Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Boletim do Museu Paraense Emílio Goeldi Ciências Naturais
versão impressa ISSN 1981-8114
Bol. Mus. Para. Emilio Goeldi Cienc. Nat. v.5 n.3 Belém dez. 2010
Vertical distribution and ecology of vascular epiphytes in a lowland tropical rain forest of Brazil
Distribuição vertical e ecologia de epífitas vasculares em uma floresta tropical do Brasil
Edwin Theodoor PosI; Adrianus David Michel SleegersII
IUtrecht University. Institute of Environmental Biology. Department of Biology. Utrecht, The Netherlands (E.TPos@uu.nl)
IIUtrecht University. Plant Ecology & Biodiversity Group. Utrecht, The Netherlands (A.D.M.Sleegers@students.uu.nl)
ABSTRACT
In this study we investigated the vertical distribution and ecology of vascular epiphytes. Ten trees were sampled within the Brazilian National Forest of Caxiuanã, Brazil, using single rope climbing techniques. In total, 476 epiphyte individuals were sampled distributed over 60 species and 19 families. Alpha diversity (Fisher's alpha) of the vascular epiphytes was 18.16. Trees were divided into six separate height zones, species and families were distributed in a clear vertical zonation pattern which was confirmed by both a Detrended Correspondence Analysis (DCA) and Weighted Averaging (WA). Araceae and Orchidaceae showed a similar pattern to sites in Guyana and French Guiana. However, the Pteridophyte/Angiosperm ratio was far higher and no Bromeliaceae were found. Furthermore, trees in the study area appeared to contain a relative small number of epiphytes; nevertheless, overall species richness was relatively high. Only one species (Elaphoglossum styriacum Mickel) appeared to be a true indicator species for a specific height zone, because this species had far more sampled individuals. Other species could not be considered as indicator species, because they were far less abundant.
Keywords: Brazil. Lowland Tropical Rain Forest. Community structure. Vertical zonation. Alpha diversity. Vascular epiphytes.
RESUMO
Neste estudo foi investigada a distribuição vertical e ecologia de epífitas vasculares. Dez árvores foram amostradas na Floresta Nacional de Caxiuanã, Brasil, utilizando técnica de escalada com uma única corda (single rope). No total, 476 epífitas foram amostradas e distribuídas em 60 espécies e 19 famílias. A diversidade alfa (Fisher's alpha) de epífitas vasculares foi 18,16. As árvores amostradas foram divididas em seis zonas distintas de altura; espécies e famílias estavam distribuídas em um claro padrão de zonação vertical, o qual foi confirmado por análise de correspondência 'destendenciada' (Detrended Correspondence Analysis - DCA) e média ponderada (WeightedAveraging - WA). Araceae e Orchidaceae mostraram padrão similar de outros estudos semelhantes realizados na Guiana e Guiana Francesa. Porém, neste estudo, a proporção de Pteridofitás/Angiopermas foi superior e nenhuma Bromeliaceae foi encontrada. Além disso, as árvores do presente estudo tiveram um menor número relativo de epífitas. Apesar disso, a riqueza de espécies foi relativamente alta. Só uma espécie (Elaphoglossumstyriacum Mickel) pareceu ser uma verdadeira indicadora de especificidade com zona de altura, pelo fato de seus indivíduos terem sido mais amostrados. As outras espécies não puderam ser consideradas como indicadoras, pois não tiveram amostragem significativa.
Palavras-chave: Brasil. Floresta tropical. Estrutura de comunidades. Zonação vertical. Diversidade alfa. Epífitas vasculares.
