personal, food and material, and removing all extraneous material from the campsites. Personnel of SEMA-Pará gave us all support within their possibilities.
REFERENCES
ANGULO, A., J.-C. MASSARY & R. BOISTEL, 2006. Addenda and errata to "Description of a new species of the genus Adenomera (Amphibia, Anura, Leptodactylidae) from French Guiana". Acta Herpetologica 1(2): 159-162.
AVILA-PIRES, T. C. S., 1995. Lizards of Brazilian Amazonia (Reptilia: Squamata). Zoologische Verhandelingen Leiden 299: 1- 706.
AVILA-PIRES, T. C. S., 2005. Reptiles. In: T. HOLLOWELL & R. P. REYNOLDS (Eds.): Checklist of the terrestrial vertebrates of the Guiana Shield. Bulletin of the Biological Society of Washington 13: 22-40.
AVILA-PIRES, T. C. S., M. S. HOOGMOED & L. J. VITT, 2007. Herpetofauna da Amazônia. In: L. B. NASCIMENTO & M. E. OLIVEIRA (Eds.): Herpetologia no Brasil II: 13-43. Sociedade Brasileira de Herpetologia, Belo Horizonte.
AVILA-PIRES, T. C. S., L. J. VITT, S. S. SARTORIUS & P. A. ZANI, 2009. Squamate (Reptilia) from four sites in southern Amazonia, with a biogeographic analysis of Amazonian lizards. Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais 4(2): 99-118.
AZEVEDO-RAMOS, C., L. A. COLOMA, S. RON, F. CASTRO & C. GASCON, 2004. Ameerega hahneli. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. Disponible in: <www.iucnredlist.org>. Acessed on: 23 August 2009.
BAILEY, J. R. & A. L. CARVALHO, 1946. A new Leptotyphlops from Mato Grosso, with notes on Leptotyphlops tenella Klauber. Boletim do Museu Nacional. Nova Série Zoologia 52: 1-7.
BARTLETT, R. D. & P. BARTLETT, 2003. Reptiles and amphibians of the Amazon. An ecotourist guide: 1-291. University Press of Florida, Gainesville.
BOISTEL, R., J.-C. MASSARY & A. ANGULO, 2006. Description of a new species of the genus Adenomera (Amphibia, Anura, Leptodactylidae) from French Guiana. Acta Herpetologica 1(1): 1-14.
CALDWELL, J. P. & M. S. HOOGMOED, 1998. Allophrynidae, Allophryne, A. ruthveni. Catalogue of American Amphibians and Reptiles 666: 1-3.
CAMPBELL, J. A. & B. T. CLARKE, 1998. A review of frogs of the genus Otophryne (Microhylidae) with the description of a new species. Herpetologica 54(3): 301-317.
CAMPBELL, J. A. & W. W. LAMAR, 2004. The venomous reptiles of the western hemisphere: i-xviii, 1-476, [1-28], i-xiv, 477-870, [1-28]. Cornell University Press, Ithaca and London.
CARAMASCHI, U. & C. A. G. CRUZ, 2001. A new species of Chiasmocleis from Brazilian Amazônia (Amphibia, Anura, Microhylidae). Boletim do Museu Nacional, Nova série Zoologia 469: 1-8.
CARVALHO, C. M., 1997. Uma nova espécie de Microteiideo do gênero Gymnophthalmus do estado de Roraima, Brasil (Sauria, Gymnophthalmidae). Papéis Avulsos de Zoologia 40(10): 161-174.
CARVALHO, C. M., 2002. Descrição de uma nova espécie de Micrurus do estado de Roraima, Brasil (Serpentes, Elapidae). Papéis Avulsos de Zoologia 42(8): 183-192.
CARVALHO, V. T.; L. BONORA & R. C. VOGT, 2007. Geographic distribution: Otophrynepyburni. Herpetological Review 38(3): 349.
CECHIN, S. Z. & M. MARTINS, 2000. Eficiência de armadilhas de queda (pitfall traps) em anfíbios e répteis no Brasil. Revista Brasileira de Zoologia 117(3): 729-740.
CHIPPEAUX, J. P., 1986. Les serpents de la Guyane française: 1-165. Editions de l'ORSTOM (Faune Tropicale, 27), Paris.
COLE, C. J. & P. J. R. KOK, 2006. A new species of gekkonid lizard (Sphaerodactylinae: Gonatodes) from Guyana, South America. American Museum Novitates 3524: 1-13.
COLWELL, R. K., 2005. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.5. Sinauer Associates, Sunderland, Massachusetts.
CORN, P. S., 1994. Straight line drift fences and pitfall traps. In: W. R. HEYER, M. A. DONNELLY, R. W. MCDIARMID, L. C. HAYEK & M. S. FOSTER (Eds.): Measuring and monitoring biological diversity. Standard methods for amphibians: 109-117. Smithsonian Institution Press, Washington.
CRUMP, M. L. & N. J. SCOTT, 1994. Visual encounter surveys. In: W. R. HEYER, M. A. DONNELLY, R. W. MCDIARMID, L.-A. C. HAYEK & M. S. FOSTER (Eds.): Measuring and monitoring biologiocal diversity. Standard methods for amphibians: 84-92. Smithsonian Institution Press, Washington.
CUNHA, O. R. & F. P. NASCIMENTO, 1978. Ofídios da Amazônia. X. As cobras da região leste do Pará. Publicações Avulsas do Museu Paraense Emílio Goeldi 32: 1-218.
