Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.1 no.1 Ananindeua Mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100014
Salmonella serovars of human origin identified in Pará State, Brazil from 1991 to 2008
Edvaldo Carlos Brito LoureiroI; Nathalia Danielly Borges MarquesI; Francisco Lúzio de Paula RamosI; Eliane Moura Falavina dos ReisII; Dália dos Prazeres RodriguesII; Ernesto HoferIII
ISeção de Bacteriologia
e Micologia, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IILaboratório de Enterobactérias, Instituto Oswaldo
Cruz, Fundação Oswaldo Cruz, Ministério da Saúde,
Rio de Janeiro, Rio de Janeiro, Brasil
IIILaboratório de Zoonoses Bacterianas, Instituto Oswaldo
Cruz, Fundação Oswaldo Cruz, Ministério da Saúde,
Rio de Janeiro, Rio de Janeiro, Brasil
Endereço para
correspondência
Correspondence
Dirección para correspondencia
Original Title: Sorovares de Salmonella de origem humana identificados no Estado do Pará, Brasil, no período de 1991 a 2008. Translated by: American Journal Experts
ABSTRACT
Salmonellosis presents a cosmopolitan distribution and affects all age groups, not only in developed countries, but also in developing ones. This study aimed to identify the serovars of Salmonella isolated from human infections occurring in 43 areas of Pará State from 1991 to 2008. Eight hundred and ninety samples of Salmonella isolated in coprocultures and blood cultures were analyzed, including 55 isolations of S. Typhi from feces and blood of symptomatic individuals, simultaneously. The cases of Salmonella infections were distributed into 13 serogroups. The majority of them were in group O:9 (68.1%), and 47 serovars of Salmonella were identified, including S. Typhi (58.9%), S. Enteritidis (5.4%) and S. Saintpaul (2.5%). S. Typhi was the most prevalent (58.9%) among the 47 identified serovars, which demonstrates that typhoid fever is a serious public health problem in northern Brazil and requires increased attention from health agencies regarding epidemiological and environmental surveillance as effective measures for its prevention and control.
Keywords: Salmonella; Serovars; Salmonella Infections.
INTRODUCTION
The genus Salmonella belongs to the Enterobacteriaceae family and comprises Gram-negative, glucose-fermenting bacilli. Most of these organisms move by means of peritrichous flagella. The current classification is based on phenotypic and genotypic studies, which divide the genus into two species: Salmonella enterica, which consists of six subspecies, and Salmonella bongon4,27, in accordance with the following written convention: Salmonella enterica subspecies enterica serovar Typhimurium, or simply, Salmonella Typhimurium, with the genus name in italics and the serovar in Roman type. Routinely, the Kauffmann-White scheme is used for the antigenic characterization of Salmonella by identifying the representative polysaccharide antigens (O) and those associated with flagella (H), which are proteinaceous in naturea9.
Salmonellosis has a cosmopolitan distribution, affecting all age groups, both in developed and developing countries, and is an important public health problem. Clinical presentations most commonly include acute gastroenteritis and enteric fevers (typhoid and paratyphoid).
Some Salmonella serovars, such as Salmonella Typhi and Salmonella Paratyphi A, B and C are exclusively adapted to human hosts, whereas other serovars, such as S. Pullorum, S. Gallinarum, S. Abortusovis and S. Choleraesuis, are more adapted to domestic or wild animals that can transmit infection to humans13,16,15,18.
Salmonellosis, therefore, is classified as a zoonosis, and is characterized by its spread by food consumption, particularly raw or undercooked20.
An estimated 1.4 million cases of salmonellosis occur annually in the United States6, with the most recent outbreaks attributed to serovar S. Enteritidis,and milk, meat and chicken eggs are its main vehicles of transmission2,3,5.
In Brazil, food-borne disease epidemics caused by S. Enteritidis have been registered in São Paulo1,24, Brasília4, Blumenau28 and Curitiba21. In the state of São Paulo, 3,554 cases of human salmonellosis were studied from 1996 to 2003, and 68 serovars were identified. Of these, S. Enteritidis was the most commonly found serovar8.
Studies conducted in the state of Pará from 1975 to 1986 indicate the importance of typhoid fever in northern Brazil where, overall, 59 human infectious Salmonella serovars were identified, the most common being S. Typhi (14.6%), followed by S. Typhimurium (9.6%), S. Give (9.0%), S. Agona (7.4%) and S. Newport (6.2%)17.
Typhoid fever affects about 20 to 30 million people in developing countries. The highest incidence is found in African, Asian, Caribbean and Central and South American countries. In the year 2000, more than 2.16 million cases of typhoid fever were estimated to occur worldwide and resulted in 216,000 deaths, with more than 90% of these occurring in Asia6,29.
In Brazil, typhoid fever is endemic, with overlapping epidemics occurring especially in the north and northeast regions20, and is associated with low socioeconomic backgrounds and poor basic sanitation.
In the Amazon region, the state of Pará has registered the greatest number of cases of typhoid fever. A total of 443 cases of typhoid fever were identified from 1987 to 2004; originating from various municipalities in the state of Pará, including epidemics in Marabá, Óbidos, Abaetetuba, Moju, Limoeiro do Ajuru and Anajás . In 1981, during the construction of the Tucuruí hydroelectric dam, the Tucuruí municipality registered the first outbreak of paratyphoid A fever in Brazil, with 101 cases identified25.
Knowledge of the geographical distribution of Salmonella serovars of human origin is important to assess their individual incidence and prevalence, as well as to identify any risk to the health of the population. This information constitutes an important epidemiological indicator of salmonellosis in any given community12. However, very few studies are performed in the Amazon that allow the recognition of Salmonella serovars involved in human and animal infections as well as their mechanisms of transmission. To address this problem, the objectives of the present research were to identify Salmonella serovars isolated at the Bacteriology Section of the Evandro Chagas Institute (IEC) from cases of human infection that occurred between 1991 and 2008 in various municipalities of the State of Pará.
MATERIALS AND METHODS
SAMPLES
A total of 890 Salmonella isolates were obtained from 10,709 stool cultures (663 positive samples) and 6,285 blood cultures (227 positive samples) of symptomatic individuals living in different areas of the state of Pará. Identification was performed in the Bacteriology Section of the IEC from 1991 to 2008.
STOOL CULTURE
Naturally collected stool samples were stored in appropriate containers and sent to the laboratory for stool culture. No more than 2 h after the collection, the feces were plated onto MacConkey (MC) agar or Salmonella-Shigella (SS) agar and cystine selenite broth, followed by plating onto the SS agar after incubation at 37° C for 24 h. The suspected colonies (lactose negative) were then seeded onto triple-sugar-iron (TSI) agar, followed by biochemical identification according to the recommendations of Ewing7. Confirmation of the Salmonella genus was performed by seroagglutination testing, using somatic and flagellar polyvalent antisera (Difco and Bio-Rad).
BLOOD CULTURE
Immediately after collection, 10 mL of peripheral blood were used to inoculate flasks containing 50 mL of tryptose phosphate broth, which were then incubated at 37° C. Inoculated cultures were monitored daily for the presence of growth and/or turbidity for up to 15 days. Cultures from turbid flasks were used to inoculate SS agar plates, upon confirmation of Gram-negative bacilli growth by bacterioscopy (Gram staining).
SEROLOGICAL IDENTIFICATION
The characterization of Salmonella serovars was performed at the Enterobacteria Laboratory of the Instituto Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, by detection of somatic and flagellar antigens and using polyvalent and monovalent antisera with or without flagellar phase induction.
RESULTS
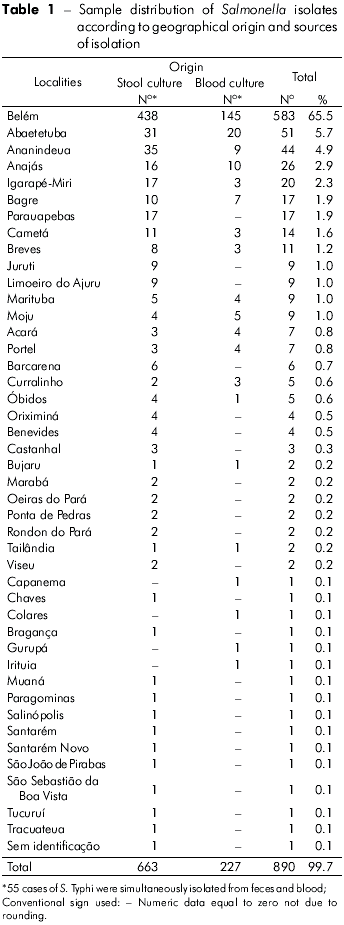
Firstly, it was determined that the 890 Salmonella isolates from human infections occurred in 43 municipalities of the state of Pará, with the highest prevalence rate in Belém (65.5%), followed by Abaetetuba (5.7%), Ananindeua (4.9 %) and Anajás (2.9%). These samples represented 79% (704 samples) of the total analyzed (Table 1).
