Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.1 Ananindeua mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100016
Surveillance of visceral leishmaniasis in epidemiologically distinct locations in Juruti, a mining municipality in Pará State, Brazil
Lourdes Maria GarcezI; Joyce Favacho CardosoII; Anadeiva Portela ChagasII; Jefferson Francisco Correia MirandaII; Gilberto César Rodrigues de SouzaII; Daniela Cristina SoaresIII; Lucilândia Maria BezerraIV; Habib FraihaV; Jeffrey Jon ShawVI; Hiro GotoVI
IInstituto Evandro Chagas/SVS/MS, Ananindeua,
Pará, Brasil.Universidade
do Estado do Pará, Belém, Pará, Brasil
IIInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIIInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil.Secretaria de
Estado de Saúde Pública do Pará (SESPA)
IVUniversidade Federal do Tocantins, Palmas, Tocantins, Brasil
VUniversidade Federal do Pará, Belém, Pará, Brasil
VIUniversidade de São Paulo, São Paulo, São Paulo, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
Original Title: Vigilância da leishmaniose visceral em localidades epidemiologicamente distintas em Juruti, um município minerário do Estado do Pará, Brasil. Translated by: American Journal Experts
ABSTRACT
Surveillance actions for human visceral leishmaniasis (HVL) were carried out in Juruti, a mining municipality in Pará State. A peri-urban (Santa Maria-SM) and a rural (Capiranga-CA) location were selected with or without HVL, respectively, for the execution of four biannual serologic inquiries (lysate ELISA) in canine populations (SM = 94, CA = 45) and three entomological surveys (CDC light traps, 18-6 h x4). Subsequently, the clinical status, as well as the infection by Leishmania, was investigated in 53 dogs (SM = 28; CA = 25) with parasitological (bone marrow/lymph, Giemsa), molecular (peripheral blood leukocytes, kDNA-PCR) and serological (ELISA) diagnoses assessing different antigens (lysate, K39, Hsp83 - screen test, ROC curve). Seroprevalence varied in SM (45; 40; 15; 15%) and in CA (22; 30; 8.5; 0%), presenting increasing average IgG rates in SM (320; 378; 951; 1866; p <0.05) despite the euthanasia of dogs after the second survey, and stable average IgG rates in CA (100; 159; 141; 0), where euthanasia was not conducted. The frequency rates of Lutzomyia longipalpis/Lutzomyia spp. differed in SM (279/296) and CA (4/6). Clinical and laboratory results were similar for dogs from SM and CA, respectively: infection (parasitological examination: 86 and 84%; kDNA-PCR: 100%), clinical status (asymptomatic: 43 and 56%; symptomatic: 57 and 44%), and specificity by ELISA (100%). On the other hand, sensitivity (lysate: 44 and 18%; Hsp83: 48 and 27%; K39: 48 and 41%) and IgG levels (≤ 6,400; ≤ 200) varied, respectively. The profile of canine infection in localities with or without HVL transmission differed only in terms of the level/evolution of IgG, which makes the temporality of investigations necessary, especially in quiet and isolated areas that present a low vector density and where the euthanasia of dogs would become unnecessary. The best serological test was ELISA-K39.
Keywords: Leishmaniasis, Visceral; Dogs; Enzyme-Linked Immunosorbent Assay; Polymerase Chain Reaction; Insect Vectors; Epidemiologic Surveillance.
INTRODUCTION
Human visceral leishmaniasis (HVL) is a severe and potentially fatal disease in cases where there is no early diagnosis or treatment. In the American continent, the disease is caused by the protozoan Leishmania (Leishmania) infantum chagasi, and its transmission to vertebrate hosts is carried out by the species Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae), the best characterized vector for HVL in the Americas. The crab-eating fox (Cerdocyon thous) is a reservoir for the parasite in the maintenance of its sylvatic cycle14,11. The domestic dog (Canis familiaris) has epidemiological importance in the transmission to humans because it is the reservoir in the domestic cycle of the protozoan and the main source of infection for vectors15.