CUNHA, O. R., F. P. NASCIMENTO & A. R. HOGE, 1980. Ofidios da Amazonia. XI. Ofidios de Roraima e notas sobre Erythrolamprus bauperthuisii (Linnaeus, 1752). Boletim do Museu Paraense Emílio Goeldi, série Zoologia 120: 1-21.
DIXON, J. R. & C. P. KOFRON, 1983. The Central and South American Anomalepid snakes of the genus Liotyphlops. Amphibia-Reptilia 4(2-4): 241-264.
DIXON, J. R., J. A. WIEST, JR. & J. M. CEI, 1993. Revision of the neotropical snake genus Chironius Fitzinger (Serpentes, Colubridae). Museu Regionale di Scienze Naturali Monografie 13: 1-279.
DONNELLY, M., M.CHEN, C. WATSON & G.G. WATKINS, 1998. Amphibians and Reptiles of the Iwokrama forest. In: ANONYMOUS (Ed.) The vertebrate fauna of the Iwokrama Forest. Final report from work carried out in the Iwokrama Forest by the Academy of Natural Sciences of Philadelphia 1996-1998: 18-36.
DONNELLY, M. A., M. H. CHEN & G. G. WATKINS, 2005a. Sampling amphibians and reptiles in the Iwokrama Forest ecosystem. Proceedings of the Academy of Natural Sciences of Philadelphia 154: 55-69.
DONNELLY, M. A., M. H. CHEN & G. G. WATKINS, 2005b. The Iwokrama herpetofauna: an exploration of diversity in a Guyanan rainforest. In: M. A. DONNELLY, B. I. CROTHER, C. GUYER, M. H. WAKE & M. E. WHITE (Eds.): Ecology and evolution in the tropics: a herpetological perspective: 428-460. University of Chicago Press, Chicago.
DONNELLY, M. A., R. D. MACCULLOCH, C. A. UGARTE & D. KIZIRIAN, 2006. A new riparian Gymnophthalmid (Squamata) from Guyana. Copeia 2006(3): 396-403.
DUELLMAN, W. E., 1972. South American frogs ofthe Hyla rostrata group (Amphibia, Anura, Hylidae). Zoologische Mededelingen Leiden 47: 177-192.
DUELLMAN, W. E., 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publications, Museum of Natural History, University of Kansas 65: 1-352.
DUELLMAN, W E., 1990. Herpetofaunas in Neotropical rainforests: comparative composition, history, and resource use. In: A. H. GENTRY (Ed.): Four Neotropical Rainforests: 455-505.Yale University Press, New Haven.
DUELLMAN, W. E., 1999. Distribution patterns of amphibians in South America. In: W. E. DUELLMAN (Ed.): Patterns of distribution of amphibians: a global perspective: 255-328. John Hopkins University Press, Baltimore.
DUELLMAN, W. E., 2005. Cusco Amazónico. The lives of amphibians and reptiles in an Amazonian forest: i-xv, 1-433. Comstock Publishing Association, Ithaca and London.
DUELLMAN, W. E. & M. S. HOOGMOED, 1992. Some hylid frogs from the Guiana highlands, northeastern South America: new species, distributional records, and a generic reallocation. Occasional Papers of the Museum of Natural History, University of Kansas 147: 1-21.
DUNN, E. R., 1949. Notes on South American frogs of the family Microhylidae. American Museum Novitates 1419: 1-21.
EMC (Environmental Management Consultants), 2006. Rapid biodiversity assessment of Halcrow and Guysuco conservancies (E1751). Final Report: 1-47. Disponible in: <http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2007/11/12/000020439
_20071112132643/Rendered/PDF/E17510Final0Report010GUYSUCO0RBA.pdf>. Accessed on: 12 March 2010.
ERNST, R., M.-O. RÕDEL & D. ARJOON, 2005. On the cutting edge - The anuran fauna ofthe Mabura Hill Forest Reserve, Central Guyana. Salamandra 41(4): 179-194.
ERNST, R., T. KONRAD, K. E. LINSENMAIR & M.-O. RÕDEL, 2007. Impacts of selective logging on three sympatric species of Leptodactylus in a Central Guyana rainforest. Amphibia-Reptilia 28: 51-64.
EVA, H. D. & O. HUBER (Eds.), 2005. EUR 21808 A proposal for defining the geographical boundaries of Amazonia. Synthesis of the results from an Expert Consultation Workshop organized by the European Commission in collaboration with the Amazon Cooperation Treaty Organization: i-x + 1-39. Office for Official Publications of the European Communities, Luxembourg.
FAIVOVITCH, J., C. F. B. HADDAD, P. C. D. A. GARCIA, D. R. FROST, J. A. CAMPBELL & W. C. WHEELER, 2005. Systematic review of the frog family Hylidae, with special reference to Hylinae: a phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History 294: 1-240.
FERREIRA-YUKI, V. L., 1993. Realocação genérica de Xenodon werneri Eiselt, 1963 (Serpentes: Colubridae). Comunicações do Museu de Ciências da PUCRS 50-54: 39-47.
FOUQUET, A., P. GAUCHER, M. BLANC & C. M. VELEZ-RODRIGUES, 2007a. Description of two new species of Rhinella (Anura: Bufonidae) from the lowlands of the Guiana Shield. Zootaxa 1663: 17-32.
FOUQUET A., M. VENCES, M.-D. SALDUCCI, A. MEYER, C. MARTY M. BLANC & A. GILLES, 2007b. Revealing cryptic diversity using molecular phylogenetics and phylogeography in frogs of the Scinax ruber and Rhinella margaritifera species groups. Molecular phylogenetics and Evolution 45: 567-582.