Overall, the identified Salmonella serovars were distributed among 13 serogroups, with group O9 comprising 68.1% of isolates, with emphasis on the serovars S. Typhi (492), S. Enteritidis (45) and S. Panama (18) (Table 2).
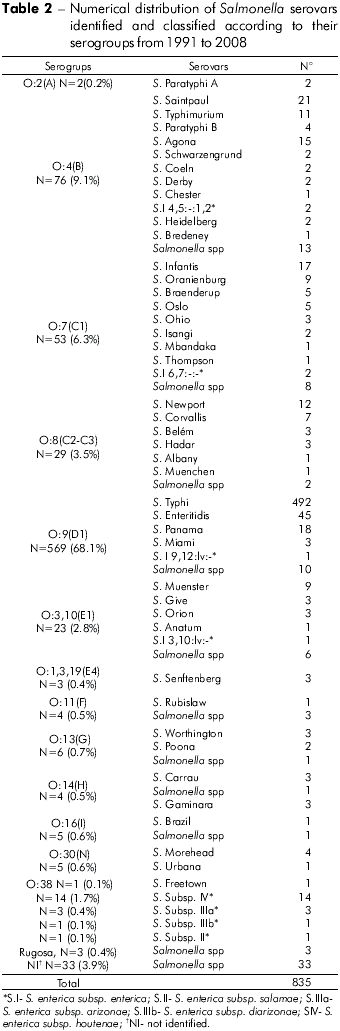
The importance of Salmonella serovar characterization should be noted as it allowed the recognition of 492 (58.9%) cases of typhoid fever out of the total of 835 samples analyzed within the same period.
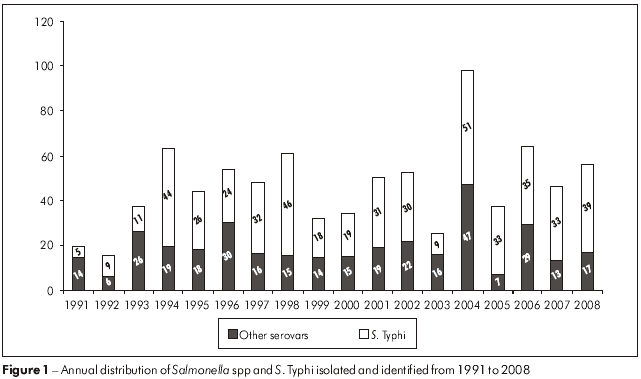
In the analysis of the annual distribution of Salmonella spp species isolates from 1991 to 2008, 2004 had the greatest number of cases (98), followed by the years 2006 (64), 1994 (63) and 1998 (61) (Figure 1). Cases of typhoid fever occurred in all years, and the highest incidences were observed in 2004 (51 cases), 1998 (46), 1994 (44) and 2008 (39), comprising 36.6% of all reported cases (Figure 1).
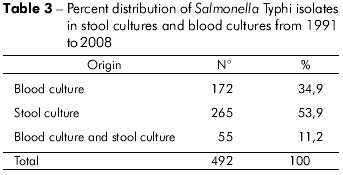
Of the cases of typhoid fever, 53.9% were detected in stool cultures, 34.9% in blood cultures and 11.2% in both stool cultures and blood cultures (Table 3).