Visceral leishmaniasis has expanded, tending to become an urban disease in the State of Pará. Some areas with high transmission rates of the disease comprise municipalities in the western region of the state. In this region, Juruti is classified as a sporadic transmission municipality for HVL; however, it has a boundary in the east with Santarém, the second largest city in the state and an area that shows high transmission rates. Juruti's great mining potential (bauxite) has attracted enterprises that contribute to the economic development of the region, but also cause rapid environmental transformations, with impacts on public health.
Socioeconomic and environmental characteristics affect the efficacy of the strategies that are implemented in Brazilian municipalities to control HVL20'7. For this reason, an understanding of the development of the disease in the context of large environmental changes would guide actions related to the surveillance, prevention, and control of visceral leishmaniasis20,4.
Over a monitoring period of 18 months, risk factors for HVL (domestic reservoir and vector) were analyzed in two microenvironments in Juruti, represented by rural sentinel locations, with and without transmission of HVL and under direct and indirect influence of mining enterprises. Then, the profile of the infected dogs was investigated in each location with the use of clinical and laboratory (parasitological, molecular, and serological) diagnostic methods. The performance of crude and recombinant antigens in the immunosorbent assay (ELISA) for the serological diagnosis in these epidemiologically distinct areas was measured. The implications of dog euthanasia among control options were also discussed.
MATERIALS AND METHODS
STUDY AREAS
The research on canine visceral leishmaniasis (CVL) was performed in two primary sentinel locations, Santa Maria and Capiranga, in the municipality of Juruti/Pará, 12 and 60 km away from the urban center, respectively (Figure 1). In the rural settlement of Capiranga, located in the surrounding area of a mineral prospecting site, there were no cases of HVL reported by the resident community, and there had been no notifications of the disease in the last five years under investigation. In Santa Maria, a peri-urban location classified as a transmission location of the disease, there was one case of HVL, which was reported three years ago, according to data provided by the Health Secretariat of the Municipality of Juruti.
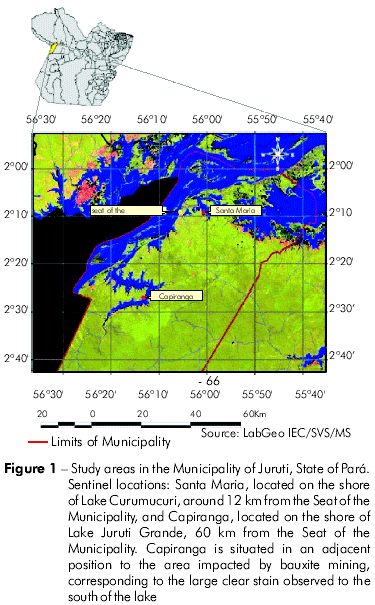
CANINE POPULATION
The canine population corresponded to 30% of the human population, both in Santa Maria (94/303) and in Capiranga (45/149) at the beginning of the study (November, 2006).
STUDY AND SAMPLING DESIGN
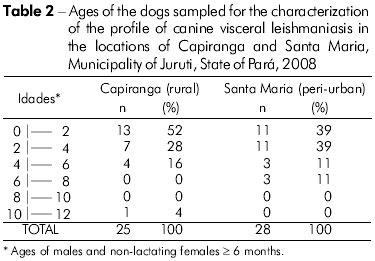
Two sample groups were analysed in the two locations. The first group (G1) was used for the monitoring of CVL by means of serological surveys, whereas the second (G2) was based on the determination of CVL profile and the diagnostic performance of different antigens in serological tests. The G1 sample consisted exclusively of plasma. As such, in a monitoring period of 18 months, four serological surveys were performed at biannual intervals: November 2006, April 2007, October 2007, and April 2008. The number of animals varied with each survey, depending on the size of the canine population and the finding of dogs in the vicinity of the residences. In Santa Maria, the samples from the first to fourth surveys totalled 217 plasmas (55, 57, 47, and 58), while in Capiranga they totaled 90 (27, 10, 23, and 30). G2 was composed of different specimens: plasma, peripheral blood leukocytes (PBL), and lymphatic/marrow aspirate from 53 dogs (28 from Santa Maria; 25 from Capiranga), obtained two months after completing serological monitoring. The CVL profile of the two locations was investigated in this sample group, considering clinical and laboratory criteria for the determination of infection and/or disease.
