Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.1 no.1 Ananindeua Mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100009
Outbreak of human toxoplasmosis in the District of Monte Dourado, Municipality of Almeirim, Pará State, Brazil
Ediclei Lima do CarmoI; Marinete Marins PóvoaI; Nair Salgado MonteiroI; Rodrigo Rodrigues MarinhoI; José Maria NascimentoI; Sued Nazaré FreitasI; Cléa Nazaré Carneiro BicharaII
IInstituto Evandro Chagas/SVS/MS, Ananindeua,
Pará,
Brasil
IIInstituto
Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil.
Universidade do Estado do Pará, Belém, Pará, Brasil
Endereço para
correspondência
Correspondence
Dirección para correspondencia
Original Title: Surto de toxoplasmose humana no Distrito de Monte Dourado, Município de Almeirim, Pará, Brasil. Translated by: American Journal Experts
ABSTRACT
OBJECTIVE: To report an outbreak of human
toxoplasmosis that occurred in the District of Monte Dourado, Municipality
of Almeirim, Pará State, Brazil.
MATERIALS AND METHODS: After the
positive diagnosis of five patients with symptoms
suggestive of toxoplasmosis, clinical research and epidemiology were executed
in the locality. A total of 186 individuals
were evaluated, including symptomatic patients, their relatives and/or close
contacts. All subjects underwent
epidemiological inquiry, clinical assessment and serology by enzyme-linked
immunosorbent assay (ELISA) for detection of
anti-Toxoplasma gondii IgG and IgM.
RESULTS: A total of 40 individuals
presented a serological profile of acute
toxoplasmosis. Epidemiological analysis indicated that the cases could be related
to infection with oocysts eliminated by
cats, whose population density was very high in the surveyed locality. The
most likely hypothesis of transmission would be
through direct contact with oocysts of the parasite, either by the ingestion
of contaminated food or by the inhalation of these
forms in the soil. The possibility of water transmission through the local
supply system was discarded because the system is
inaccessible to cats. Infected individuals were treated at the local health
care units. Moreover, local health authorities were
instructed to implement measures to control stray cats in order to prevent
new cases or outbreaks.
CONCLUSION: The
outbreak that occurred between February and March 2004 in Monte Dourado was
caused by T. gondii. The hypothesis of
contamination via oocysts of the parasite is supported by several factors,
such as a high population density of cats in the
surveyed District, frequent gardening habits and a lack of reports of ingestion
of raw or undercooked meat.
Keywords: Toxoplasmosis; Seroepidemiologic Studies; Enzyme-Linked Immunosorbent Assay; Health Surveillance.
INTRODUCTION
Toxoplasmosis is an important zoonotic disease that is caused by the coccidian protozoan Toxoplasma gondii, a cosmopolitan parasite capable of infecting several species of homeothermic animals, including humans27,16.
The worldwide seroprevalence of this infection in humans is relatively high, reaching 90% in some regions27,2,17,25. Similar to what was observed in other areas, in the Brazilian Amazon, epidemiology studies have demonstrated that toxoplasmosis, in its other forms, is a very frequent infection with seroprevalence above 70%4,10,5,9.
Humans acquire the infection primarily through the ingestion of infecting forms of T. gondii, such as oocysts, which are eliminated in the feces of felids and contaminate foods, water and soil27,26,14, and through the ingestion of raw or undercooked meat containing the parasite's cystic tissue27,26,1. Among other mechanisms, besides the congenital form of exposure, some researchers consider the possibility of inhalation of the oocysts19,23. In general, toxoplasmosis tends to be asymptomatic or manifests itself with symptoms common to other pathologies, with patient developing a clinical diagnosis known as Mononucleosis-Like Syndrom22. However, in some situations, the infection can become considerably severe, especially in the congenital form or when the infected individual is immunosuppressed, in which case the manifestation of the disease in general compromises the central nervous system, sometimes even causing death26,3,23. Numerous human toxoplasmosis outbreaks have been reported in some countries, including Brazil, due to the consumption of meat containing parasite cysts or due to consumption of other food and water contaminated with the T. gondii, oocysts, with the emphasis on the outbreak that occurred in Santa Izabel do Ivaí-PR, which is considered the greatest outbreak ever registered in the whole world13,20,7,6,21,15.
The objective of this study was to describe a human toxoplasmosis outbreak that occurred in the Monte Dourado District, Almeirim municipality-PA, Brazil, due to its uncommon presentation in the northern region of the country, which involved a significant number of cases.
MATERIALS AND METHODS
AREA OF STUDY
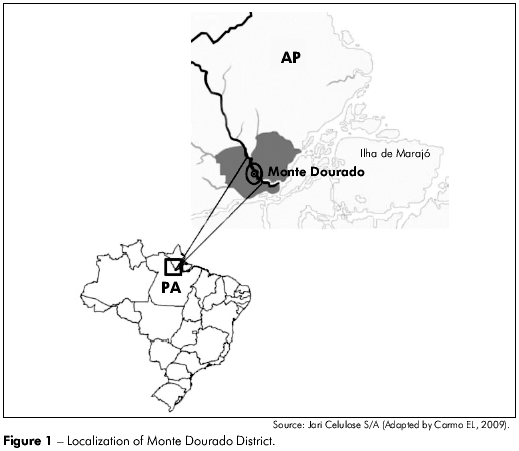
The investigated area was the Monte Dourado District, which belongs to the Almeirim municipality and is about 450 km from the capital city, Belém. Monte Dourado is situated in the north of Pará state (0o 53' 22'' S, 52o 36' 6'' W) and has an estimated population of 12 thousand inhabitants (Figure 1). Jari Celulose S/A, a multinational cellulose-producing company, is located in this district in the Amazonian Region.
OUTBREAK INVESTIGATION
Five company employees, residents of Monte Dourado, presented with the first signs and suggestive symptoms of acute toxoplasmosis between February 20 and March 10, 2004. During clinical evaluation, high and persistent fever (38o C a 40o C) prostration, anorexia, weight loss and lymphadenopathy, especially around the neck, were observed. Patients were sent to a hospital in Belém where laboratory tests for several infectious pathologies were performed, and a diagnosis of acute toxoplasmosis was confirmed.
Due to the observation of similar cases in the same region, the company appealed to the Evandro Chagas Institute (ECI) for instructions on how to proceed. A technical team from ECI, composed of a medical infectologist, an ophthalmologist, a biologist and laboratory technicians, was sent to Monte Dourado to conduct clinical, laboratorial and epidemiological evaluations in the area and to establish the following investigation strategies:
a) Visit the neighborhoods of the district for observation of the soil condition (dry or humid, etc); visit the system of water abstraction, treatment and distribution; and observation of the presence of felines and other stray animals in the urban area of the District;
b) Clinical and epidemiological evaluation of patients, family members and close contacts with completion of individual questionnaires and blood sampling;
c) Educational lectures on toxoplasmosis for health professionals from the municipality, engineers, professors, veterinarians and community leaders of the Jari Company;
d) Definition of control measures made together with the authorities of the Health and Agriculture Secretaries of Pará state and of the Jari Company.
LABORATORIAL METHODS
Total blood (5 ml) was collected by venous puncture from each investigated person, resulting in two serum aliquots (100 µL/each). The serological test performed was an enzyme immunoassay (ELISA) to detect anti-T. gondii antibodies. Indirect ELISA was used for detection of IgG antibodies and immunocapture ELISA for IgM detection. Commercial kits (Toxoplasma gondii IgG/IgM-In vitro Diagnostica/Human) were used, and the technical procedural methods were done according to the manufacturer's recommendations.
Furthermore, smear and thick blood drop slides were collected to exclude a diagnosis of malaria, which is severely endemic in the region with signs and symptoms similar to that of acute toxoplasmosis in some cases.
RESULTS
EPIDEMIOLOGICAL ANALYSIS OF THE ARE:
After analysis, it was observed:
a) Innumerable población felina doméstica errante, habitando casas abandonadas, jardines y plazas, favoreciendo la intensa eliminación de ooquistes;
b) The lack of pavement in the streets in the District allowed for higher survival of oocysts in the soil;
c) The distribution of cases in various neighborhoods of the District, not only in punctual agglomerates;
d) The impossibility of feline access to any of the phases of water abstraction and distribution systems destined to the local population;
e) The first cases appeared concurrently with a period of intense rain, which can cause erosion of the soil and suspension of feline feces and consequently, the dispersion of the oocytes to the majority of neighborhoods in the District;
f) There was a greater frequency of cases after this period of rain when the Brazilian carnival holiday was celebrated, which was during a dry spell, and several families decided to garden or clean their backyards;
g) Coincidence between the outbreak and the reported large number of cat births, which generally disperse the majority of the oocysts, favoring the contamination of the environment.
CLINICAL EVALUATION
A total of 186 patients, varying between 1 and 65 years of age (average: 23,3 ± 16,3), were clinically evaluated. Forty-one (22%) presented suggestive clinical symptoms of acute toxoplasmosis ((lymphadenopathy, persistent fever, hepatosplenomegaly, exanthema and others); twelve (6.45%), with unspecific symptoms (respiratory symptoms, nausea, migraine, others); and the remaining cases were asymptomatic.
LABORATORIAL EVALUATION
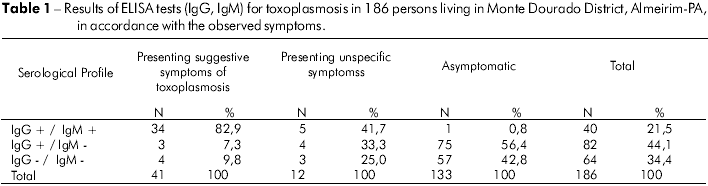
Results from the serological analysis of the 186 persons are presented in table 1. In relation to the plasmodium research, all collected slides (thick blood drop and smear) presented negative results.
DISCUSSION
The serological results demonstrated an elevated frequency in individuals with a profile compatible with acute toxoplasmosis (21.5%, 40/186). Of these individuals, 85% (34/40) presented classic clinical symptoms, 12.5% (5/40) showed unspecific symptoms and 2.5% (1/40) did not present any symptoms. This evidence is in accordance with what has been observed in other outbreaks of toxoplasmosis, in which the majority of persons diagnosed with acute toxoplasmosis presented classic symptoms of the disease13,20. Due to the fact that the majority of cases are clinically symptomatic and present high levels of IgG and IgM, there was no doubt that residual IgM characterized the persistence, and thus, the IgG test for avidity was not performed.
The observation of symptomatic individuals with a profile of immunity towards the infection (8.5%; 7/82) can be justified by the possibility that some cases had not yet established the seroconversion; in relation to symptomatic individuals with an immunity profile (reactive IgG and non-reactive IgM), there is a possibility that these were cases of re-infection because the region belongs to an area of primary forest, and these patients could have come in contact with atypical strains that were, until then, restricted to the wild environment. However, the lack of isolation and molecular analysis of the strains do not allow for confirmation of this hypothesis.
The epidemiologic analysis performed in Monte Dourado demonstrated a different reality than what is observed in other national and international outbreak reports. In these outbreaks, the confirmed form of infection has been directly linked to oral transmission, consumption of oocyte-contaminated water and/or meat and derivatives of different origins containing parasites cystic tissue13'20'7'6'24. In Monte Dourado, it was established that the transmission of the infection by T. gondii oocytes was related to the association of factors, such as: the high population of felines in all the urban areas of the District and in its surrounding forest; a period of high procreation and birth of cats; erosion of the soil due to abundant rain with probably re-suspension of oocytes that were disseminated by dust in the air due to gardening and/or wind; and contamination of commercialized foods in the area with parasite oocytes. These factors can relate the means of infection by oocytes to the ingestion of contaminated foods, the possible inhalation of oocytes dispersed by the air and even with the direct contact with cats and other mechanical vectors (flies and roaches) present both outdoors and in. These observations can be corroborated by the results from studies done both in Europe and the United States11,18.
At first, the possibility of infection associated with the ingestion of meat containing parasite cysts was excluded because there were no accounts of raw or undercooked meat ingestion, even though the local inhabitants, especially the natives of other regions of the country, ingest meat of different origins (bovine, swine and ovine) in barbecue form.
In 2002, the largest outbreak of toxoplasmosis in the world was registered in Santa Izabel do Ivai, municipality of Parana state, in which 70% of 600 persons who looked for medical assistance presented an acute infection profile. In this outbreak, the transmission occurred through water contaminated by oocysts eliminated in the feces of kittens that inhabited the area around the cistern that holds the water that is distributed to the city20. In Monte Dourado, the infection via the local water distribution network was excluded because there was no possibility of direct contact between cats and oocysts during the process. However, it was observed that in some houses, water for consumption was not adequately stored and was exposed to the risk of oocyte contamination.
It was also observed that stray cats were frequently seen in the surrounding local forest and could have been infected in the wild environment with oocytes of virulent atypical strains of T. gondii, disseminated by wild felids, and upon returning to the urban areas, they contaminated the environment with the atypical strains of oocytes. This possibility can justify the occurrence of the severe cases of toxoplasmosis and possibly even the cases of re-infection. The severe form of toxoplasmosis in humans, which is associated with atypical virulent strains, has been previously reported in forest areas, on the border of Suriname with French Guiana, including accounts of death8,12. This occurrence is also possible in Monte Dourado, especially due to the geographic proximity and similarity between the forests of the Almeirim municipality and of Suriname and French Guiana, which favor the circulation of these strains in both areas.
Through the dispersion of cases throughout the District, it was not possible to perform a case-controlled study to determine the common risk factors associated with the transmission of toxoplasmosis in the outbreak region.
After the etiology clarification, lectures and technical meetings were established between the public and private institutions involved in the investigation to implement of measures aimed at avoiding the upsurge of new cases or even other outbreaks of the disease, such as stray animal control and preventive orientations for the habitants and workers of the District.
Future studies still need to be performed to isolate and genotype the strains found in the area. In addition, it will be necessary to re-evaluate the individuals who were sick or susceptible (seronegative) during the outbreak so that possible ocular sequelae or seroconversion can be detected.
CONCLUSION
The observed laboratorial, clinical and epidemiological evidence indicates that the protozoan T. gondii was the pathogen responsible for the outbreak in the Monte Dourado District between February and March of 2004. The transmission of the infection possibly occurred through the ingestion of food containing the parasite's oocyte, direct contact with dirty hands after gardening activities or even the inhalation of oocytes suspended and dispersed through dust in the air.
ACKNOWLEDGEMENTS
We thank Jari Celulose S/A for their logistic support during the outbreak investigation.
REFERENCES
1 Aspinall TV, Marlee D, Hyde JE, Syms PE. Prevalence of Toxoplasma gondii in commercial meat products as monitored by polymerase chains reaction - food for thought? Int J Parasitol. 2002 Aug;32(9):1193-9. DOI:10.1016/S0020-7519(02)00070-X [ Links ]
2 Beaman M, McCabe RE, Wong SY, Remington JS. Toxoplasma gondii. In: Mandell GL, Douglas RG, Bennet JE, editors. Enfermidades Infecciosas-Principios y Práctica. Buenos Aires: Editorial Medica Panamericana; 1997. p. 2754-70.
3 Bhopale GM. Pathogenesis of toxoplasmosis. Comp Immunol Microbiol Infect Dis. 2003 Jul;26(4):213-22. DOI:10.1016/S0147-9571(02)00058-9 [ Links ]
4 Bichara CNC. Perfil epidemiológico da toxoplasmose humana na área metropolitana de Belém-Pará: a experiência no Serviço de Parasitologia do Instituto Evandro Chagas [dissertação]. Belém: Universidade Federal do Pará, Centro de Ciências Biológicas; 2001. [ Links ]
5 Bóia MN, Carvalho-Costa FA, Sodré FC, Pinto GMT, Amendoeira MRR. Seroprevalence of Toxoplasma gondii infection among indian people living in Iauareté, São Gabriel da Cachoeira, Amazonas, Brazil. Rev Inst Med Trop Sao Paulo. 2008 Jan-Feb;50(1):17-20. DOI:10.1590/S0036-46652008000100004 [ Links ]
6 Bonametti AM, Passos JN, Silva EMK, Bortoliero AL. Surto de toxoplasmose aguda transmitida através da ingestão de carne crua de gado ovino. Rev Soc Bras Med Trop. 1997 jan-fev;30(1):21-5. DOI:10.1590/S0037-86821997000100005 [ Links ]