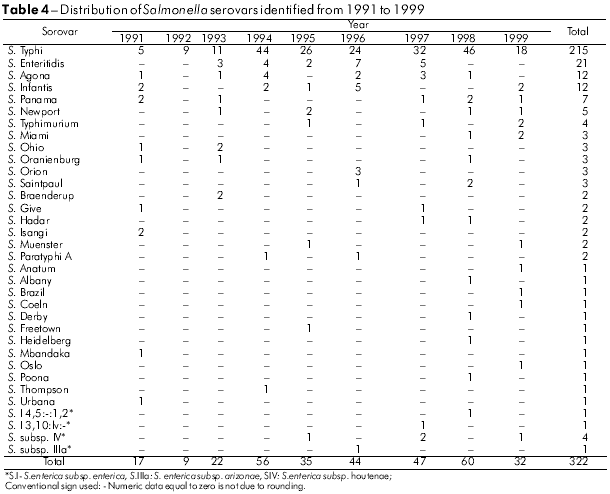
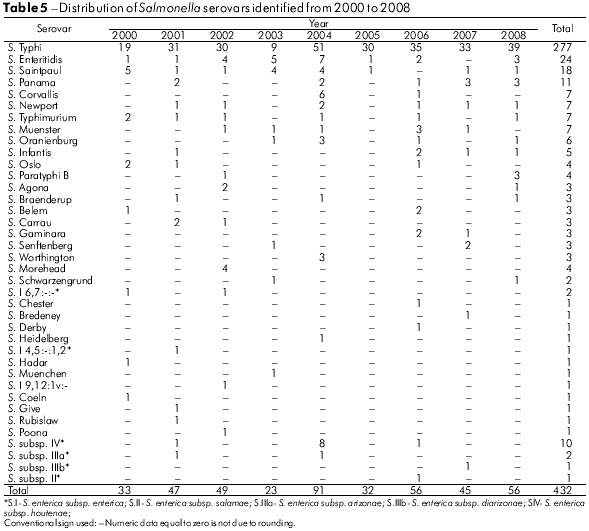
The analysis method used for these samples allowed the recognition of 47 Salmonella serovars. As can be seen in tables 4 and 5, S. Typhi (58.9%), S. Enteritidis (5.4%) and S. Saintpaul (2.5%) were the serovars most frequently detected from 1991 to 2008.
DISCUSSION AND CONCLUSION
In the epidemiological surveillance of human salmonellosis, it is important to know the prevalent and/or incident serovars, as well as the transmission routes, so that the health services involved in surveillance can intervene with more effective measures of disease prevention and control. At the same time, regular identification of serovars reveals the introduction of new serological types into a region12,11.
This study analyzed 835 strains of Salmonella responsible for human infections over a period of 18 years, and demonstrated its occurrence in 43 municipalities in the state of Pará. Cases appeared to be clustered, especially in those municipalities with larger populations, such as Belém, with 65.5% of the isolates, followed by Abaetetuba (5.7%), Ananindeua (4.9%) and Anajás (2.9%) (Table 1). It is likely that these occurrences result from poor urban sanitation conditions. However, the observed higher incidence may result from more frequent diagnoses and laboratory testing, given the greater number of medical facilities in these areas. Overall, from the 47 Salmonella serovars identified (Tables 4 and 5), S. Typhi (65.3%), S. Enteritidis (6.0%) and S. Saintpaul (2.8%) were the most prevalent. From 1999 to 2003, S. Enteritidis was identified in 67.4% of cases of gastrointestinal and extraintestinal infections in the State of São Paulo, followed by S. Typhimurium (5.2%)8. In recent years, S. Enteritidis has been a concern to health authorities because it is the most common serovar in human infections in Africa, Asia, Europe, Latin America and the Caribbean; the S. Typhi serovar was the third most frequent in Africa, Latin America and the Caribbean10. It is interesting to note that in the regions studied, the predominant Salmonella serovars found belong to serogroup O:9 (Table 2), comprising 68.1% of the cases analyzed. Within this group, it is important to highlight the occurrence of S. Typhi (58.9%).
In the state of São Paulo, S. typhi was the fourth most frequent serovar and the second most isolated Salmonella strain from blood cultures8, whereas in the state of Pará, all 227 cases of blood culture isolates were characterized as S. Typhi, and they were detected in all the years of the study (Tables 4 and 5). It is important to note the occurrence detected in 2004 (Figure 1) when, among the 98 isolates, 51 (52%) were from cases of typhoid fever, distributed among 15 municipalities in the state of Pará, with the highest incidence in Belém (52.9%), followed by Anajás (13.7%) and Ananindeua (7.8%). This highlights the importance of the problem of typhoid fever in the state of Pará, considering that of the 835 cases of salmonellosis that occurred from 1991 to 2008 in 43 municipalities, typhoid fever was diagnosed by laboratory testing in 34 (79.1%) locations. In light of this, we emphasize that typhoid fever was, and continues to be, endemic in Belém during the 18 years of this study, and is, to some extent, undoubtedly directly related to the precarious conditions of basic sanitation and low levels of education in the community22.
These results are consistent with other studies previously conducted in the region, which have demonstrated the high prevalence of infection by S. typhi compared to other studied serovars17,26. In developed countries, there are few reported cases of typhoid infection in contrast to the high frequency of typhoid fever in the developing countries of Africa and Asia6,22,23.
The cases of typhoid fever originating from routine medical visits and those referred by Brazil's Unified Health System (known as SUS) comprise the majority of patients treated at the IEC. At the time of admission, they had been ill for an average of 20 to 25 days26. These data may explain the greater success in isolating S. typhi from stool samples (53.9%) as compared to blood samples (34.9%), as was found in this study (Table 3). However, although the stool samples showed a higher positive rate during the third week of illness, it should be noted that this study showed significant positive incidence rates during the first and, particularly, second weeks26.
Considering that humans are the only natural host of S. typhi, prophylactic measures should be directed towards water treatment, basic sanitation, adequate personal hygiene and health education, as well as enlightening the population about the disease and vaccination30. Undoubtedly, the implementation of these measures will allow minimization of the incidence of typhoid fever in the Amazon region, which will thereby help to prevent social and economic losses.
REFERENCES
1 Araújo E, Pacheco MASR, Boni RF, Fonseca YSK, Gelli DS, Fernandes AS, et al. Surtos alimentares por Salmonella Enteritidis associados ao consumo de alimentos à base de ovos, em Sorocaba, SP. Hig Aliment. 1995;9(40):24-6.
2 Badrinath P, Sundkvist T, Mahgoub H, Kent R. An outbreak of Salmonella Enteritidis phage type 34a infection associated with a Chinese restaurant in Suffolk, United Kingdom. BMC Public Health. 2004 Sep;4:40. DOI:10.1186/1471-2458-4-40 [ Links ]
3 Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006 Aug;43(4):512-7. DOI:10.1086/505973 [ Links ]
4 Carmo LS, Vieira AC, Reis JDP, Nascimento RS, Pereira ML, Santos EJ, et al. Staphylococcus aureus and Salmonella Enteritidis present in food implicated in food poisoning. Rev Microbiol. 1996 Apr-Jun;27(2):122-5. [ Links ]
5 Centers for Disease Control and Prevention. Outbreak of Salmonella serotype Enteritidis infections associated with raw almonds United States and Canada, 2003-2004. MMWR Morb Mortal Wkly Rep. 2004 Jun;53(22):484-7. [ Links ]
6 Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004 May;82(5):346-53. [ Links ]
7 Ewing WH. Edward and Ewing's identification of Enterobacteriaceae. 4th ed. New York: Elsevier; 1986. 536 p.
8 Fernandes SA, Tavechio AT, Ghilardi AC, Dias AM, Almeida IA, Melo LC. Salmonella serovars isolated from humans in São Paulo State, Brazil, 1996-2003. Rev Inst Med Trop Sao Paulo. 2006 Jul-Aug;48(4):179-84. DOI:10.1590/S0036-46652006000400001 [ Links ]
9 Ferreira EO, Campos LC. Salmonella. In: Trabulsi LR, Alterthum F, editores. Microbiologia. 5. ed. São Paulo: Atheneu; 2008. p. 329-38.
10 Galanis E, Lo Fo Wong DMA, Patrick ME, Binsztein N, Cieslik A, Chalermchaikit T, et al. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerg Infect Dis. 2006 Mar;12(3):381-8. [ Links ]
11 Hald T, Vose D, Wegener HC, Koupeev T. A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Anal. 2004 Feb;24(1):255-69. DOI:10.1111/j.0272-4332.2004.00427 [ Links ]
12 Herikstad H, Motarjemi Y, Tauxe RV. Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect. 2002 Aug;129(1):1-8. [ Links ]
13 Humphrey T. Public-health aspects of Salmonella infection. In: Wray C, Wray A, editors. Salmonella in domestic animals. Florida: CABI Publishing; 2000. p. 245-63.