SPECIMEN COLLECTION AND LABORATORY TESTS
A total of 5 ml of blood (for G1 or G2) was collected from each animal by means of cephalic vein puncture in an empty vacuum tube containing EDTA (Shandong, USA). The plasma was then obtained by centrifugation at room temperature (3700 g/10'/RT) and maintained at -20o C for subsequent analysis through an immunoenzymatic assay (ELISA) using the crude antigen Leishmania, which consisted of a promastigote lysate of L. (L.) infantum chagas18. For G2, an ELISA was also carried out; in addition to the crude antigen Leishmania, the recombinant antigens Hsp832 and k3923 were used. The performance of the serological tests for the diagnosis of CVL with the three antigens was compared in the different areas in relation to the established gold standard. For this group (G2), PBLs were also obtained. They were stored at -20o C until the moment of use and were designated for diagnosis by kDNA-PCR13. Lymph node (pre-scapular and/or popliteal) and bone marrow samples were obtained from the same dogs by aspirate puncture. This material was designated for the preparation of Giemsa-stained smears and was examined under an optical microscope (40x) for amastigote research16.
CLINICAL EXAMINATION OF THE DOGS
The dogs of the G2 group were examined focusing mainly on six signs of disease: alopecia, dermatitis, skin ulcers, conjunctivitis, onychogryphosis, and lymphadenopathy. Each sign was scored on a semiquantitative scale from 0 (absent) to 3 (severe), and the sum revealed the total clinical score. Dogs with a total score from 0 to 2 were arbitrarily classified as asymptomatic, those with a score from 3 to 6 as oligosymptomatic, and those with a score from 7 to 18 as polysymptomatic19.
GOLD STANDARD FOR TEST COMPARISON
For the sample obtained after monitoring, a direct parasitological exam and an ELISA-lysate assay were used in conjunction to determine a panel of gold standard samples. A consensus was established between the two tests, in which the plasmas positive for at least one test and those negative for both were considered the positive and negative gold standard groups, respectively.
STATISTICS
The Fisher's exact test and the analysis of variance (ANOVA) were used for the comparison between groups, with a significance level of 5%. As part of the analysis of the infection/disease profile in the sample of dogs from Santa Maria (28) and Capiranga (25), the results of the ELISA tests with the three antigens for this sample were compared to those obtained with the gold standard to determine the performance of the test with each antigen (ELISA-lysate, ELISA-Hsp83, and ELISA-k39). Thus, a screening test was used where a = true positive, b = false positive, c = false negative, and d = true negative. The sensitivity (a/a + c x 100), specificity (d/b + d x 100), and the positive prediction values (a/a + b x 100) and negative prediction values (d/c+d x 100) were calculated, in addition to the prevalence rate (a+c/a+b+c+d). The counterbalance between sensitivity and specificity was expressed in an ROC (receiver operator characteristic) curve for the definition of the test with a higher discriminatory level in each location8.
ENTOMOLOGICAL SURVEYS
Three entomological surveys with CDC light traps were performed in the Amazonian summer (April and July, 2007) and Amazonian winter (January, 2008) periods. Five entomological collection stations were used in each location, and each collection period lasted four nights (18:00 to 6:00 h x 4), totaling 144 hours of capture effort in each location. The CDC light traps were set in pairs within the houses or outside of them, near the annexes housing animals within a radius of 20 m. The identification of sandflies was performed following the morphological method22.
RESULTS
The results obtained through surveillance of the canine reservoir (Canis familiaris) and the sandfly vector (Luzomyia longipalpis) in Santa Maria and Capiranga revealed risk factors for human visceral leishmaniasis in both locations.
