Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Pan-Amazônica de Saúde
versión impresa ISSN 2176-6215versión On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.1 Ananindeua mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100006
New evidences on the diagnostic value of indirect immunofluorescence test and delayed hypersensitivity skin test in human infection by Leishmania (L.) infantum chagasi in the Amazon, Brazil
Raimundo Nonato Pires BarbosaI; Zuila de Jesus Coelho CorrêaI; Roseli Conceição dos Santos de JesusI; Domingas Ribeiro EverdosaI; João Alves BrandãoI; Raimundo Negrão CoelhoI; Antonio Júlio de Oliveira MonteiroI; Raimundo Sérgio MachadoI; João Batista Palheta da LuzI; Antonio Francisco Pires MartinsI; Roberto Carlos Feitosa BrandãoI; José Aprígio Nunes LimaI; Iorlando da Rocha BarataI; Maria Sueli Barros PinheiroI; Edna de Freitas LeãoI; Fábio Márcio Medeiros da SilvaI; Maria das Graças Soares da SilvaI; Marliane Batista CamposI; Adelson Alcimar Almeida de SouzaI; Ralph LainsonI; Fernando Tobias SilveiraII
ISeção de Parasitologia, Instituto
Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IISeção de Parasitologia,
Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil. Núcleo
de Medicina Tropical, Universidade Federal do Pará, Belém, Pará,
Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
Original Title: Novas evidências sobre o valor diagnóstico da reação de imunofluorescência indireta e reação intradérmica de hipersensibilidade tardia na infecção humana por Leishmania (L.) infantum chagasi na Amazônia, Brasil. Translated by: American Journal Experts
ABSTRACT
This is a prospective study on a cohort of 1099 individuals of both genders, aged 1-84 years (mean 24.4 years), living in an endemic area of American visceral leishmaniasis (AVL) in the municipality of Cametá, Brazil, from May 2006 to September 2008. It aimed to analyze the prevalence and incidence rates of human infection by Leishmania (L.) infantum chagasi, as well as the evolutional process of its previously defined clinical and immunological profiles: 1. Asymptomatic infection (AI); 2. Symptomatic infection (SI = AVL); 3. Subclinical oligosymptomatic infection (SOI); 4. Subclinical resistant infection (SRI); and 5. Indeterminate initial infection (III). The diagnosis was based on the simultaneous use of indirect immunofluorescence assay (IFA) and delayed hypersensitivity skin test. A total of 304 cases of infection were diagnosed during the period studied (187 for prevalence and 117 for incidence), generating an accumulated prevalence rate of 27.6%. The distribution regarding their clinical and immunological profiles presented the following order: AI 51.6%; III 22.4 %; SRI 20.1%; SOI 4.3%; and SI (= AVL) 1.6%. Based on the dynamics of the infection, the main discovery was about the III profile, which had an instrumental role in its evolution, directing it either to the resistant immunological pole - SRI (21 cases - 30.8%) and AI (30 cases - 44.1 %) profiles - or to the susceptible immunological pole - SI (1 case - 1.5%) profile. In addition, 16 cases remained within the III profile until the end of the study. It was concluded that this diagnostic approach can help monitor the infection in endemic areas, aiming mainly at preventing morbidity caused by AVL, and reducing the treatment time and expenses.
Keywords: Leishmania (L.) infantum chagasi; Infection; Immunologic Tests; Hypersensitivity, Delayed; Fluorescent Antibody Technique, Indirect.
INTRODUCTION
Currently one of the most important aspects related to the interaction between Leishmania (L.) infantum chagasi Shaw 2002 (= Leishmania chagasi Cunha and Chagas 1937), the etiological agent of American visceral leishmaniasis (AVL), and the immune response in humans is the clinical and immunological spectrum that may result from this interaction. It seems clear that a good understanding of this spectrum, especially with respect to the repertoire of human immune system responses against infection with this agent, may be of crucial importance in treating clinical cases. Until recently, the clinical spectrum of infection was thought to vary between asymptomatic infection, found in resistant individuals with a strong cellular immune response (consisting of delayed hypersensitivity, lymphocyte proliferation, and the production of gamma-interferon), and a symptomatic form found in susceptible individuals in whom suppression of this cellular immune response may lead to classical AVL15,33. However, in addition to these extreme forms of infection, individuals may present with an intermediate, "borderline" form known as subclinical oligosymptomatic infection, whose clinical and immunological characteristics are still not entirely clear27,10.
There are some studies in Brazil that have attempted to characterize the clinical and immunological spectrum of human infection with L. (L.) i. chagasi. However, these studies have been based mainly on i) the IgG antibody response (humoral response = susceptibility) or ii) the delayed hypersensitivity skin response (cellular immune response = resistance) of infected individuals. This has made it more difficult to gain a broader understanding of the human immune response against infection7,14,19. In other words, these studies have generally used either serological methods such as the immunoenzymatic ELISA assay or cellular immunity ones such as the delayed-type hypersensitivity skin test in order to obtain a diagnosis of active L. (L.) i. chagasi infection. However, it is known that these types of diagnostic methods underestimate the possibility that certain individuals who reside in an endemic area could simultaneously exhibit both types of immune responses (humoral and cellular) against L. (L.) i. chagasi infection. Hence, these types of approaches do not provide a realistic view of infections in an endemic area.
More recently, however, it has been demonstrated that simultaneous use of an indirect immunofluorescence test (IFT) and a delayed hypersensitivity skin reaction (=Montenegro skin test - MST) can provide an immunodiagnosis of symptomatic and asymptomatic human infection with L. (L.) i. chagasi in an endemic area. The high specificity of this diagnostic approach, based on the use of species-specific antigens from amastigote (for IFT) and promastigote (for MST) forms of L. (L.) i. chagasi, has permitted the identification of a broader clinical and immunological spectrum of human infection with L. (L.) i. chagasi in the Brazilian Amazon; the following clinical-immunological profiles of infection were thus defined: Asymptomatic Infection (AI), Symptomatic Infection (SI) (=AVL), Subclinical Oligosymptomatic Infection (SOI), Subclinical Resistant Infection (SRI) and Indeterminate Initial Infection (III)11,29. Although, as previously mentioned, the first three profiles (AI, SI and SOI) have already been presented in the literature, the last two (SRI and III) represent new profiles of the clinical-immunological spectrum of infection.