FRANÇA, F. G. R., D. O. MESQUITA & G. R. COLLI, 2006. A checklist of snakes from Amazonian savannas in Brazil, housed in the Coleção Herpetológica da Universidade de Brasília, with new distribution records. Occasional Papers Sam Noble Oklahoma Museum of Natural History 17: 1-13.
FRANCO, F. L., M. G. SALOMÃO & P. AURICCHIO, 2002. Répteis. In: P. AURICCHIO & M. G. SALOMÃO (Eds.): Manual de Técnicas e Preparação de Vertebrados: 77-121. Instituto Pau-Brasil de História Natural, São Paulo.
FRANCO, F. L. & R. R. PINTO, 2009. Stenostoma albifrons Wagler in Spix, 1824 as nomen dubium and recognition of the name Leptotyphlops tenellus Klauber, 1939 (Serpentes: Leptotyphlopidae). Salamandra 45(4): 239-244.
FROST, D. R., 2009. Amphibian species of the world: an online reference. American Museum of Natural History, New York. Version 5.3. Disponible in: <http://research.amnh.org/herpetology/amphibia/index.php>. Access on: 12 February 2009.
FROST, D. R., T. GRANT J. FAIVOVITCH, R. H. BAIN, A. HAAS, C. F B. HADDAD, R. O. DE SÁ, A. CHANNING, M. WILKINSON, S. C. DONNELLAN, C. J. RAXWORTHY, J. A. CAMPBELL, B. L. BLOTTO, P. E. MOLER, R. C. DREWES, R. A. NUSSBAUM, J. D. LYNCH, D. M. GREEN & W. C. WHEELER, 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History 297: 1-370.
GANS, C., 1971. Redescription of three monotypic genera of amphisbaenians from South America: Aulura Barbour, Bronia Gray, and Mesobaena Mertens. American Museum Novitates 2475: 1-32.
GARDNER, T. A., M. A. RIBEIRO-JUNIOR, J. BARLOW, T. C. S. AVILA-PIRES, M. S. HOOGMOED & C. A. PERES, 2007. The value of primary, secondary, and plantation forests for a neotropical herpetofauna. Conservation Biology 21(3): 775-787.
GASC, J.-P, 1990. Les lézards de Guyane: 1-76. Ed. Raymond Chabanaud, Paris.
GASC, J.-P. & M. T. RODRIGUES, 1980. Liste préliminaire des serpents de la Guyane française. Bulletin Museum National d'Histoire Naturelle (4) 2A(2): 559-598.
GORZULA, S. & J. C. SEÑARIS, 1999. Contribution to the herpetofauna of the Venezuelan Guayana. I. A data base. Scientia Guaianae 8: i-xviii, 1-269.
GOVERNO DO ESTADO DO PARÁ, 2006. Pará cria maior área contínua de preservação do mundo. Disponible in: <http://www.amazonia.org.br/noticias/noticia.cfm?id=227652>. Accessed on: 4 February 2010.
GRANT, T., D. R. FROST, J. P. CALDWELL, R. GAGLIARDO, C. F. B. HADDAD, P. J. R. KOK, D. B. MEANS, B. P. NOONAN, W. E. SCHARGEL & W. C. WHEELER, 2006. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athespatanura: Dendrobatidae). Bulletin of the American Museum of Natural History 299: 1-262.
HAAS, W., 2004. Beitrag zur Biologie und Verbreitung dreier Vertreter des neotropischen Bufo typhonius-Komplexes (Anura: Bufonidae). Salamandra 40(3/4): 207-216.
HADDAD, C. F. B. & M. MARTINS, 1994. Four species of Brazilian poison frogs related to Epipedobates pictus (Dendrobatidae): taxonomy and natural history observations. Herpetologica 59(3): 282-295.
HALLER, E. C. P. & M. T. RODRIGUES, 2005. Podocnemis uniplis (Yellow-spotted River Turtle). Nests and Nesting. Herpetological Review 36(1): 60.
HALLER, E. C. P. & M. T. RODRIGUES, 2006. Reproductive biology of the Amazonian Turtle Podocnemis sextuberculata (Testudinata: Pelomedusidae) in the Biological Reserve of Rio Trombetas, Para, Brazil. Chelonian Conservation and Biology 5: 280-284.
HEATWOLE, H., H. SOLANO & A. HEATWOLE, 1965. Notes on amphibians ofthe Venezuelan Guayanas with description oftwo new forms. Acta Biologica Venezuelica 4(12): 349-364.
HEDGES, S. B., W. E. DUELLMAN & M. P. HEINICKE, 2008. New world direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa 1737: 1-182.
HEINICKE, M. P., W. E. DUELLMAN & S. B. HEDGES, 2007. Major Caribbean and Central American frog faunas originated by ancient oceanic dispersal. Proceedings of the Academy of Natural Sciences 104: 10092-10097.
HENDERSON, R. W., P. PASSOS & D. FEITOSA, 2009. Geographic variation in the emerald treeboa, Corallus caninus (Squamata: Boidae). Copeia 2009(3): 572-582.
HEYER, W. R., 1994. Variation within the Leptodactylus podicipinus-wagneri complex of frogs (Amphibia: Leptodactylidae). Smithsonian Contributions to Zoology 546: 1-124.
HEYER, W. R., 1995. South American rocky habitat Leptodactylus (Amphibia: Anura: Leptodactylidae) with description oftwo new species. Proceedings of the Biological Society of Washington 108(4): 695-716.
HEYER, W. R., 2005. Variation and taxonomic clarification ofthe large species of the Leptodactylus pentadactylus species group (Amphibia: Leptodactylidae) from Middle America, northern South America, and Amazonia. Arquivos de Zoologia 37(3): 269-348.
HOLLOWELL, T. & R. P REYNOLDS (Eds.), 2005. Checklist of the terrestrial vertebrates ofthe Guiana Shield. Bulletin of the Biological Society of Washington 13: i-ix, 1-98.
HOOGMOED, M. S., 1969a. Notes on the fauna of Surinam II. - On the occurrence of Allophryne ruthveni Gaige (Amphibia, Salientia, Hylidae) in Surinam. Zoologische Mededelingen Leiden 44(5): 75-81.
HOOGMOED, M. S., 1969b. Notes on the herpetofauna of Surinam III. - A new species of Dendrobates (Amphibia Salientia, Dendrobatidae) from Surinam. Zoologische Mededelingen Leiden 44(9): 133-141.
HOOGMOED, M. S., 1971a. Dendrobates, eine farbenreiche Gattung. Die Aquarien- und Terrarien-Zeitschrift (DATZ) 24(1): 1-7.
HOOGMOED, M. S., 1971b. Dendrobates, een kleurrijk genus. Het Aquarium 41(8): 182-189.
HOOGMOED, M. S., 1973. Notes on the herpetofauna of Surinam. IV The lizards and amphisbaenians of Surinam: 1-417. Dr. W. Junk Publishers (Biogeographica, v. 4), The Hague.
HOOGMOED, M. S., 1977. On a new species of Leptotyphlops from Surinam, with notes on the other Surinam species of the genus (Leptotyphlopidae, Serpentes). Notes on the herpetofauna of Surinam V Zoologische Mededelingen Leiden 51(7): 99-123.
HOOGMOED, M. S., 1979a. Resurrection ofHylaornatissima Noble (Amphibia, Hylidae) and remarks on related species of green tree frogs from the Guiana area. Notes on the herpetofauna of Surinam VI. Zoologische Verhandelingen Leiden 172: 1-46.
HOOGMOED, M. S., 1979b. The herpetofauna ofthe Guianan Region. In: W. E. DUELLMAN (Ed.): The South American herpetofauna: its origin, evolution and dispersal. Museum of Natural History, The University of Kansas Monographs 7: 241-279.
HOOGMOED, M. S., 1980. Revision ofthe genus Atractus in Surinam, with the resurrection of two species (Reptilia, Colubridae). Notes on the herpetofauna of Surinam VII. Zoologische Verhandelingen Leiden 175: 1-47.
HOOGMOED, M. S., 1983 [1982]. Snakes of the Guianan Region. Memorias do Institute Butantan 46: 219-254.
HOOGMOED, M. S., 1985. Xenodon werneri Eiselt, a poorly known snake from Guiana, with notes on Waglerophis merremii (Wagler) (Reptilia: Serpentes: Colubridae). Notes on the Herpetofauna of Surinam IX. Zoologische Mededelingen Leiden 59(8): 79-88.
HOOGMOED, M. S., 1986. Biosystematic studies ofthe Bufo "typhonius" group. A preliminary progress report. In: Z. ROČEK (Ed.): Studies in Herpetology: 147-150. Societas Europaea Herpetologica, Prague.
HOOGMOED, M. S., 1990a. Resurrection of Hyla wavrini Parker (Amphibia: Anura: Hylidae), a gladiator frog from northern South America. Zoologische Mededelingen Leiden 64(6): 71-93.
HOOGMOED, M. S., 1990b. Biosystematics of South American Bufonidae, with special reference to the Bufo "typhonius" group. In: G. PETERS & R. HUTTERER (Eds.): Vertebrates in the tropics: 113-123. Museum Alexander Koenig, Bonn.
HOOGMOED, M. S. & T. C. S. AVILA-PIRES, 1989. Observations on the nocturnal activity of lizards in a marshy area in Serra do Navio, Brazil. Tropical Zoology 2: 165-173.
HOOGMOED, M. S. & T. C. S. AVILA-PIRES, 1991a. A new species of Amphisbaena (Reptilia: Amphisbaenia: Amphisbaenidae) from western Amazonian Brazil. Boletim do Museu Paraense Emflio Goeldi, série Zoologia 7(1): 77-94.
HOOGMOED, M. S. & T. C. S. AVILA-PIRES, 1991b. Annotated checklist of the herpetofauna of Petit Saut, Sinnamary River, French Guiana. Zoologische Mededelingen Leiden 65(5): 53-88.
HOOGMOED, M. S. & T. C. S. AVILA-PIRES, 1992. Studies on the species of the South American lizard genus Arthrosaura Boulenger (Reptilia: Sauria: Teiidae), with the resurrection of two species. Zoologische Mededelingen Leiden 66(35): 453-484.
HOOGMOED, M. S. & S. J. GORZULA, 1979. Checklist of the savanna inhabiting frogs of the El Manteco region with notes on their ecology and the description of a new species of tree frog (Hylidae, Anura). Zoologische Mededelingen Leiden 54(13): 183-216.
HOOGMOED, M. S. & J. LESCURE, 1975. An annotated checklist of the lizards of French Guiana, mainly based on "two recent collections. Zoologische Mededelingen Leiden 49(13): 141-171.
HOOGMOED, M. S. & T. MOTT, 2003. On the identity of Amphisbaena hugoi Vanzolini, 1990 (Reptilia: Squamata: Amphisbaenidae). Zoologische Mededelingen Leiden 77(28): 455-457.