INTRODUCTION
Tropical rain forests are generally accepted to be among the most species rich terrestrial habitats of the world. Of all the species found in tropical rain forests, a considerable amount appears to be represented by vascular epiphytes (Benzing, 1990; Gentry & Dodson, 1987a; Croat, 1978; Whitmore et al., 1985). In some montane cloud forests this can even reach up to 50 percent (Kelly et al., 1994). Considering this apparent importance of vascular epiphytes for local diversity, the number of papers that investigate epiphytes in a natural habitat is relatively small, although the last decade there seems to have been an increase in interest (e.g., ter Steege & Cornelissen, 1989; Catling & Lefkovitch, 1989; Kelly et al., 1994, 2004; Hietz & Hietz-Seifert, 1995a; Freiberg, 1996; Freiberg & Freiberg, 2000; Engwald, 2000; Acebey et al., 2003; Barthlott et al., 2001; Krömer et al., 2005; Arevalo & Betancur, 2006; Kreft et al., 2004; Küper et al., 2004; Benavides et al., 2005; Cardelüs et al., 2006; Köster et al., 2009).
The vertical distribution of vascular epiphytes in a tree is not random and is arguably related to humidity, branch characteristics and photon-flux densities (Gentry & Dodson, 1987b; ter Steege & Cornelissen, 1989; Hietz & Hietz-Seifert, 1995b; Freiberg, 1996, 1999; Cardelüs & Chazdon, 2005; Cardelüs, 2007; Krömer et al., 2007; Martinez-Melendez et al., 2008). The characteristics of the environment change from the bottom of the rainforest upwards into the canopy (Allee, 1926), e.g.: only a limited amount of sunlight penetrates the dense canopy and reaches the forest floor (Montgomery & Chazdon, 2001); small twigs pose problems for the settlement of the bigger epiphytes; in the canopy temperatures are higher and humidity is lower then on the forest floor (Kumagai et al., 2001). Because each species or species group has a unique set of characteristics they will all respond in a different way to these environmental factors.
Here we describe the epiphytic communities of a Tropical Lowland Rain Forest in Caxiuanä, Para, Brazil. Although Caxiuanä has been extensively studied, no inventory of vascular epiphytes and their ecology has been made before. We did not measure environmental characteristics such as photon-flux densities, humidity or substratum characteristics. Instead, we used the vertical separation of different parts of the tree (zones) to act as a surrogate for the differences in climatic conditions. Zones were sampled for the occurrence of vascular epiphyte species and their abundances.
METHODS
STUDY AREA
The study area is situated within the Brazilian national forest of Caxiuanã (1o 42' 30" S, 51o 31' 45" W; altitude approximately 60 meters), located in the municipality of Melgaço, approximately 350 km W of Belém. Total mean annual rainfall is 2,272 mm (±193 mm) with a distinct dry season between July and December, when the average rainfall drops to 555 mm (±116 mm) (Fisher et al., 2006). Mean annual temperature is approximately 25.7 oC (with a minimum of 22 oC and a maximum of 32 oC). Relative humidity averages 80% and the prevailing wind-direction is from the northeast. The amount of sunlight exceeds 2.100 hours year-1 (Oliveira et al., 2008).
The major part of the Caxiuanã Forest is Terra Firme forest, which represents approximately 85% of the total area. However, prior studies also identified flooded forests (várzea and igapó) and savannah-like vegetation. In addition, there is some residual vegetation of previously existing orchards (Lisboa et al., 1997). All data were collected in the Terra Firme Forest.
The Terra Firme forest is found on a flat surface with yellow oxisol soils of tertiary origin. The average tree density is 450-500 individuals ha-1 with c. 150-160 species (Almeida et al., 2003; Viana et al., 2003). The most dominant tree species in the Terra Firme parts of the forest belong to the families Lecythidaceae, Caesalpiniaceae and Burseraceae (Viana et al., 2003). The forest structure is formed by emergent trees (40-50 m), canopy trees (30-35 m), sub-canopy trees (20-25 m) and understory trees (up to 5 m). The opening of the canopy is approximately 10%; consequently illumination of the forest floor is very poor (Viana et al., 2003).
DATA COLLECTION
Between March and June 2009, ten trees were selected within the Caxiuanã National Forest and sampled for vascular epiphytes. The number of trees to be sampled was based on a comparison of the results of Nieder etal. (2000) and Freiberg & Freiberg (2000) which showed that ten trees should yield the majority of epiphytic species. More trees only results in the collection of the (very) rare species and fewer trees d ramatically decreases the amount of species of the area to be found within the sampling selection. Sampling effort was checked by a species-accumulation curve with species estimation based on Fisher's alpha. Total coverage area of the research area was approximately 2 km2.