7 Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997 Jul;350(9072):173-7. DOI:10.1016/S0140-6736(96)11105-3 [ Links ]
8 Carme B, Demar-Pierre M. Toxoplasmosis in French Guiana. Atypical (neo-) tropical features of a cosmopolitan parasitosis. Med Trop (Mars). 2006 Oct;66(5):495-503. [ Links ]
9 Carmo EL, Silva MCM, Xavier UAM, Costa BO, Póvoa MM. Inquérito sorológico de toxoplasmose em candidatos a transplante renal no Hospital Ofir Loyola, Belém, Pará, Brasil. Rev Panam Infectol. 2004;6(4):15-7. [ Links ]
10 Cavalcante GT, Aguiar DM, Camargo LMA, Labruna MB, Andrade HF, Meireles LR, et al. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. J Parasitol. 2006 Jun;92(3):647-9. [ Links ]
11 Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenun PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. BMJ. 2000 Jul;321(7254):142-7. DOI:10.1136/bmj.321.7254.142 [ Links ]
12 Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007 Oct;45(7):88-95. DOI:10.1086/521246 [ Links ]
13 Dias RA, Freire RL. Surtos de toxoplasmose em seres humanos e animais. Semina Cienc Biol Saude. 2005 out-dez;26(2):239-48. [ Links ]
14 Dubey JP. Toxoplasmosis - a waterborne zoonosis. Vet Parasitol. 2004 Dec;126(1-2):57-72. DOI:10.1016/j.vetpar.2004.09.005 [ Links ]
15 Franco RMB. Protozoários de veiculação hídrica: relevância em saúde pública. Rev Panam Infectol. 2007;9(1):36-43. [ Links ]
16 Frenkel JK. Toxoplasmosis in Humans Beings. J Am Vet Med Assoc. 1990 Jan;196(2):240-8.
17 Jenum PA, Kapperud G, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiol Infect. 1998 Feb;120(1):87-92. DOI:10.1017/S0950268897008480 [ Links ]
18 Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154(4):357-65. [ Links ]
19 Kolbekova P, Kourbatova E, Novotna M, Kodym P, Flegr J. New and old risk-factors for Toxoplasma gondii infection: prospective cross-sectional study among military personnel in the Czech Republic. Clin Microbiol Infect. 2007 Oct;13(10):1012-7. DOI:10.1111/j.1469-0691.2007.01771.x [ Links ]
20 Ministério da Saúde (BR). Fundação Nacional de Saúde. Surto de Toxoplasmose no município de Santa Isabel do Ivaí-Paraná. Bol Eletro Epidemiol. 2002;2(3):2-9. [ Links ]
21 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Surto intra familiar de toxoplasmose, Santa Vitória do Palmar-RS, julho de 2005. Bol Eletro Epidemiol. 2006 out;6(3):2-7. [ Links ]
22 Neves ES, Bicudo LN, Curi AL, Carregal E, Bueno WF, Ferreira RG, et al. Acute acquired toxoplasmosis: clinical-laboratorial aspects and ophthalmologic evaluation in a cohort of immunocompetent patients. Mem Inst Oswaldo Cruz. 2009 Mar;104(2):393-6. DOI:10.1590/S0074-02762009000200039 [ Links ]
23 Remington RM, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: Sanders; 1997. p. 140-267.
24 Robson JMB, Wood RN, Sullivan JJ, Nicolaides NJ, Lewis BR. A probable foodborne outbreak of toxoplasmosis. Commun Dis Intell. 1995;19:517-22.
25 Sobral CAQ, Amendoeira MRR, Teva A, Patel BN, Klein CH. Seroprevalence of infection with Toxoplasma gondii in indigenous brazilian populations. Am J Trop Med Hyg. 2005 Jan;72(1):37-41. [ Links ]
26 Sukthana Y. Toxoplasmosis: beyond animals to humans. Trends Parasitol. 2006 Jun;22(3):137-42. DOI:10.1016/j.pt.2006.01.007 [ Links ]
27 Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000 Nov;30(12-13):1217-58. DOI:10.1016/S0020-7519(00)00124-7 [ Links ]
 Correspondência/Correspondence/Correspondencia:
Correspondência/Correspondence/Correspondencia:
Ediclei Lima do Carmo
Instituto Evandro Chagas
Seção de Parasitologia / Laboratório de Toxoplasmose
Rodovia BR 316, km 7, s/no,
Bairro: Levilândia
CEP: 67030-000
Ananindeua-Pará-Brasil
Fones: 55 (91) 3214-2148
E-mail:edicleicarmo@iec.pa.gov.br
Recebido em/Received/Recibido en: 17/07/2009
Aceito em/Accepted/Aceito en: 21/09/2009











 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI