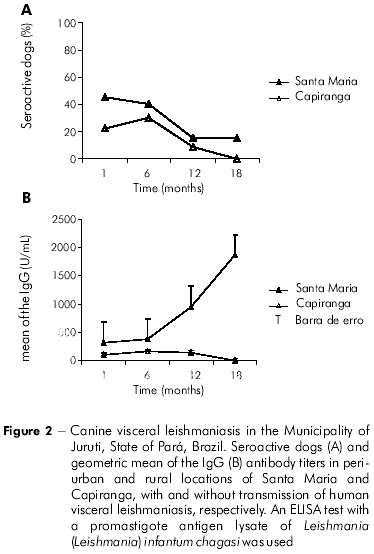
The monitoring of the dogs from the populations of Santa Maria and Capiranga for 18 months, by means of serological surveys, revealed higher seropositivity rates for CVL (ELISA-Lysate) in Santa Maria than in Capiranga, especially at the first two collection points (Figure 2A). The IgG antibody levels were also high in the dogs from Santa Maria ( ≤ 6400), with a clear rise in the geometric mean over time (320, 378, 951, 1866; p <0.05). Low levels of IgG and no significant alterations were detected ( < 200) in the animals from Capiranga at any time during monitoring period (10, 159, 141, 0; p >0.05), as shown in figure 2B.
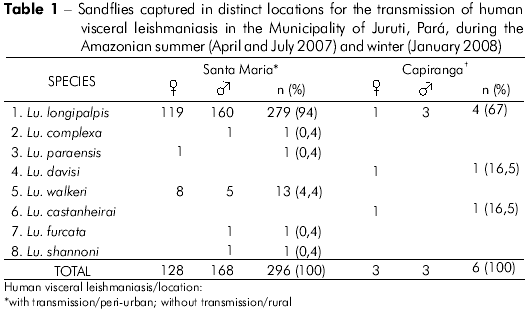
The entomological collections carried out during the monitoring of the dogs, two in the Amazonian summer and one in Amazonian winter, revealed the presence of synanthropic flies in both locations, with a predominance of Lutzomyia longipalpis over Lutzomyia spp. The frequency rate of the former in the sample was greater in Santa Maria (279/296) than in Capiranga (4/6), with a higher density rate within peridomestic areas. Indoors, the species was captured at a low frequency in both locations (Santa Maria: 3/279; Capiranga: 2/4). table 1 presents the eight species identified, as well as their frequency rates in the sample. No specimen presented natural Leishmania infection.
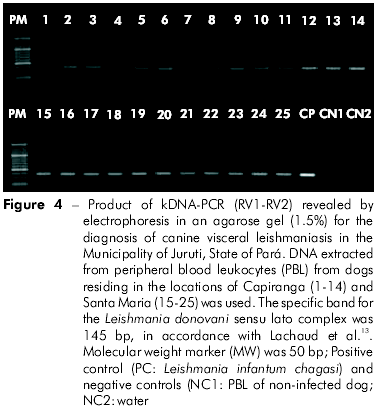
The transversal approach for the comparison of the profile of infection/disease indicated similarities among the dogs of Santa Maria (28) and Capiranga (25). The majority, approximately 80%, had an age equal to or less than four years in the two locations (Table 2). In the comparison between the two locations, the number of animals in different clinical categories also did not differ: asymptomatic (SM = 12 and CA = 14), oligosymptomatic (SM = 11 and CA = 9), and polysymptomatic (SM = 5 and CA = 2) with similar asymptomatic (SM = 43% and CA = 56%) and symptomatic (SM = 57% and CA = 44%) percentages. The frequency of confirmed parasitological infection was equally high in Santa Maria (86%) and Capiranga (84%) and attained 100% when kDNA-PCR was used (Figure 3). The specific amplification of DNA (145 base pairs) confirmed the presence of infection by L. (L) infantum chagasi (Figure 4).