HOOGMOED, M. S., R. R. PINTO, W. A. ROCHA & E. G. PEREIRA, 2009. A new species of Mesobaena Mertens, 1925 (Squamata: Amphisbaenidae) from Brazilian Guiana, with a key to the Amphisbaenidae of the Guianan Region. Herpetologica 65(4): 436-448.
KOK, P. J. R., 2000. A survey ofthe anuran fauna of Montagne Belvédère, county of Saül, French Guiana: field list with comments on taxonomy and ecology. The British Herpetological Society Bulletin 71: 6-26.
KOK, P. J. R., 2005. A new genus and species of gymnophthalmid lizard (Squamata: Gymnophthalmidae) from Kaieteur National Park, Guyana. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie 75: 35-45.
KOK, P. J. R., 2006a. A new species of Hypsiboas (Amphibia: Anura: Hylidae) from Kaieteur National Park, eastern edge of the Pakaraima Mountains, Guyana. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie 76: 191-200.
KOK, P. J. R., 2006b. A new snake of the genus Atractus Wagler, 1828 (Reptilia: Squamata: Colubridae) from Kaieteur National Park, Guyana, northeastern South America. Zootaxa 1378: 19-35.
KOK, P. J. R., 2008a. A new highland species of Arthrosaura Boulenger, 1885 (Squamata: Gymnophthalmidae) from Maringma tepui on the border of Guyana and Brazil. Zootaxa 1909: 1-15.
KOK, P. J. R., 2008b. Lizard in the clouds: a new highland genus and species of Gymnophthalmidae (Reptilia: Squamata) from Maringma tepui, western Guyana. Zootaxa 1992: 53-67.
KOK, P. J. R., 2009. A new species of Oreophrynella (Anura: Bufonidae) from the Pantepui region of Guyana, with notes on O. macconnelli Boulenger, 1900. Zootaxa 2071: 35-49.
KOK, P. J. R., R. D. MACCULLOCH, P. GAUCHER, E. H. POELMAN, G. R. BOURNE, A. LATHROP & G. L. LENGLET, 2006a. A new species of Colostethus (Anura: Dendrobatidae) from French Guiana with a redescription of Colostethus beebei (Noble, 1923) from its type locality. Phyllomedusa 5(1): 43-66.
KOK, P. J. R., H. SAMBHU, I. ROOPSIND, G. L. LENGLET & G. R. BOURNE, 2006b. A new species of Colostethus (Anura: Dendrobatidae) with maternal care from Kaieteur National Park, Guyana. Zootaxa 1238: 35-61.
KOK, P. J. R., M. N. C. KOKUBUM, R. D. MACCULLOCH & A. LATHROP, 2007. Morphological variation in Leptodactylus lutzi (Heyer, 1975) (Anura: Leptodactylidae) with description of its advertisement call and notes on its courtship behavior. Phyllomedusa 6(1): 45-60.
KOK, P. J. R. & R. ERNST, 2007. A new species of Allobates (Anura: Aromobatidae: Allobatinae) exhibiting a novel reproductive behaviour. Zootaxa 1555: 21-38.
KOK, P. J. R. & S. CASTROVIEJO-FISHER, 2008. Glassfrogs (Anura: Centrolenidae) of Kaieteur National Park, Guyana, with notes on the distribution and taxonomy of some species of the family in the Guiana Shield. Zootaxa 1680: 25-53.
KOK, P. J. R. & M. KALAMANDEEN, 2008. Introduction to the taxonomy of the amphibians of Kaieteur National Park, Guyana. Abc Taxa 5: 1-278.
KOK, P. J. R. & G. RIVAS FUENMAYOR, 2008. Typhlophis ayarzaguenai Seharis, 1998 is a junior synonym of Typhlophis squamosus (Schlegel, 1837). Amphibia-Reptilia 29(4): 555-558.
LA MARCA, E., C. AZEVEDO-RAMOS, L. A. COLOMA & S. RON, 2004. Scinax garbei. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. Disponible in: <www.iucnredlist.org>. Accessed on: 23 August 2009.
LATHROP, A. & R. D. MACCULLOCH, 2007. A new species of Oreophrynella (Anura: Bufonidae) from the highlands of Guyana. Herpetologica 63: 87-93.
LAURENTI, J. N., 1768. Specimen medicum, exhibens synopsin Reptilium emendatam cum experimentis circavenena et antidota reptilium austriagorum: 1-214. Trattern, Vienna.
LEMA, T., 2001 Fossorial snake genus Apostolepis from South America (Serpentes: Colubridae: Elapomorphinae). Cuadernos de Herpetologia 15(1): 29-43.
LEMA, T. & M. F. RENNER, 1998. O status de Apostolepis quinquelineata Boulenger, 1896, A. pymi, Boulenger, 1903, e A. rondoni Amaral, 1925 (Serpentes, Colubridae, Elapomorphini). Biociencias 6(1): 99-121.
LESCURE, J. & C. MARTY, 2000. Atlas des amphibiens de Guyane. Patrimoines Naturels 45: 1-388.
LIMA, A. P., W. E. MAGNUSSON, M. MENIN, L. K. ERDMANN, D. J. RODRIGUES, C. KELLER & W. HÖDL, 2006. Guia de sapos da Reserva Adolpho Ducke, Amazônia Central: 1-168. INPA, Manaus.