Tree selection
Trees were selected using several criteria. Because the trees should represent the average circumstances of the forest, trees that either did not reach the upper canopy or emerged above it were not selected. Furthermore, trees were selected upon the accessibility of the crown and maturity of the tree. Branches should slope less then 45o for safety reasons and trees that were either too young or too dangerous to climb were not selected. Trees were climbed using single-rope techniques (Perry, 1978; ter Steege & Cornelissen, 1988).
Sampling
After ter Steege & Cornelissen (1989) trees were divided into six height zones. Because this scheme is based on conspicuous differences in epiphyte community composition and the structure of the tree rather than absolute height, it allows easy application onto many trees (Gradstein et al., 2003; Zotz, 2007). The tree is subdivided in the lower base of the tree (zone 1), the lower and upper trunk (zones 2 and 3), the lower canopy (zone 4), middle canopy (zone 5) and outer canopy (zone 6). Within each height zone the tree was searched for vascular epiphytes. Because of the relative low number of epiphytes per tree, each zone is considered a plot, i.e. per tree there are 6 plots. Zones 1-4 were sampled directly by hand, only zones 5 and 6 could not be sampled this way. Branches from these zones were sampled using a long pole with a cutter attached to the end. Of all species, the cover percentages and number of individuals were estimated and counted. From each height zone a number of individuals of each observed species were collected for identification. Collected individuals were photographed and dried on site before being transported to the herbarium in Belém (Museu Paraense Emílio Goeldi - MG). Before drying, the first identification of species was also done on site. Confirmation and secondary identification was done at the herbarium in Belém using the materials collected in earlier studies as well as at the University of Utrecht with the use of photographs.
DATA ANALYSIS
To investigate vertical distribution patterns two different analyses were carried out: DECORANA (DCA) and Weighted Averaging (WA). The DCA is an eigenvector ordination technique which creates a multidimensional space of the data-set and searches for the axes that explain the most variance. Considering the plots separately, the number of used segments for the DCA was set on 26. Many plots contained only 1-3 individuals, therefore plots were combined by artificially dividing the forest into layers according to the height zones. In this way all sampled trees are considered at once (number of used segments was then set to 22).
Weighted Averaging is based on the abundance and occurrence of each species in the specific plots and the number indicates the mean zone-preference. An Indicator Species Analysis (ISA) was used to search for species occurring faithfully in a specific height zone. ISA combines species abundances with the occurrence of the species in the particular groups and produces an indicator value. This value is tested for statistical significance by a randomization procedure. A species-accumulation curve was used to test the sampling effort. The extrapolation for the expected number of species of the area was calculated by making use of the total number of individuals collected and Fisher's alpha calculated for the area. The expected number of individuals collected was estimated by doubling the number of trees and, hence, the number of individuals. The expected number of species was calculated as: S = 1 1 exp a * ((1 + N)/a). All analyses were performed by PCORD 5.0 except WA and the species-accumulation curve, which were carried out with Microsoft Office Excel 2007.
RESULTS
FLORISTICS
The ten sampled trees of average canopy height yielded a total number of 476 individuals belonging to 60 different species of vascular epiphytes distributed over 19 families (Appendix). Species belonged to eight Angiosperm families, seven Pteridophyte families and four unknown families. Fisher's alpha was 18.16. Of all species found, there are only a few common species and a large tail of rare species, similar to the log series distribution (Fisher et al., 1943). The species-accumulation curve for the area, based on Fisher's alpha, indicated that, although a relative small number of trees has been sampled, the number of collected species is not extremely low. A doubling of the sampled trees (i.e. 20 trees) would result in an estimated increase of 12 species, even a four-fold increase of the amount of trees (i.e. 40 trees) is estimated to yield 25 more species. This suggests that, although a relative low number of trees was sampled, biases of a lack of sampling are unlikely to have a very strong effect on the results. Pteridophytes were the most important group (17 species; 28% of the total number of species). Of the Angiosperms, the most represented families were Araceae (15 species; 25%) and Orchidaceae (13 species; 22%). Other families represented 15 species (25%) in total. However, individual families in this group each represented less than 10% of the total number of species. Mean number of species found on the trees was ten species per tree with a standard deviation of three. The maximum number of species found on a single tree was fifteen and the minimum was six.
VERTICAL DISTRIBUTION
The highest number of species was found in zone 5, or the middle canopy (26 species; 28% of all species), followed by zone 4, the lower canopy (22 species; 24%). The lowest number of species was found on the trunk itself, in zone 2 (ten species; 11%). Number of species in zones 1, 3 and 6 were approximately similar (Table 1). The majority of species appear to be restricted to one specific height zone (38 out of 60 species). Although such species are found along the complete vertical gradient, zones 1, 4 and 5 included most of these specialist-species (respectively seven, nine and nine species). Indicator Species Analysis (ISA), however, showed that only one of these species could be considered as a sign ificant i nd icator species (Elaphoglossum styriacum in zone 4; P = 0.02). The remaining 22 species were found in more than one height zone, although none of the species were found in all six height zones.
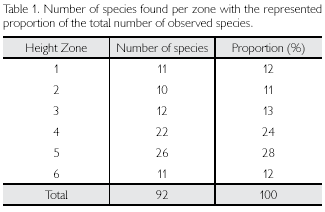
To reveal patterns in the distribution of species over the specific height zones, a DCA was performed. Considering all plots as separate sampling units, no strong vertical zonation pattern was found even after deletion of plots with fewer than three individuals. Grouping all plots in their specific height zone, however, gave a distinct pattern. DCA Axis 1 scores (zone 1 = 614.9, zone 2 = 490.1, zone 3 = 129, zone 4 = 44.3, zone 5 = 17.2, zone 6 = 0) showed zones 1-6 in exact order. This vertical zonation can be considered significant, as the probability of finding such an order at random is 1/6! (1/720 < 0.05). This vertical gradient also became apparent when performing Weighted Averaging. Species showed a preference for one or more height zones forming a vertical distribution pattern along the tree axis (Appendix). Furthermore, DCA showed that zones 1 and 2 are more related to each other than they are to zones 3, 4, 5 and 6 and vice versa.
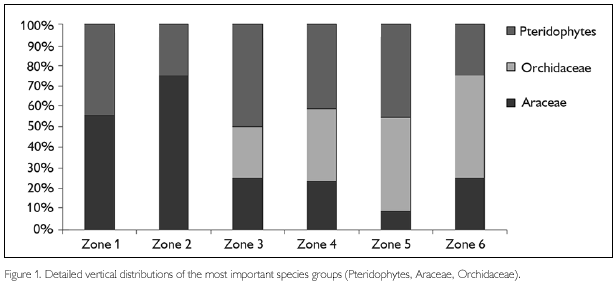
The community composition of vascular epiphytes at the family level also showed a clear gradient along the vertical axis. For Angiosperms, the numbers of Araceae were higher on the lower parts of the tree (buttress and lower trunk). In contrast, the numbers of Orchidaceae were higher in the canopy of the tree and none were found in zone 1 and 2 (Figure 1). Pteridophytes also show an interesting distribution pattern. They were well represented on the lower parts of the tree and the lower and middle canopy, but were less abundant on the trunk (i.e. zone 2) and the outer canopy (zone 6). In addition, the distribution of families within the Pteridophyte group also shows a distinct pattern on the vertical axis. The Hymenophyllaceae and Pteridaceae are most abundant in the first two zones, whereas Dryopteridaceae, Aspleniaceae, Gesneriaceae and Polypodiaceae are most abundant in the canopy zones (Appendix).
DISCUSSION
The species richness (60) of the study area is comparable to other sites in Guyana and French Guiana, where similar research was performed with approximately the same amount of trees. In Wallaba Forest (WA - Guyana), 11 sampled trees yielded 62 different species (ter Steege & Cornelissen, 1989), in Mora Forest (MO - Guyana) ten sampled trees showed 76 different species (J. C. Biesmeijer, unpublished data) and Saül (SA - French Guiana) resulted in 152 species sampled on 26 trees (R. C. Ek & D. Montfoort, unpublished data). Of the 60 species found in Caxiuanã only 12 species were also encountered in the sites of Guyana and French Guiana. Fisher's alpha for local diversity showed that biodiversity in Caxiuanã was higher in comparison to Wallaba, Guyana (18.16 compared to 11.58), but lower than in Saül (SA), French Guiana (18.16 compared to 31.99). Although the number of individuals per tree in Caxiuana was much lower, species richness was relatively higher.
Lowest species richness was found on the lower trunk (i.e. zone 2) while highest species richness was found in de middle of the canopy, zone 5. Similar results were found in previous studies by ter Steege & Cornelissen (1989), Freiberg (1996), Acebey et al. (2003) and Kromer etal. (2007). The vertical distribution of vascular epiphytes is strongly related to the microclimatic gradients such as temperature, wind velocity, humidity and light intensity (Gentry & Dodson, 1987b; ter Steege & Cornelissen, 1989; Hietz & Hietz-Seifert, 1995b; Freiberg, 1996, 1999; Cardelus & Chazdon, 2005; Cardelus, 2007; Kromer et al., 2007; Martinez-Melendez et al., 2008). The outer canopy of trees is characterized by a relatively extreme microclimate, with higher light intensities due to more direct sunlight and as a consequence higher temperatures and a lower humidity. The middle canopy however benefits from the protection of the outer canopy and its inhabitants, i.e. it benefits from within-crown shading (Cardelus & Chazdon, 2005). As a consequence, the amount of sunlight is less direct and the microclimate is less extreme and variable and provides a more suited environment for the colonization of epiphytes. This may lead to the observed higher species richness in the middle canopy. In contrast, the lower trunk provides much more difficulties in the settlement for epiphytes as a result of the simple structure. The vertical trunks are often relatively smooth and provide little suitable spots for epiphytes to anchor themselves, as suggested by Kromer et al. (2007). As a consequence, apart from a number of Hymenophyllaceae and Araceae, hardly any vascular epiphytes were found on these parts of the tree (see also Appendix).
Because of the low density of epiphytes per tree, it was not possible to find a significant vertical zonation pattern. DCA per tree only showed separation between zones 1-2 and zones 3-6. Only after combining all plots in the sampling area according to their specific height zones, a stronger vertical zonation was discovered. On a species level only one species was a true indicator species for a specific height zone (E. styriacum), probably as a consequence of having far more sampled individuals. Other species were much less abundant and could therefore not be considered as indicator species. This could be caused by a lack of sampling, which appears to be unlikely based on the species-accumulation curve calculated for the area, or because these species are truly rare species. Despite the lack of true indicator species, species did appear to have a preference for a specific part of the tree. Out of the 60 species found, 38 were found only in a single specific zone and 22 were found in more than one zone. Further analysis of the latter showed a clear distinction in species that were true generalists (8), i.e. occurring in both the canopy and on the trunk, and species which occurred either only on the trunk (5) or in the canopy (9). Species occurring solely on the trunk belonged to Araceae and Hymenophyllaceae, while species found only in the canopy belonged to Aspleniaceae, Gesneriaceae, Dryopteridaceae, Polypodiaceae or Orchidaceae. True generalists belonged to Aspleniaceae, Araceae, Clusiaceae, Pteridaceae and Orchidaceae (and one Moraceae).
Trees in the Forest of Caxiuana apparently contain a relative small number of epiphytic species in comparison to other areas (CA 15, WA 35, MO 41 and SA 61). This was also observed while exploring the forest in search for suitable trees. However, vertical distribution of the three important epiphyte groups Araceae, Orchidaceae, and Pteridophytes, was similar in all sites. Araceae were most abundant in the lower zones 1, 2 and 3 while Orchidaceae and Pteridophytes were far more abundant in the upper zones 4, 5 and 6. Despite the fact that Bromeliaceae appear to be an important epiphyte family in the other three sites, no species of the family was collected in Caxiuana on the sampled trees. In addition, no Bromeliad species were observed in the area which was sampled. In terms of individuals, the ratio between Pteridophytes and Orchidaceae in CA was 1.07 while the other three areas had far lower ratios (0.12, 0.53 and 0.27 for WA, MO and SA, respectively). Also, in terms of number of species, WA has the lowest and CA the highest ratio with MO and SA having intermediate values (WA 0.14, MO 0.56, SA 0.56 and CA 1.14). The high ratio found in CA indicates that there appear to be approximately the same number of both individuals and species of Orchidaceae and Pteridophytes in this area. Although this is unusual, it is not rare, since earlier studies indicated that ratios of Orchidaceae and Pteridophytes can vary between 0.20 and 0.84 across Neotropical epiphyte Flora in Western Amazonia (Kreft et al., 2004).
ACKNOWLEDGMENTS
We like to thank MPEG for giving us the opportunity to do fieldwork and for all the arrangements in Brazil and helpful advice we like to thank Anna Luiza Ilkiu-Borges. For the great help with collecting and identification we particularly want to thank Ana Kelly Koch, Adeilza Felipe Sampaio and José Leonardo Lima Magalhães. Furthermore, we like to thank the Herbarium of University of Utrecht and the Herbarium of MPEG for making final identification possible. For helpful comments, criticism and advice at various stages in the completion of this project we would like to thank Hans ter Steege. This project was funded by Alberta Mennega Stichting, Van Eeden Fund, Foundation Kronendak and Miquel Fonds and was part of our MSc. project at the University of Utrecht.
REFERENCES
ACEBEY, A., S. R. GRADSTEIN, & T. KRÖMER, 2003. Species richness and habitat diversification of bryophytes in submontane rain forest and fallows of Bolivia. Journal of Tropical Ecology 19(1): 9-18.
ALLEE, W. C., 1926. Measurement of environmental factors in the Tropical Rain-Forest of Panama. Ecology 7(3): 273-302.
ALMEIDA, S. S., M. A. D. FREITAS, A. S. L. SILVA & E. S. G. CAJUEIRO, 2003. Monitoring forest dynamics with permanent forest plots: the TEAM vegetation protocol and preliminary findings from Caxiuanã. Seminário Estação Científica Ferreira Penna, Dez anos de Pesquisa na Amazônia: Contribuições e Novos Desafios: 1: 13-15. Available in: <http://www.museu-goeldi.br/semicax/CBO_005.pdf>. Access on: 12 December 2010.
ARÉVALO, R. & J. BETANCUR, 2006. Vertical distribution of vascular epiphytes in four forest types of the Serranía de Chiribiquete, Colombian Guayana. Selbyana 27(2): 175-185.
BARTHLOTT, W., V. SCHMIT-NEUERBURG, J. NIEDER & S. ENGWALD, 2001. Diversity and abundance of vascular epiphytes: a comparison of secondary vegetation and primary montane rain forest in the Venezuelan Andes. Plant Ecology 152(2): 145-156.
BENAVIDES, D., A. M., A. J. DUQUE M, J. F. DUIVENVOORDEN, A. VASCO & R. CALLEJAS, 2005. A first quantitative census of vascular epiphytes in rain forests of Colombian Amazonia. Biodiversity and Conservation 14(3): 739-758.
BENZING, D. H., 1990. Vascular epiphytes. Cambridge University Press, Cambridge.
CARDELÚS, C. L., 2007. Vascular epiphyte communities in the inner-crown of Hyeronima alchorneoides and Lecythis ampla at La Selva Biological Station, Costa Rica. Biotropica 39(2): 171-176.
CARDELÚS, C. L. & R. L. CHAZDON, 2005. Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica 37(2): 238-244.
CARDELÚS, C. L., R. K. COLWELL & J. E. WATKINS JR., 2006. Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. Journal of Ecology 94(1): 144-156.
CATLING, P. M. & L. P. LEFKOVITCH, 1989. Associations of vascular epiphytes in a Guatemalan Cloud Forest. Biotropica 21(1): 35-40.
CROAT, T. B., 1978. Flora of Barro Colorado Island. Stanford University Press, Stanford.
ENGWALD, S., V. SCHMIT-NEUERBURG & W. BARTHLOTT, 2000. Epiphytes in rain forests of Venezuela–diversity and dynamics of a biocenosis: 1-425 In: S. W. BRECKLE, E. SCHWEIZER & U. ARNDT (Eds.): Results of worldwide ecological studies. Proceedings of the first symposium by the AFW Foundation. Günter Heimbach, Hoheneim, Holland.
FISHER, R. A., M. WILLIAMS, R. L. VALE, A. L. COSTA & P. MEIR, 2006. Evidence from Amazonian forests is consistent with isohydric control of leaf water potential. Plant, Cell & Environment 29(2): 151-165.
FISHER, R. A., A. S. CORBET & C. B. WILLIAMS, 1943. The relation between the number of species and the number of individuals in a random sample of an animal population. Journal of Animal Ecology 12(1): 42-58.
FREIBERG, M., 1996. Spatial distribution of vascular epiphytes on three emergent canopy trees in French Guiana. Biotropica 28(3): 345-355.
FREIBERG, M., 1999.The vascular epiphytes on a Virola michelii tree (Myristicaceae) in French Guiana. Ecotropica 5: 75-81.
FREIBERG, M. & E. FREIBERG, 2000. Epiphyte diversity and biomass in the canopy of lowland and montane forests in Ecuador. Journal of Tropical Ecology 16: 673-688.
GENTRY, A. H. & C. DODSON, 1987a. Contribution of Nontrees to species richness of a tropical rain forest. Biotropica 19(2): 149-156.
GENTRY, A. H. & C. H. DODSON, 1987b. Diversity and biogeography of neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74(2): 205-233.
GRADSTEIN, S. R., N. M. NADKARNI, T. KHRÖMER, I. HOLZ & N. NÖSKE, 2003. A protocol for rapid and representative sampling of vascular and non-vascular epiphyte diversity of tropical rain forests. Selbyana 24(1): 105-111.
HIETZ, P. & U. HIETZ-SEIFERT, 1995a. Structure and ecology of epiphyte communities of a cloud forest in central Veracruz, Mexico. Journal of Vegetation Science 6(5): 719-728.
HIETZ, P. & U. HIETZ-SEIFERT, 1995b. Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. Journal of Vegetation Science 6(4): 487-498.
KELLY, D. L., E. V. J. TANNER, E. M. N. LUGHADHA & V. KAPOS, 1994. Floristics and biogeography of a rain-forest in the Venezuelan Andes. Journal of Biogeography 21(4): 421-440.
KELLY, D. L., G. O’DONOVAN, J. FEEHAN, S. MURPHY, S. O. DRANGEID & L. MARCANO-BERTI, 2004. The epiphyte communities of a montane rain forest in the Andes of Venezuela: patterns in the distribution of the flora. Journal of Tropical Ecology 20(6): 643-666.
KÖSTER, N., K. FRIEDRICH, J. NIEDER & W. BARTHLOTT, 2009. Conservation of epiphyte diversity in an Andean landscape transformed by human land use. Conservation Biology 23(4): 911-919.
KREFT, H., N. KÖSTER, W. KÜPER, J. NIEDER & W. BARTHLOTT, 2004. Diversity and biogeography of vascular epiphytes in Western Amazonia, Yasuní, Ecuador. Journal of Biogeography 31(9): 1463-1476.
KRÖMER, T., M. KESSLER, S. R. GRADSTEIN & A. ACEBEY, 2005. Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. Journal of Biogeography 32(10): 1799-1809.