Based on the above-mentioned observations, it is important to present new evidence on the diagnostic value of the combined use of IFT and MST for human infection with L. (L.) i. chagasi, as obtained from a prospective study conducted in an AVL-endemic area in the Municipality of Cameta, Para State, Brazil. This study has reinforced previous findings about this diagnostic approach for human infection with L. (L.) i. chagasi, used primarily for the early diagnosis of infection; it consisted of recognizing recently infected individuals with the potential to develop an active form of the infection, the AVL. This study discusses the relevance of a diagnostic tool for recently infected cases in an endemic area — those represented by the clinical-immunological profile III — to prevent the morbidity of severe AVL and decrease the time and costs of treatment.
MATERIALS AND METHODS
STUDY AREA
This study was conducted in four small towns (Ajó, Vacaria, Vacajo and Enseada) located in the Municipality of Cameta (01o 56' S: 54o 45' W), which is situated on the banks of the Tocantins River in the northeast region of Para State, Brazil. The climate is typically equatorial, with an average temperature of 28o C and high humidity. The rainy season in this region, from January to June, has a rainfall of around 2,500 mm or more. The primary forest has almost totally been destroyed, and there are some plantations left in the midst of secondary forests. Approximately 70% of the inhabitants reside in wooden houses constructed on dry land, whereas the other part of the population live in varzea areas (floodplains) covered mainly by low vegetation. The land is flooded twice a day by the Tocantins River. Thus, the climate and the environmental conditions in this area are very similar to those described in another study conducted in the Municipality of Barcarena, Para State, located approximately 150 km from this area, where the dynamics of human L. (L.) i. chagasi transmission was previously studied28.
POPULATION AND STUDY DESIGN
The population analyzed by this study consisted of a cohort of 1,099 individuals (92.2% of the total population, 596 males and 503 females) between the ages of 1 and 84 (average 24.4 years) - a relatively young population. When the study started, there were a total of 1,192 inhabitants in the area16.
Considering that this study was designed to analyze the prevalence and incidence of human infection with L. (L.) i. chagasi, as well as the evolutionary dynamics of its clinical-immunological profiles (AI, SI=AVL, SOI, SRI, and III), it was necessary to plan a prospective study to follow up the cohort (1,099 individuals) over a two-year period (May 2006-September 2008). Thus, I FT and MST were used simultaneously to determine both the prevalence and the incidence. In other words, all individuals were previously selected for prevalence investigation, and the incidence was investigated after 12 and 24 months. Thereafter, these tests were only conducted on individuals who were found to be negative in the prevalence study and in the first incidence study (at 12 months). Therefore, individuals presenting reactivity only to MST, which represents a genetic characteristic of immunological resistance against the infection18, were removed from the subsequent assessments with this technique similarly to what was done in a longitudinal study conducted in Sudan34. Individuals presenting reactivity in both tests were tested again only for IFT, since it is unnecessary to inject new antigen loads into them, as would be the case with MST. In cases of reactivity only with IFT, which, in contrast to MST, represents an immunological state of susceptibility to infection, the individuals remained in the cohort for both tests in order to analyze the evolution of humoral and cellular immune responses. Almost 5% (54 individuals) of our original cohort were excluded from our study during follow-up period due to several reasons, such as holidays or trips.
Finally, the population was stratified into three age groups, 1-10 (303 individuals), 11-20 (252), and ≥ 21 years of age (544 to analyze the distribution of infection according to age.
We designed the current study in a similar manner as our two previous studies; one has already been published28 and the other was accepted for publication29.
CLINICAL ASSESSMENT OF INFECTED INDIVIDUALS
All the individuals who presented with some type of immunological response for IFT and/or MSR were clinically examined (mainly physically) to identify some sign or symptom recognized as a classical clinical pattern of AVL or a subclinical oligosymptomatic infection. However, it is important to highlight that only the typical cases of AVL underwent conventional therapy with pentavalent antimony, as recommended by the Brazilian National Program to Control AVL24. The cases that presented a diagnosis of subclinical oligosymptomatic infection were followed for a period of up to three months to confirm spontaneous clinical resolution, as observed in another study in Maranhão State, Brazil14.
CRITERIA FOR THE IDENTIFICATION OF HUMAN INFECTION
IFT-reactivity is indicative of a humoral response (susceptibility) and MST-reactivity is indicative of a cellular immune response (resistance)3, therefore a human case of L. (L.) i. chagasi infection was assumed as the presence of reactivity for one or both immunological tests. However, considering that co-infection with human immunodeficiency virus (HIV) can interfere with this diagnostic approach, we should note that there were no human cases of HIV co-infection within the community at the beginning of the study, as recorded by the Health Department of the Municipality of Cametá.
Considering the importance of establishing the specificity of both immunological tests (IFT and MST), we used a semi-quantitative scale scoring results from + to + + + + in the following manner: for IFT, serological titers (IgG) from 80-160 and from 320-640 received + and + + , and those from 1,280-2,560 and from 5,12010,240 received +++ and ++++, respectively. For MST, weak skin reactions (5-8 mm) received +, moderate reactions (9-12 mm) ++ , strong reactions (13-15 mm) +++, and exacerbated reactions (16 mm) ++++ . Thus, we assumed that serological reactions from a titer designation of 80 (IgG) and skin reactions forming indurations ≥ 5 mm in diameter were considered positive cut-off points for IFT and MST, respectively23,31,28. Therefore, when combining the clinical state of the infected individuals with the semi-quantitative scale of the IFT and MST results, we could identify the following immunological profiles in the clinical infection groups: AI (MST+/+ +++ and IFT-), SI (=AVL) and SOI with the same immunological profile (MST- and IFT+++/++++), SRI (MST+/++++, and IFT+/++) and III (MST- and IFT+/++)11,29.
IMMUNOLOGICAL TESTING PROCEDURES
The implementation of MST followed the same technical steps described in other studies for the diagnosis of American cutaneous leishmaniasis (ACL)31,28. Notwithstanding, considering that this study area has similar epidemiological characteristics that suggest the possibility of ACL transmission, we used a highly specific antigen for AVL, generated from promastigote forms of a stationary phase culture (RPMI 1640 medium) of a regional strain of L. (L.) i. chagasi (MCAO/BR/2003/M22697/Barcarena, Pará State) isolated from a dog infected with visceral leishmaniasis in the Municipality of Barcarena. The promastigote forms of the parasite were fixed with a merthiolate solution (1/10,000), at a final concentration of approximately 10x106 parasites/mL. As a control antigen, we used an equal dose of 0.1 mL of merthiolate solution (1/10,000) injected intradermally into the opposite forearm of each individual. It should be noted that since the Instituto Evandro Chagas (IEC) is a laboratory connected to the Secretariat of Health Surveillance of Brazil's Ministry of Health (MS, Brazil), all reagents destined for human research were previously assessed by a quality control program before use in humans.
The implementation of IFT was based on a previous study23, which demonstrated that antigens from the amastigote forms of L. (L.) i. chagasi have a greater sensitivity and specificity than antigens from the promastigote forms of the same parasite and from Leishmania (L.) major-like (Bio-Manguinhos/FIOCRUZ, Brazil), as well as those from the amastigote forms of L. (L.) amazonensis. Briefly, the antigens from the amastigote forms were fixed onto the surface of IFT slides by applying small fragments from the liver, spleen (L. i. chagasi) and skin (L. amazonensis) of a hamster (Mesocricetus auratus) infected with these parasites. When used for the laboratory diagnosis of canine visceral leishmaniasis, this procedure was also shown to be more specific than commercial kits for IFT and ELISA from Bio-Manguinhos, Brazil20.
These procedures for conducting the two immunological tests, MST and IFT, have been previously published11,28.
STATISTICAL ANALYSIS
The results were analyzed using the program Bio-Stat 4.04 and X2, and binomial tests were used to determine the significance of the differences between the clinical-immunological profiles of the infection, with a confidence interval of 95%.
APROVAL BY THE ETHICS COMMITTEE
This work was approved by the Ethics Committee in Human Research of the IEC, with the protocol number: CEP/IEC 16/2003.
RESULTS
DISTRIBUTION OF THE CLINICAL-IMMUNOLOGICAL PROFILES OF HUMAN INFECTION WITH L. (L.) I. CHAGASI: PREVALENCE
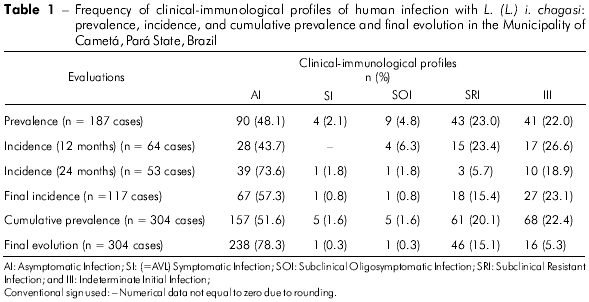
The prevalence rate of infection was 17% (187 cases/1,099 individuals). This accounts for 90 cases diagnosed only by MST (AI clinical-immunological profile), 54 diagnosed only by IFT (four cases with a SI=AVL profile, nine with a SOI profile, and 41 with a III profile), and 43 diagnosed by both tests (SRI profile). Thus, the distribution of these profiles showed a higher frequency (P < 0.05) for the AI profile (48.1%) than the others: SRI (23%), III (22%), SOI (4.8%), and SI=AVL (2.1%) (Table 1). The results also showed that the frequencies of the SRI and III profiles were higher (P < 0.05) than those of the SOI and SI profiles; however, no statistically significant difference was observed (P > 0.05) between the SRI and III profiles, and between the SOI and SI profiles. In addition, these results demonstrated that the vast majority of infected individuals (93%, 174) were asymptomatic (AI, SRI and III profiles).
DISTRIBUTION OF CLINICAL-IMMUNOLOGICAL PROFILES OF HUMAN INFECTION WITH L. (L.) I. CHAGASI: INCIDENCE
The first incidence rate, estimated 12 months after the beginning of the study, was 7.2% (64 new cases / 892 uninfected individuals in the initial prevalence study). This accounted for 28 new cases were diagnosed only by MST (AI profile), 21 only by IFT (four cases with a SOI profile and 1 7 with a III profile), and 1 5 by both tests (SRI profile). The distribution of clinical-immunological profiles showed that the AI profile once again had a higher frequency (43.7%) (P < 0.05) than the other profiles, III (26.6%), SRI (23.4%) and SOI (6.3%) (Table 1). We note, therefore, that there were no cases of AVL (=SI profile) among the new cases of infection (first year of the study). These results also demonstrated that the frequencies of the III and SRI profiles were higher (P < 0.05) than that of the SOI profile; no statistically significant difference was observed (P > 0.05) between the frequencies of the III and SRI profiles.
The second incidence measurement, calculated 24 months after the beginning of the study, was 6.6% (53 new cases / 802 uninfected individuals in the first incidence study). Overall, 39 new cases of infection were diagnosed only by MST (AI profile); 11 were diagnosed only by IFT (one case with a SI profile=AVL and ten cases with a III profile) and three were positive for both tests (SRI profile). The distribution of clinical-immunological profiles demonstrated, once again, that the AI profile (73.6%) was more frequent (P < 0.05) than the others: III (18.9%), SRI (5.7%), and SI (1.8%) (Table 1). This time, the SOI profile was not present among the new cases of infection (second year of the study). These results showed that the III profile (18.9%) was more frequent (P < 0.05) than the SRI (5.7%) and SI (1.8%) profiles, and, finally, that the SRI profile was more frequent (P < 0.05) than the SI profile.
As an explanation for the number of individuals who were assessed for incidence compared to the number expected, it is important to highlight that, based on the initial prevalence measurement, we expected to examine 912 uninfected individuals for the first incidence study. However, only 892 (97.8%) were examined, signifying a loss of 20 (2.2%) individuals. In the second incidence measurement, based on the outcomes of the first incidence study, we expected to examine 828 uninfected individuals. However, we examined only 802 (96.8%), with a loss of 26 (3.2%) individuals. Thus, during the follow-up of the cohort (1,099 individuals) over the two years of the study, there was a total loss of 46 (5.4%) individuals; we considered this to be a small, non-significant loss.
In summary, the two measurements of incidences recorded a total of 117 new cases during the two years of study; the AI profile had the highest frequency rate (57.3%), followed by the III (23.1%), SRI (15.4%), SOI (3.4%) and SI=AVL (0.8%) profiles (Table 1). Therefore, we observed that the vast majority of new cases of infection (95.7%) were also asymptomatic (AI, III and SRI profiles).
DISTRIBUTION OF CLINICAL-IMMUNOLOGICAL PROFILES OF HUMAN INFECTION WITH L. (L.) I. CHAGASI: CUMULATIVE PREVALENCE
After three assessments (one for prevalence and two for incidence), a total of 304 cases of human infection with L. (L.) i. chagasi were recorded, with an cumulative prevalence rate of 27.6%; the AI profile was the most prevalent (51.6%), followed by III (22.4%), SRI (20.1%), SOI (4.3%), and, finally, SI=AVL (1.6%) (Table 1).
DISTRIBUTION OF CLINICAL-IMMUNOLOGICAL PROFILES OF HUMAN INFECTION WITH L. (L.) I. CHAGASI BY AGE: PREVALENCE AND INCIDENCE
When considering the age distribution of clinical-immunological profiles of infection in the prevalence study (187 cases), we observed that, in the 1-10 age group (23%, 43 cases), there was no difference (P > 0.05) between the frequency rates of the AI (30.2%, 13), SRI (30.2%, 13) and III (25.6%, 11) profiles, which were higher (P < 0.05) than those of the SI (9.3%, 4) and SOI (4.7%, 2) profiles. In the 11-20 age group (23.5%, 44 cases), there was no difference (P > 0.05) between the frequencies of the AI (47.7%, 21) and III (34.1%, 15) profiles, which were higher (P > 0.05) than those of the SRI (1 3.6%, 6) and SOI (4.6%, 2) profiles. Finally, in the ≥ 21 age group (53.5%, 100 cases), we noted a higher frequency (P < 0.05) of the AI profile (56%, 56) than the other profiles: SRI (24%, 24), III (15%, 15), and SOI (5%, 5). On the other hand, when comparing the age distribution of cases of infection within the same profile, three findings stood out in the prevalence study: i) we noted an increasing frequency of AI-profile cases with age, with 13 cases (14.5%) in the 1-10 age group, 21 cases (23.3%) in the 11-20 age group, and 56 cases (62.2%) in the ≥ 21 age group, which suggests an increasing trend of the AI profile with age; ii) the four cases with a SI (=AVL) profile were diagnosed in individuals in the youngest age group, 1-10 years; and iii) among the cases with the III profile (44 cases), there was no difference (P > 0.05) in the frequency between age groups, with 1 1 cases (26.8%) in the 1-10 age group, 1 5 cases (36.6%) in the 1 1 -20 age group, and 1 5 cases (36.6%) in the ≥ 21 age group (Table 2).
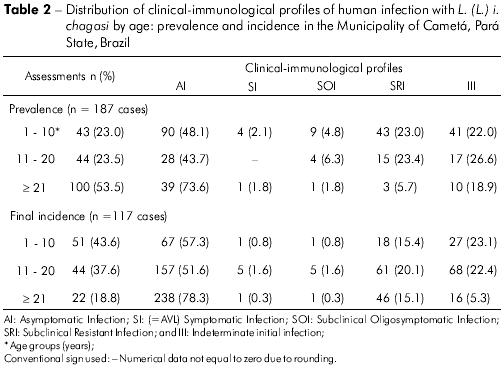
As for the two incidence measurements (117 cases), we note that the highest frequency of cases occurred in the two lowest age groups: i) in the 1-10 age group (43.6%, 51 cases), the AI profile was more frequent (58.8%, 30 cases) (P<0.05) than the III (27.5%, 14 cases), SRI (5.9%, three cases), SOI (5.9%, three cases) and SI (1.9%, one case) profiles; ii) in the 11-20 age group (37.6%, 44 cases), the AI profile was more frequent (68.2%, 30 cases) (P<0.05) than the SRI (18.2%, eight cases) and III (13.6%, six cases) profiles. The ≥21 age group (18.8%, 22 cases) showed not only the lowest infection frequency, but also equal rates (P<0.05) for the AI (31.8%, seven cases), SRI (31.8%, seven cases) and III (31.8%, seven cases) profiles, with the lowest frequency for the SOI profile (4.6%, one case). On the other hand, when the infection frequency was compared for the same profiles, two findings stood out: i) for both the AI and III profiles, the highest frequency of cases, 89.5% (60 cases) and 74% (20 cases) respectively, occurred in the lowest age groups (1-10 and 11-20 years), which indicates a higher frequency of new infections in individuals under 21 years of age; and ii) the only disease case (SI=AVL profile) occurred, once again, in the lowest age group, 1-10 years (Table 2).
DYNAMICS OF THE CLINICAL AND IMMUNOLOGICAL PROGRESSION OF HUMAN INFECTION WITH L. (L.) I. CHAGASI
The results related to the clinical and immunological progression of the infection are shown in table 1 and figure 1 (final evolution of the infection). However, to clarify this process, we decided to evaluate the results obtained for the cumulative prevalence (total number of cases diagnosed in the prevalence and incidence studies) in order to eventually elucidate the final evolution of the cases of infection. Thus, starting with the recent cases of infection with the III profile (IFT+/++ and MST-), we observed that, of the 68 (22.4%) diagnosed cases (41 in the prevalence and 27 in the incidence studies), 21 (30.9%) evolved to the SRI profile (converted to MST+/+ + + +), 30 (44.1%) to the AI profile (converted to MST+/+ + + + and IFT-), 1 (1.5%) to the SI (=AVL) profile (amplified to IFT+ + +/+ + + +), and 16 (23.5%) retained the same profile until the end of the study. Next, of the 61 (20.1%) cases (43 in the prevalence and 18 in the incidence studies) displaying the SRI profile (IFT+/++ and MST +/+ + + +), 47 (77%) evolved to the AI profile (converted to IFT-) and 14 (23%) maintained the same profile. As 21 cases of the III profile evolved to the SRI profile, and other 8 of the SOI profile and 3 of the SI profile also evolved to the SRI profile, the final evolution of the SRI profile was of 15.1% (46 cases). With respect to the SOI profile (IFT+ + +/+ + + + and MST-), of the 13 (4.3%) diagnosed cases (nine in the prevalence and four in the incidence studies), eight (61.5%) evolved to the SRI profile (converted to MST+/+ + + + and IFT reaction decreased to +/++), two (15.4%) evolved to the AI profile (converted to MST+/+ + + + and I FT-), and 3 (23.1%) maintained the same profile. Of the five (1.6%) cases of AVL (IFT+ + +/+ + + + e MST-), four were diagnosed in the prevalence study and one in the incidence studies; clinical treatment with pentavalent antimony resulted in three (60%) conversions to the SRI profile (converted to MST+/+ + + + and IFT reaction decreased to +/++), one (20%) to the AI profile (converted to MST+/+ + + + and IFT-), and one (20%) case maintained the initial immune response profile until the end of the study but was asymptomatic (clinically cured). Finally, regarding the 157 cases (51.6%) of the AI profile (MST+/+ + + + and IFT-) (90 diagnosed in the prevalence study and 67 in the incidence studies) they did not evolve to other profiles because they already represent the pool of individuals with genetic resistance to infection. However, 30 cases were added from the III profile, 47 from the SRI profile, two from the SOI profile, and one from the SI (=AVL) profile, yielding a final frequency of 78.3% (238 cases).
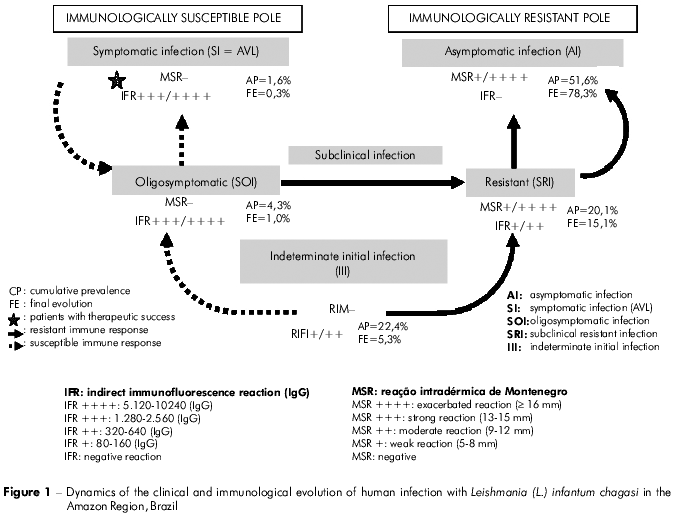
In summary, the only profile that became more frequent over the progression of the clinical and immunological response was the AI profile (51.6% - 78.3%); the frequency of the other profiles decreased: SI (1.6% - 0.3%), SOI (4.3% - 1%), SRI (20.1% - 15.1%), and, with the most significant decrease, III (22.4% - 5.3%).
Finally, it is important to point out that, throughout the study period, no cases of cutaneous leishmaniasis were detected among MST- and IFT-reactive individuals. This confirmed the specificity of the MST and IFT immunological reactions in diagnosing cases of human infection with L. (L.) i. chagasi. Similarly, there were no known cases of co-infection with HIV among the individuals who took part in the study.
DISCUSSION
There is no no doubt as to the importance of the AI profile in the clinical-immunological spectrum of human infection with L. (L.) i. chagasi. The results of this study demonstrate, once again, that the AI was the most frequent of all the profiles diagnosed, especially in the cumulative prevalence analysis (old and new cases of infection); it represented 51.6% of all diagnosed cases, followed by the III (22.4%), SRI (20.1%), SOI (4.3%), and SI (=AVL) (1.6%) profiles (Table 1). Thus, the high frequency of the AI profile may be interpreted as a short period of time that elapses between the initial stage of infection (III profile) and its final stage (AI profile). This indicates that the majority of the III profile cases present with a short period of humoral response (IFT+), followed by rapid conversion of the delayed-type hypersensitivity response (SRI profile) and, lastly, a negative conversion of I FT-.
In a previous study29, a similar situation was observed in an AVL-endemic area in the Municipality of Barcarena, Pará, around 150 km from the location of the present study; in that study, the AI profile corresponded to 73.2% of all cases of infection regarding their cumulative prevalence. Therefore, considering that delayed-type hypersensitivity represents a strong expression18 of cellular immune resistance33,10 against infection with L. (L.) i. chagasi, these results confirm that the vast majority (final distribution of the AI profile, 78.3%) of infected individuals in an endemic area are genetically resistant to infection. Also, considering that the SRI profile (final distribution, 15.1%) represents an evolutionary stage of infection with respect to the resistant pole (AI profile), the AI frequency could reach 90% in an endemic area.
Accordingly, if delayed hypersensitivity can be considered a definitive genetic characteristic of cellular immune resistance against infection, it is probable that the loss of this response (delayed hypersensitivity) may result in immunosuppression2 or in low specificity of response to Leishmania antigens used to stimulate delayed hypersensitivity13. In the Brazilian Amazon, however, a much greater specificity was observed for delayed hypersensitivity reactions (16 x 12 mm) using L. (L.) i. chagasi species-specific antigen from this study than the reactions (11 x 6 mm) produced by the L. (L.) amazonensis antigen, a causative agent of ACL, in two adult individuals (30 years of age) native to an AVL-endemic area (Municipality of Igarapé-Miri) in Pará State, Brazil. This not only suggests that cellular immunity has a long duration (immunological memory), but also that it results from a species-specific immune response30.
As for the SRI profile, which represents a new stage of infection for this diagnostic approach, its performance was not very different from that observed in previous studies29. Its frequency rate in the prevalence study (23%) was relatively higher than in the final incidence (15.4%), suggesting that it is a stage more often found among older cases of infection and, consequently, with a higher probability of evolving towards infection resistance (AI profile). This fact was confirmed in 47 (77%) of the 61 SRI cases diagnosed in this study through the negative conversion of the humoral response and the maintenance of delayed hypersensitivity (IFT- and MST+/+ + + +). Thus, since the expression of delayed hypersensitivity is genetically controlled, from the moment that a recently infected individual (III profile) converts the delayed hypersensitivity (SRI profile), the infection will naturally progress to the resistance profile (AI profile).
With respect to the III profile, which not only constitutes the other new stage of infection, but also the most recent stage of infection in this diagnostic context, we found it interesting that, in contrast to a previous study carried out in the Municipality of Barcarena (Pará State, Brazil), where the prevalence rate of infection was 12.6%, the recent cases of infection varied little throughout the phases of this study: initial prevalence, 22%; incidence at 12 months, 26.6%; incidence at 24 months, 18.9%; final incidence, 23.1%; and cumulative prevalence, 22.4%. This is indicative of stable transmission at a higher level in the current study area, in the Municipality of Cametá, where the prevalence of infection was also higher (17%). In addition, considering that the final incidence of the III profile of 23.1%, we expected that at least 20-23% of the new cases of infection in this area would require clinical monitoring, since approximately 5-6% of these cases presented with the potential to develop into susceptible clinical forms of the infection, SOI and SI (=AVL). Thus, these findings should be taken into consideration when developing new programs to control AVL.
The SOI and SI (=AVL) profiles presented the lowest frequency rates in all phases of this study, although the former had a cumulative prevalence (4.3%) almost three times higher than the latter (1.6%). Nonetheless, similarly to the first study29, both profiles combined (cumulative prevalence of 5.9%) did not surpass the range of 6% of the total number of cases in the endemic area of the Brazilian Amazon. This finding appears to be very different from the data collected in the northeast of Brazil. In Bahia State, the subclinical oligosymptomatic form (SOI) was detected in 60% of 86 infected children under the age of 156. In Maranhão State, this form was diagnosed in 1 7.4% of infected children in the same age group14. We should stress, however, that in both cases the final diagnosis of disease (SOI) was based on clinical parameters and supported only by a serological test (ELISA). Therefore, it is possible that the differences observed in the two studies from the northeast region of Brazil are due to the age group restriction (up to 15 years) of the individual participants (the children presented with a greater susceptibility to symptomatic infection), although in this study we observed that seven (53.8%) of the 1 3 SOI profile cases were in the youngest age groups (1-10 and 1 1 -20 years). On the other hand, the incidence of the SI (=AVL) profile was almost negligible (0.2 / 1,000 inhabitants) when compared to the much higher rates recorded in the northeast region of Brazil6,9,25. Th is indicates a more intense transmission of infection and/or an increased susceptibility of infected individuals to develop active disease.
When the distribution of the clinical-immunological profiles was considered with respect to age, it was demonstrated once more that the AI profile was more frequent than the other profiles in almost all the groups analyzed (1-10, 11-20 and ≥ 21 years), in terms of both prevalence and incidence (Table 2), with exception of the prevalence in the 1-10 age group (30.2%), which was equal to the frequency rate of the SRI profile, and the incidence in the ≥ 21 age group (31.8%), which was also equal to the frequency of the SRI and III profiles. Thus, the AI profile was the most frequent across age groups and phases of the study (prevalence and incidence measurements).
On the other hand, when the frequency of infection was compared within the same profiles, the following notable findings were observed. First, the prevalence of the AI profile increased with age. Although this may be interpreted as clear evidence that delayed hypersensitivity (acquired cellular immunity) increases with age6,13,26,1, in fact it only reflects the fact that that older individuals (≥ 21 years of age) have had a longer exposure than younger individuals (1-10 and 11-20 years of age). In contrast, the incidence of the AI profile decreased with age, with a significant reduction of the number of cases from the two youngest groups (1-10 and 11-20 years of age: 30 cases each, 44.8%) to the oldest one (≥ 21 years: seven cases, 10.4%). This demonstrates that children and adolescents constitute the majority of the AI profile among new cases of infection. Second, the four cases of AVL (SI profile) occurred in the youngest age group (1-10 years), which confirms that AVL is a disease typical of young children. Third, among the SOI profile cases, we noted an almost equal distribution between the age groups, with seven (53.8%) cases in the two youngest groups (1-1 0 and 11-20 years of age) and six (46.2%) cases in the older group (≥21 years). Fourth, we noted that the prevalence of the SRI profile was greater in the ≥ 21 age group (55.8%) compared to the 1-10 and 11-20 age groups (44.2%). This suggests a greater frequency of the AI profile among individuals who have been infected longer in the older age group. However, this tendency was inverted when examining the incidence, with a greater frequency in the 110 and 11-20 age groups (61.1%) compared to the older group (38.9%). Finally, with respect to the III profile, a significantly greater frequency of cases in the 1-10 and 1120 age groups (74%) compared to the 21 age group (26%) was only evident in the incidence measurements (new cases of infection). Again, this suggests that the transmission of infection occurs mainly in or close to the home, where children and adolescents are infected21,22,32.
With respect to the dynamics of the evolution of infection, the importance of the findings related to the III profile cannot be overlooked because, for this diagnostic approach this clinical-immunological profile plays a fundamental role in the transition to other profiles of human infection. Thus, this study shows that, of the 68 diagnosed cases, 30 (44.1%) evolved to the AI profile and 21 (30.8%) into the SRI profile. This accounts for almost 75% (74.9%) of the cases that progressed to the infection resistance (AI profile) pool, which constituted 78.3% of the final distribution of profiles. Additionally, one (1.5%) case progressed to the infection susceptibility pool (SI-AVL profile) and 16 (23.5%) did not change over the course of the study. Thus, these results appear to agree completely with the rationale behind this diagnostic appraoch29, in that the progression of infection from the III profile to the resistance pool (AI profile) or to the susceptibility pool (SI profile) may depend on the genetic profile of the individual's cellular immune reponse8,17 (Figure 1).
Based on the above premise, we consider the progression of the susceptible profiles (SOI and SI) to be a priority. Although these groups show the same immunological profile (MST- and IFT+ + +/+ + + +), they can be distinguished by the fact that the former (SOI) presented with spontaneous clinical progression towards cure in ten (77%) of the 13 diagnosed cases (eight evolved to the SRI profile, two into the AI profile, and three remained unaltered until the end of the study). For the latter group (AVL), pentavalent antimony therapy was needed in order to favorably progress towards cure (three progressed to the SRI profile, one into the AI profile, and one maintained the initial immunological profile but remained asymptomatic). In this respect, it is important to mention the results obtained on the evolution of the SOI form from the states of Maranhão14 and Ceará15 : 33 cases in Maranhão and 12 cases in Ceará showed a similar progression to the evolution pattern observed in the present study. In addition, another study conducted in Bahia State5 reported that individuals with the SOI form were able to produce more interferon-gamma  in peripheral blood mononuclear cell cultures than individuals with AVL, which can help to better understand the performance of these symptomatic forms of human infection with L. (L.) i. chagasi.
in peripheral blood mononuclear cell cultures than individuals with AVL, which can help to better understand the performance of these symptomatic forms of human infection with L. (L.) i. chagasi.
With respect to the profiles showing resistance to infection (SRI and AI), our impression was that SRI appears to represent a developmental stage towards the AI profile, since the majority (77%) of cases converted to IFT-, evolving to the immune status of the AI profile. This may help to explain the high frequency of the AI profile found in this study and in a previous study29. On the other hand, a few cases with the AI profile in the prevalence study exhibited transitory sero-conversion to IFT+ at low levels (+/++), which was followed by negative reconversion; this finding is interpreted as resulting from a possible antigen impulse of short duration caused by a reinfection aborted by the cellular immune response of these individuals. However, the vast majority of AI cases retained an unaltered immune profile, suggesting that the AI profile represents the end of the developmental process of the infection (Figure 1).
Finally, the infection dynamics showed that only the AI profile increased in frequency over the course of infection, rising from cumulative prevalence of 51.6% to a final distribution of 78.3%, whereas the frequency of the other profiles decreased significantly: SI (=AVL) from 1.6% to 0.3%; SOI from 4.3% to 1.0%; SRI from 20.1% to 15.1%; and III from 22.4% to 5.3%. Therefore, considering the role of the SRI and III profiles in the context of this diagnostic approach, the importance of these new clinical-immunological stages in promoting the evolution of the infection, especially the III profile, appears irrefutable. This fact may be of great value in preventing AVL morbidity, as well as in reducing the time and costs required for treatment.
FINANCIAL SUPPORT
This work was conducted with financial support from IEC/SVS/MS and the Wellcome Trust Foundation (London, UK).
REFERENCES
1 Ali A, Ashford RW. Visceral leishmaniasis in Ethiopia. I. Cross-sectional leishmanin skin test in an endemic locality. Ann Trop Med Parasitol. 1993 Apr;87(2):157-61. [ Links ]
2 Ali A, Ashford RW. Visceral leishmaniasis in Ethiopia. II. Annual leishmanin transformation in a population: is positive leishmanin reaction a life-long phenomenon? Ann Trop Med Parasitol. 1993 Apr;87(2):163-7. [ Links ]
3 Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J Med Res. 2004 Jun;119(6):238-58. [ Links ]
4 Ayres M, Ayres Júnior M, Ayres D, Santos AS. Bioestat 4.0: aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: Sociedade Civil Mamirauá; 2004.
5 Bacellar O, Barral-Neto M, Badaró R, Carvalho EM. Gamma interferon production by lymphocytes from children infected with L. chagasi. Braz J Med Biol Res. 1991;24(8):791-5. [ Links ]
6 Badaró R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, et al. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003-11. [ Links ]
7 Badaró R, Jones TC, Lorenço R, Cerf BJ, Sampaio D, Carvalho EM, et al. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986 Oct;154(4):639-49. [ Links ]
8 Blackwell JM, Mohamed HS, Ibrahim ME. Genetics and visceral leishmaniasis in the Sudan: seeking a link. Trends Parasitol. 2004 Jun;20(6):268-74. DOI:10.1016/j.pt.2004.04.003 [ Links ]
9 Caldas AJM, Costa JML, Silva AAM, Vinhas V, Barral A. Risk factors associated with infection by Leishmania chagasi in north-east Brazil. Trans Roy Soc Trop Med Hyg. 2002 Jan-Feb;96(1):21-8. [ Links ]
10 Costa SR, D'Oliveira Júnior A, Bacellar O, Carvalho EM. T cell response of asymptomatic Leishmania chagasi infected subjects to recombinant leishmania antigens. Mem Inst Oswaldo Cruz. 1999 May-Jun;94(3):367-70. [ Links ]
11 Crescente JAB, Silveira FT, Lainson R, Gomes CMC, Laurenti MD, Corbett CEP. A cross-sectional study on the clinical and immunological spectrum of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans Roy Soc Trop Med Hyg. 2009 Dec; 103(12):1250-6. DOI:10.1016/j.trstmh.2009.06.010 [ Links ]
12 Cunha AM, Chagas E. Nova espécie de protozoário do gênero Leishmania patogênico para o homem. Leishmania chagasi n.sp. Nota prévia. Hospital (Rio J). 1937;11:3-9.
13 Davies CR, Mazloumi Gavgani AS. Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology. 1999 Sep;119(Pt 3):247-57. [ Links ]
14 Gama MEA, Costa JML, Gomes CMC, Corbett CEP. Subclinical form of the American visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2004 Dec;99(8):889-93. DOI:10.1590/S0074-02762004000800018 [ Links ]
15 Holaday BJ, Pompeu MM, Evans T, Braga DN, Texeira MJ, Sousa AQ, et al. Correlates of Leishmania-specific immunity in the clinical spectrum of infection with Leishmania chagasi. J Inf Dis. 1993 Feb;167(2):411-7. [ Links ]
16 Instituto Brasileiro de Geografia e Estatística. Contagem nacional de populações. Rio de Janeiro: Superintendência de Estudos Geográficos e Sócio-Econômicos; 2004.
17 Jamieson SE, Miller EM, Peacock CS, Fakiola M, Wilson ME, Bales-Holst A, et al. Genome-wide scan for visceral leishmaniasis susceptibility genes in Brazil. Genes Immun. 2007 Jan;8(1):84-90. DOI:10.1038/sj.gene.6364357 [ Links ]
18 Jerônimo SMB, Holst AK, Jamieson SE, Francis R, Bezerra FL, Ettinger NA, et al. Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 2007 Oct;8(7):539-51. DOI:10.1038/sj.gene.6364422 [ Links ]
19 Jerônimo SMB, Teixeira MV, Sousa AQ, Thielking P, Pearson RD, Evans TG. Natural history of Leishmania (Leishmania) chagasi infection in Northeastern Brazil: Long-term follow-up. Clin Infec Dis. 2000 Mar;30(3):608-9. DOI: 10.1086/313697 [ Links ]
20 Jesus RCS, Corrêa ZC, Everdosa DR, Martins AP, Eliseu LS, Campos MC, et al. Comparação das técnicas de RIFI (ag. IEC x ag. Bio-Manguinhos) e ELISA no sorodiagnóstico da leishmaniose visceral canina, estado do Pará, Brasil. Rev Soc Bras Med Trop. 2003;36:323.
21 Lainson R, Rangel EF, editores. Flebotomíneos no Brasil. Rio de Janeiro: FIOCRUZ; 2003. Ecologia das leishmanioses: Lutzomyia longipalpis e a ecoepidemiologia da leishmaniose visceral americana (LVA) no Brasil. p. 311-36. [ Links ]
22 Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil a review. Mem Inst Oswaldo Cruz. 2005 Dec;100(8):811-27. DOI:10.1590/S0074-02762005000800001 [ Links ]
23 Lima LVR, Souza AAA, Jennings YL, Corrêa Z, Jesus R, Everdosa D, et al. Comparison of the reactivity between antigens of Leishmania (L.) chagasi, L. (L.) amazonensis e Leishmania sp. (Bio-Manguinhos) in the serodiagnosis of visceral leishmaniasis by the indirect fluorescent antibody test (IFAT). Rev Inst Med Trop Sao Paulo. 2003;45 Suppl 13:147.
24 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de Vigilância e Controle da Leishmaniose Visceral. Brasília; 2003. p. 1-120.
25 Nascimento MDSB, Souza EC, Silva LM, Leal PC, Cantanhede KL, Bezerra GFB, et al. Prevalência de infecção por Leishmania chagasi utilizando os métodos de ELISA (rK39 e CRUDE) e intradermorreação de Montenegro em área endêmica do Maranhão, Brasil. Cad Saude Publica. 2005;21:1801-07. DOI:10.1590/S0102-311X2005000600028b [ Links ]
26 Pampiglione S, Manson-Bahr PEC, La Placa M, Borgatti MA, Musumeci S. Studies in Mediterranean leishmaniasis: 3. The leishmanin in skin test kala-azar. Trans Roy Soc Trop Med Hyg. 1975;69(1):60-8. [ Links ]
27 Pearson RD, Souza AQ. Clinical spectrum of leishmaniasis. Clin Inf Dis. 1996;22:1-13.
28 Silveira FT, Blackwell JM, Ishikawa EA, Braga RR, Shaw JJ, Quinnell RJ, et al. T cell responses to crude and defined leishmanial antigens in patients from the lower Amazon region of Brazil infected with different species of Leishmania of the subgenera Leishmania and Viannia. Parasite Immunol. 1998 Jan;20(1):19-26. DOI:10.1046/j.1365-3024.1998.t01-1-00126.x [ Links ]
29 Silveira FT, Lainson R, Souza AAA, Crescente JAB, Campos MB, Gomes CMC, et al. A prospective study on the dynamics of clinical and immunological evolution of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans Roy Soc Trop Med Hyg. In Press 2009.
30 Silveira FT, Lainson R, Souza AAA, Ishikawa EAY, Laurent MD, Corbett CEP. Failure of natural immunity induced by asymptomatic Leishmania chagasi-infection in protecting against to Leishmania braziliensis-cutaneous disease. Poster Section presented at: Immune Response. 3rd World Congress on Leishmaniasis; 2005; Palermo-Terrasini, Sicily, Italy; 2005. p. 278.
31 Silveira FT, Lainson R, Shaw JJ, Souza AA, Ishikawa EAI, Braga RR. Cutaneous leishmaniasis due to Leishmania (Leishmania) amazonensis in Amazonian Brazil, and the significance of a negative Montenegro skin-test in human infections. Trans R Soc Trop Med Hyg. 1991 Nov-Dec;85(6):735-8. [ Links ]
32 Silveira FT, Shaw JJ, Bichara CNC, Costa JML. Leishmaniose visceral americana. In: Leão RNQ, coordenador. Doenças infecciosas e parasitárias: enfoque amazônico. Belém: CEJUP; 1997. p. 631-44. [ Links ]
33 Vinhas V, Freire M, Bacellar O, Cunha S, Rocha H, Carvalho EM. Characterization of T cell responses to purified leishmania antigens in subjects infected with Leishmania chagasi. Braz J Med Biol Res. 1994 May;27(5):1199-205. [ Links ]
34 Zijlstra EE, El-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and postkala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994 Dec;51(6):826-36. [ Links ]
 Correspondência/Correspondence/Correspondencia:
Correspondência/Correspondence/Correspondencia:
Instituto Evandro Chagas,
Rodovia BR 316, km 7, s/nº, Bairro: Levilândia
CEP: 67030-000 Ananindeua-Pará-Brasil
E-mail:fernandotobias@iec.pa.gov.br
Fernando Tobias Silveira
Seção de Parasitologia
Recebido em/Received/Recibido en: 22/6/2009
Aceito em/Accepted/Aceito en: 17/10/2009










 texto en
texto en