LIMA, J. D., 2008. A herpetofauna do Parque Nacional Montanhas do Tumucumaque, Amapá, Brasil, Expedições I - V In: E. BERNARD (Ed.): Inventários Biológicos Rápidos no Parque Nacional Montanhas do Tumucumaque, Amapá, Brasil. RAP Bulletin of Biological Assessment 48: 38-50. Disponible in: <http://www.conservation. org.br/publicacoes/files/RAP_Tumucumaque.pdf>. Accessed on: 12 March 2010.
LÖTTERS, S., W. HAAS, S. SCHICK & W. BÖHME, 2002. On the systematics of the harlequin frogs (Amphibia: Bufonidae: Atelopus) from Amazonia. II. Redescription of Atelopus pulcher (Boulenger, 1882) from the eastern Andean versant in Peru. salamandra 38: 165-184.
LÖTTERS, S., R. BOISTEL, M. BLANC, C. F. B. HADDAD & A. VAN DER MEIDEN, 2005. Atelopus hoogmoedi. In: J. E. RUEDA-ALMONACID, J. V. RODRIGUEZ-MAHECHA, E. LA MARCA, S. LÖTTERS, T. KAHN & A. ANGULO (Eds.): Ranas arlequines: 132134. Conservacion Internacional (Serie libretas de campo, 5), Bogotá.
LÖTTERS, S., K.-H. JUNGFER, F. W. HENKEL & W. SCHMIDT, 2007. Poison frogs. Biology, species & captive care: 1-668. Edition Chimaira, Frankfurt.
LUGER, M., T. W. J. GARNER, R. ERNST, W. HÖDL & S. LÖTTERS, 2008. No evidence for precipitous declines of harlequin frogs (Atelopus) in the Guyanas. Studies on Neotropical Fauna and Environment 43(3): 177-180.
MACCULLOCH, R. D. & A. LATHROP, 2001. A new species of Arthrosaura (Sauria: Teiidae) from the highlands of Guyana. Caribbean Journal of Science 37: 174-181.
MACCULLOCH, R. D. & A. LATHROP, 2002. Exceptional diversity of Stefania (Anura: Hylidae) on Mount Ayanganna, Guyana: three new species and new distribution records. Herpetologica 58: 327-346.
MACCULLOCH, R. D. & A. LATHROP, 2004a. A new species of Dipsas (Squamata: Colubridae) from Guyana. Revista de Biología Tropical 52: 239-247.
MACCULLOCH, R. D. & A. LATHROP, 2004b. Micrurusibiboboca (Serpentes, Elapidae) is not a Guiana Shield species. Phyllomedusa 3(2): 141-144.
MACCULLOCH, R., R. REYNOLDS, M. T. RODRIGUES & A. MIJARES, 2004. Pristimantis marmoratus. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. Disponible in: <www.iucnredlist.org>. Accessed on: 23 August 2009.
MACCULLOCH, R. D & A. LATHROP, 2005. Hylid frogs from Mount Ayanganna, Guyana: new species, redescriptions, and distributional records. Phyllomedusa 4: 17-37.
MACCULLOCH, R. D., A. LATHROP & S. Z. KAHN, 2006. Exceptional diversity of Stefania (Anura, Cryptobatrachidae) II: six species from Mount Wokomung, Guyana. Phyllomedusa 5(1): 31-41.
MACCULLOCH, R. D., A. LATHROP, R. P. REYNOLDS, J. CELSA SEÑARIS & G. SCHNEIDER, 2007. Herpetofauna of Mount Roraima, Guiana Shield Region, Northeastern South America. Herpetological Review 38(1): 24-30.
MACCULLOCH, R. D., A. LATHROP, P. J. R. KOK, L. R. MINTER, S. Z. KHAN & C. L. BARRIO-AMORÓS, 2008a. A new species of Adelophryne (Anura: Eleutherodactylidae) from Guyana, with additional description of A. gutturosa. Zootaxa 1884: 36-50.
MACCULLOCH, R. D., A. LATHROP, L. R. MINTER & S. Z. KAHN, 2008b. Otophryne (Anura: Microhylidae) from the highlands of Guyana: redescriptions, vocalizations, tadpoles and new distributions. Papéis Avulsos de Zoologia 48(22): 247-261.
MACCULLOCH, R. D. & A. LATHROP, 2009. Herpetofauna of Mount Ayanganna, Guyana. ROM Contributions in Science 4: 1-35.
MARTINS, M. & M. E. OLIVEIRA, 1993. The snakes of the genus Atractus Wagler (Reptilia: Squamata) from the Manaus region, central Amazonia, Brazil. Zoologische Mededelingen Leiden 67(2): 21-40.
MARTINS, M. & M. E. OLIVEIRA, 1998. Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetological Natural History 6(2): 78-150.
MASSEMIN, D., D. BORDAGE & K. KUNZ, 2003. Entdeckung einer für Französch-Guyana neuen Pipa-Art. DRACO 3: 96.
MASSEMIN, D., D. BORDAGE & K. KUNZ, 2007. Report on the occurrence of Pipa snethlageae (Anura: Pipidae) in French Guiana, with notes on its natural history. salamandra 43: 139-147.
MCDIARMID, R. W. & M. A. DONNELLY, 2005. The Herpetofauna of the Guayana Highlands: Amphibians and Reptiles of the Lost World. In: M. A. DONNELLY, B. I. CROTHER, C. GUYER, M. H. WAKE & M. E. WHITE (Eds.): Ecology and evolution in the tropics. A herpetological perspective: 461-560. University of Chicago Press, Chicago.
MEDEM, F., 1983. Los Crocodylia de Sur America. Volumen II: 1-270. Universidade Nacional de Colombia & Colciencias, Bogotá.
MORALES, V. R., 2002 [2000]. Sistematica y biogeografía del grupo trilineatus (Amphibia, Anura, Dendrobatidae, Colostethus), com descripción de once nuevas especies. Publicaciones de la Asociacion de Amigos de Donaña 13: 1-59.
MYERS, C. W. & A. S. RAND, 1969. Checklist of amphibians and reptiles of Barro Colorado Island, with comments on faunal change and sampling. Smithsonian Contributions to Zoology 10: 1-11.
MYERS, C. W. & J. E. CADLE, 1994. A new genus for South American snakes related to Rhadinea obtusa Cope (Colubridae) and resurrection of Taeniophallus Cope for the "Rhadinea" brevirostris group. American Museum Novitates 3102: 1-33.
NARVAES, P. & M. T RODRIGUES, 2009. Taxonomic revision of Rhinella granulosa species group (Amphibia, Anura, Bufonidae), with a description of a new species. Arquivos de Zoologia 40(1): 1-73.
NOONAN, B. P. & P. GAUCHER, 2005. Phylogeography and demography of Guianan harlequin frogs (Atelopus): diversification within a refuge. Molecular Ecology 14: 3017-3031.
NOONAN, B. P. & P. GAUCHER, 2006. Refugiai isolation and secondary contact in the dyeing poison frog Dendrobates tinctorius.Molecular Ecology 15: 4425-4435.
NOONAN, B. P. & K. P. WRAY, 2006. Neotropical diversification: the effects of a complex history within the poison frog genus Dendrobates. Journal of Biogeography 33: 1007-1020.
NUSSBAUM, R. A. & M. S. HOOGMOED, 1979. Surinam Caecilians, with notes on Rhinatrema bivittatum and the description of a new species of Microcaecilia (Amphibia, Gymnophiona). Zoologische Mededelingen Leiden 54(14): 217-235.
OREJAS-MIRANDA, B., 1967. El genero "Leptotyphlops" en el region Amazónica. Atas do simpósio sobre a Biota Amazónica 5 (Zoologia): 421-442.
PARKER, H. W., 1940. Undescribed anatomical structures and new species of reptiles and amphibians. The Annals and Magazine of Natural History 11: 257-274.
PEEL, M. C., B. L. FINLAYSON & T. A. MCMAHON, 2007. Updated world map of the Kóppen-Geiger climate classification. Hydrology and Earth System Sciences 1: 1633-1644.
PELOSO, P. L. V. & T. C. S. AVILA-PIRES. Morphological variation in Ptychoglossus brevifrontalis Boulenger, 1912 and the status of Ptychoglossus nicefori (Loveridge, 1929) (Squamata, Gymnophthalmidae). Herpetologica (in press).
PELOSO, P. L. V. & M. J. STURARO, 2008. A new species of narrow-mouthed frog ofthe genus Chiasmocleis Méhely 1904 (Anura, Microhylidae) from the Amazonian rainforest of Brazil. Zootaxa 1947: 39-52.
PINTO, M. G. M. & W. E. QUATMAN, 2005. Geographic Distribution: Ptychoglossus brevifrontalis (Boulenger's Large-scaled Lizard). Herpetological Review 36: 202.
PRITCHARD, P. C. H. & P. TREBBAU, 1984. The turtles of Venezuela: 1-399. Society for the Study of Amphibians and Reptiles, Ithaca.
RIBEIRO-JUNIOR, M. A., T. A. GARDNER & T. C. S. AVILA-PIRES, 2008. Evaluating the effectiveness of herpetofaunal sampling techniques across a gradient of habitat change in a tropical forest landscape. Journal of Herpetology 42(4): 733-749.
RODRIGUES, M. T., R. REYNOLDS & C. GASCON, 2004. Chiasmocleis shudikarensis. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. Disponible in: <www. iucnredlist.org>. Accessed on: 23 August 2009.
RON, S., 2005. Predicting the distribution ofthe amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37(2): 209-221.
ROZE, J., 1996. Coral snakes of the Americas: i-xii, 1-328. Krieger, Malabar.
RUEDA-ALMONACID, J. V., J. L. CARR, R. A. MITTERMEIER, J. V. RODRÍGUEZ-MAHECHA, R. B. MAST, R. C. VOGT, A. G. J. RHODIN, J. OSSA-VELÁSQUEZ, J. N. RUEDA & C. G. MITTERMEIER, 2007. Las Tortugas y los cocodrilianos de los países andi nos del trópico: 1-537. Serie Guías Tropicales de Campo, Conservación Internacional, Bogotá.
SCOTT, N. J., 1994. Complete species inventories. In: W. R. HEYER, M. A. DONNELLY, R. W. MCDIARMID, L.-A. C. HAYEK & M. S. FOSTER (Eds.): Measuring and monitoring biological diversity. Standard methods for amphibians: 84-92. Smithsonian Institution Press, Washington.
SEBA, A., 1734. Locupletissimi rerum naturalium thesauri accurata descriptio, et iconibus artificiossissimis expressio, per universam physiceps historiam. Vol. 1: 1-212. J. Wetstenius, G. Smith, J. Waesbergius, Amsterdam.
SEÑARIS, C. J. & A. ACOSTA-GALVIS, 2004. Otophryne pyburni. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.2. Disponible in: <www.iucnredlist.org>. Accessed on: 17 February 2010.
SEÑARIS, J. C. & J. AYARZAGÜENA, 2005. Revisión taxonómica de la Familia Centrolenidae (Amphibia; Anura) de Venezuela: i-vii, 1-337. Publicaciones del Comité Español del Programa Hombre y Biosfera - Red IberoMaB de la UNESCO, Sevilla.
SEÑARIS, J. C. & R. MACCULLOCH, 2005. Amphibians. In: T. HOLLOWELL & R. P REYNOLDS (Eds.): Checklist ofthe terrestrial vertebrates of the Guiana Shield. Bulletin of the Biological Society of Washington 13: 9-23.
SEÑARIS, J. C., C. A. LASSO, G. RIVAS, M. KALAMANDEEN & E. MANAWANARU, 2008. Amphibians and reptiles of the Acarai Mountains, and Sipu, Kamoa and Essequibo rivers in the Konashen COCA, southern Guyana. In: L. A. ALONSO, J. N. MCCULLOUGH, P. NASKRECKI, E. ALEXANDER & W. E. WRIGHT (Eds.): A rapid biological assessment of the Konashen Community Owned Conservation Area, Southern Guyana: 55-62. Conservation International (RAP Bulletin of Biological Assessment, 51), Arlington.
SILVA, J. M. C., A. B. RYLANDS & G. A. B. FONSECA, 2005. The fate of the Amazonian areas of endemism. Conservation Biology 19(3): 689-694.
SILVERSTONE, P. A., 1976. A revision of the poison-arrow frogs of the genus Phyllobates Bibron in Sagra (Family Dendrobatidae). Natural History Museum of Los Angeles County Science Bulletin 27: 1-53.
STARACE, F., 1998. Guide des serpents et amphisbènes de Guyane: 1-449. Ibis Rouge Editions, Guyane.
STOKSTAD, E., 2008. A second chance for rainforest biodiversity. Science 329: 1436-1438.
SUDAM, 1984. Atlas climatológico da Amazônia Brasileira: 1-125. SUDAM (Projeto de hidrologia e climatologia da Amazônia, 19), Belém.
TRUEB, L. & D. C. CANNATELLA, 1986. Systematics, morphology and phylogeny of genus Pipa (Anura: Pipidae). Herpetologica 42(4): 412-449.
TWOMEY, E. & J. L. BROWN, 2008. A partial revision of the Ameerega hahneli complex (Anura: Dendrobatidae) and a new cryptic species from the East-Andean versant of Central Peru. Zootaxa 1757: 49-65.
VANZOLINI, P. E. & C. M. CARVALHO, 1991. Two sibling and sympatric species of Gymnophthalmus in Roraima, Brasil (Sauria: Teiidae). Papéis Avulsos de Zoologia 37(12): 173-226.
VIDAL, N., M. DEWYNTER & D. GOWER, 2010. Dissecting the major American snake radiation: A molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, Caenophidia). Comptes Rendus Biologies 333(2010): 48-55.
VITT, L. J., W. E. MAGNUSSON, T. C. S. AVILA-PIRES & A. P. LIMA, 2008. Guia de lagartos da Reserva Adolpho Ducke, Amazônia Central: 1-175. INPA, Manaus.
VOGT, R. C., 1994. Reproduction of the cabeçudo, Peltocephalus dumerilianus, in the Biological Reserve of Rio Trombetas, Brazil. Chelonian Conservation and Biology 1: 159-162.
VOGT, R. C., 2008. Amazon turtles: 1-104. Gráfica Biblio, Lima.
WAGLER, J., 1824. Serpentum brasiliensium species novae ou Histoire Naturelle des espèces nouvelles de serpens, recueillis et observées pendant le voyage dans l'intérieur du Brésil dans les années 1817, 1818, 1819, 1820 execute par ordre de Sa Majesté le Roi de Bavière, publiée par Jean de Spix, écrite d'après les notes du voyageur: 1-75. Typis franc. Seraph. Hübschmanni, Munich.
WOLLENBERG, K. C., M. VEITH, B. P. NOONAN & S. LÓTTERS, 2006. Polymorphism versus species richness - systematic of large Dendrobates from the eastern Guiana Shield (Amphibia: Dendrobatidae). Copeia 2006(4): 623-629.
WOLLENBERG, K. C., S. LÓTTERS, C. MORA-FERRER & M. VEITH, 2008. Disentangling composite colour patterns in a poison frog species. Biological Journal of the Linnean Society 93: 433-444.
YANEK, K., W. R. HEYER & R. O. DE SÁ, 2006. Genetic resolution of the enigmatic Lesser Antillean distribution ofthe frog Leptodactylus validus (Anura, Leptodactylidae). South American Journal of Herpetology 1(3): 192-201.
YÁNEZ-MUÑOZ, M., P. PÉREZ-PEÑA & D. CISNEROS-HEREDIA, 2009. New country records of Hyalinobatrachium iaspidiense (Amphibia, Anura, Centrolenidae) from the Amazonian lowlands of Ecuador and Peru. Herpetology Notes 2: 49-52.
ZAHER, H., F. G. GRAZIOTTIN, J. E. CADLE, R. W. MURPHY, J. C. MOURA LEITE & S. L. BONATTO, 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia 49(11): 115-153.
 Mailing address:
Mailing address:
Museu Paraense Emílio Goeldi
Editor do Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais
Av. Magalhães Barata, 376
São Braz – CEP 66040-170
Belém - PA - Brazil
Caixa Postal 399
Phone: 55-91-3182-3246
Fax: 55- 91-3249-6373
E-mail: boletim.naturais@museu-goeldi.br
Recebido: 07/09/2009
Aprovado: 16/03/2010