KRÖMER, T., M. KESSLER & S. R. GRADSTEIN, 2007. Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: the importance of the understory. Plant Ecology 189(2): 261-278.
KUMAGAI, T., K. KURAJI, H. NOGUCHI, Y. TANAKA, K. TANAKA & M. SUZUKI, 2001. Vertical profiles of environmental factors within tropical rainforest, Lambir Hills National Park, Sarawak, Malaysia. Journal of Forest Research 6(4): 257-264.
KÜPER, W., H. KREFT, J. NIEDER, N. KÖSTER & W. BARTHLOTT, 2004. Large-scale diversity patterns of vascular epiphytes in Neotropical montane rain forests. Journal of Biogeography 31(9): 1477-1487.
LISBOA, P. L. B., S. S. ALMEIDA & A. S. L. SILVA, 1997. Florística e estrutura dos ambientes. In: P. L. B. LISBOA (Org.): Caxiuanã: 163-193. Museu Paraense Emílio Goeldi, Belém.
MARTÍNEZ-MELÉNDEZ, N., M. A. PÉREZ-FARRERA & A. FLORESPALACIOS, 2008. Estratificación vertical y preferencia de hospedero de las epífitas vasculares de un bosque nublado de Chiapas, México. Revista de Biología Tropical 56(4): 2069-2086.
MONTGOMERY, R. A. & R. L. CHAZDON, 2001. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82(10): 2707-2718.
NIEDER, J., S. ENGWALD, M. KLAWUN & W. BARTHLOTT, 2000. Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland amazonian rain forest (Surumoni crane plot) of southern Venezuela. Biotropica 32(3): 385-396.
OLIVEIRA, L. L., R. F. COSTA, A. C. L. COSTA, F. A. S. SOUSA & A. P. BRAGA, 2008. Modelagem da interceptação na Floresta Nacional de Caxiuanã, no leste da Amazônia. Revista Brasileira de Meteorologia 23(3): 318-326.
PERRY, D. R., 1978. A method of access into the crowns of emergent and canopy trees. Biotropica 10(2): 155-157.
TER STEEGE, H. & J. H. C. CORNELISSEN, 1988. Collecting and studying bryophytes in the canopy of standing rain forest trees. In: J. M. GLIME (Ed.): Methods in bryology: 285-290. Hattori Botanical Laboratory, Nichinan.
TER STEEGE, H. & J. H. C. CORNELISSEN, 1989. Distribution and Ecology of vascular epiphytes in Lowland Rain Forest of Guyana. Biotropica 21(4): 331-339.
VIANA, J. S., S. S. ALMEIDA, C. CONCEIÇÃO, E. FERREIRA, N. ALVES & R. SILVA. 2003. Comparação estrutural e florística entre os ambientes de Terra-Firme e Igapó do entorno da Estação Científica Ferreira Penna – ECFPn. Seminário Estação Científica Ferreira Penna, Dez anos de Pesquisa na Amazônia: Contribuições e Novos Desafios. Available in: <http://www.museu-goeldi.br/semicax/CBO_001.pdf>. Access on: 12 December 2010.
WHITMORE, T. C., R. PERALTA & K. BROWN, 1985. Total Species Count in a Costa Rican Tropical Rain Forest. Journal of Tropical Ecology 1(4): 375-378.
ZOTZ, G., 2007. Johansson revisited: the spatial structure of epiphyte assemblages. Journal of Vegetation Science 18(1): 123-130.
 Endereço para correspondência:
Endereço para correspondência:
Museu Paraense Emílio Goeldi
Editor do Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais
Av. Magalhães Barata, 376
São Braz – CEP 66040-170
Belém - PA - Brasil
Caixa Postal 399
Telefone: 55-91-3182-3246
Fax: 55-91-3249-6373
E-mail: boletim.naturais@museu-goeldi.br
Recebido: 24/09/2009
Aprovado: 22/12/2010
Responsabilidade editorial: Anna Luiza Ilkiu-Borges