14 Le Minor L, Popoff MY. Designation of Salmonella enterica sp. nov., nom. rev., as the type and only species of the genus Salmonella: request for an opinion. Int J Syst Bacteriol. 1987;37:465-8. DOI:10.1099/00207713-37-4-465 [ Links ]
15 Lins ZC. Studies on enteric bacteria in the lower Amazon region: II. Salmonella types isolated from wild reptiles in Pará State, Brazil. Rev Microbiol. 1971;2:165-9.
16 Lins ZC. Studies on enteric bacterias in the lower Amazon Region. I. Serotypes of Salmonella isolated from wild forest animals in Pará State, Brazil. Trans R Soc Trop Med Hyg. 1970;64(3):439-43. [ Links ]
17 Loureiro ECB. Contribuição ao estudo bacteriológico de Salmonella oriundas de diferentes fontes da região Amazônica brasileira. [dissertação]. São Paulo (SP): Universidade de São Paulo, Instituto de Ciências Biomédicas; 1990.
18 Loureiro ECB. Ocorrência do gênero Salmonella em animais silvestres da ordem Edentata, na Região Amazônica, norte do Estado do Pará, Brasil. Rev Latinoam Microbiol. 1985 jan-mar;27(1):31-4. [ Links ]
19 Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999 Sep-Oct;5(5):607-25. [ Links ]
20 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de vigilância epidemiológica. Brasília; 2005. Febre Tifóide. p. 350-63.
21 Mota CC, Vieira HR, Puzyna IP, Kalache J, Konolsaisen JF, Camargo NL. Toxi-infeccao alimentar por Salmonella Enteritidis. Relato de um surto ocorrido em Curitiba - PR, Brasil/julho de 1981. Hig Aliment. 1983;2(3):123-6.
22 Mweu E, English M. Typhoid fever in children in Africa. Trop Med Intern Health. 2008;13(4):532-40. DOI:10.1111/j.1365-3156.2008.02031.x. [ Links ]
23 Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86(4):260-8. DOI:10.2471/BLT.06.039818 [ Links ]
24 Peresi JTM, Almeida IAZC, Lima SI, Marques DF, Rodrigues ECA, Fernandes AS, et al. Surtos de enfermidades transmitidas por alimentos causados por Salmonella Enteritidis. Rev Saude Publica. 1998 out;32(5):477-83. Disponível em: http://www.scielosp.org/scielo.php?. DOI:10.1590/S0034-89101998000500011 [ Links ]
25 Pessoa GVA, Lins ZC, Calzada CT, Irino K, Neme SN, Raskin M, et al. Identificação e lisotipagem de amostras de Salmonella paratyphi A, causadora de surto epidêmico em Tucuruí, Pará, Brasil, em 1980. Rev Inst Adolfo Lutz. 1983;43(1-2):105-7. [ Links ]
26 Ramos FL. Febre tifóide: a experiência do Instituto Evandro Chagas. [dissertação]. Belém (PA): Universidade Federal do Pará; 2005.
27 Reeves MW, Evins GM, Heiba AA, Plikaytis BD, Farmer JJ. Clonal nature of Salmonella typhi and its genetic relatedness to other Salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J Clin Microbiol. 1989 Feb;27(2):313-20. [ Links ]
28 Santos SM, Kupek E. Serial outbreaks of food-borne disease in Blumenau, Brazil, caused by Salmonella enteritidis. Braz J Infect Dis. 2000 Dec; 4(6):275-8. [ Links ]
29 Wilde H. Enteric fever due to Salmonella typhi and paratyphi A. A neglected and emerging problem. Vaccine. 2007 Jul;25(29):5246-7. DOI:10.1016/j. [ Links ]
30 World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. WHO/V&B/03.07. Geneva; 2003. [ Links ]
 Correspondência/Correspondence/Correspondencia:
Correspondência/Correspondence/Correspondencia:
Edvaldo Carlos Brito Loureiro
Instituto Evandro Chagas
Rodovia BR316, km 7, s/no, Levilândia
CEP: 67030-000
Ananindeua-Pará-Brasil
Tel: +55 (91) 3214-2113
E-mail:edvaldoloureiro@iec.pa.gov.br
Recebido em/Received/Recibido en: 30/07/2009
Aceito em/Accepted/Aceito en: 05/10/2009











 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI