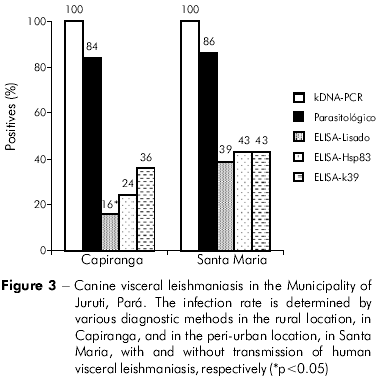
The differences between the infection/disease profiles of the dogs of Santa Maria and Capiranga were only observed in the response of IgG humoral antibodies to infection by Leishmania. The frequency of seropositive dogs differed in the two areas, according to the antigen used for the ELISA test (Figure 3). K39 was more sensitive than lysate and Hsp83 in the detection of asymptomatic infected dogs, and it was the only antigen to yield a similarity between the frequencies of asymptomatic seropositivity in both locations. The others were only sensitive to the asymptomatic infected dogs in Santa Maria (Figures 5A and 5C). Similarly to the data from serological surveys carried out during monitoring, the levels of IgG in the dogs were higher among the animals in Santa Maria than among those in Capiranga, especially when using an ELISA-lysate test (Figures 5B and 5D).
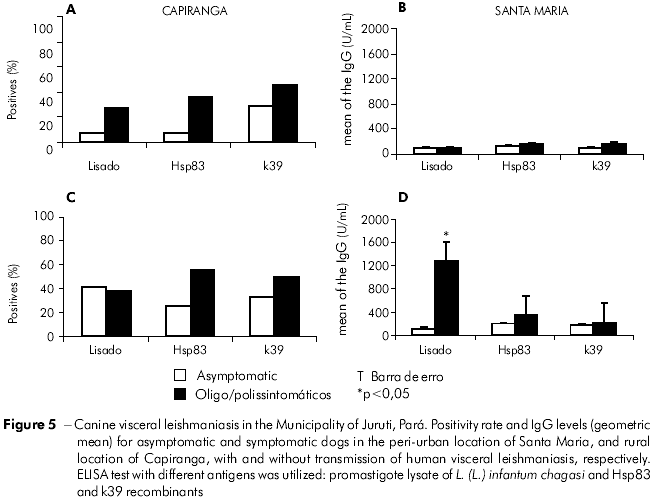
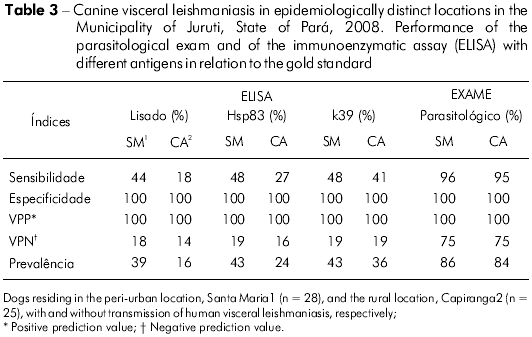
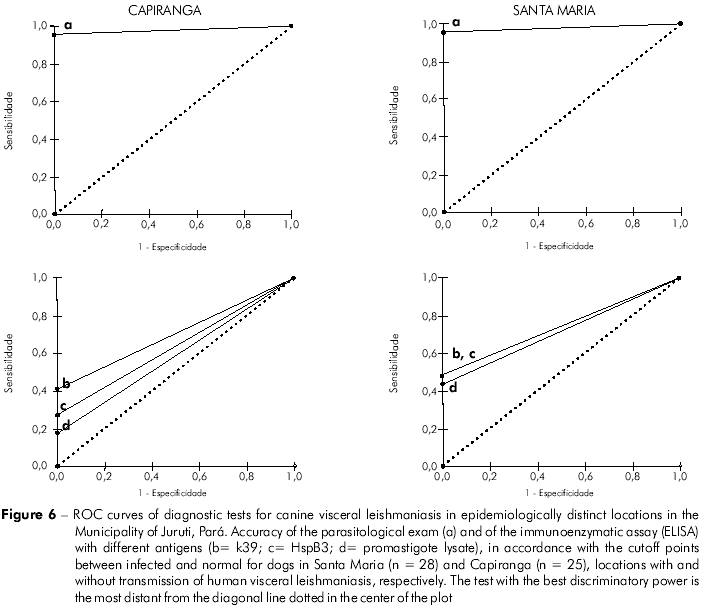
Table 3 presents the performance of the diagnostic tests in relation to the gold standard. It highlights a greater sensitivity of the parasitological exam for the detection of infection by Leishmania and variations in prelavence rates determined by the diagnostic method adopted. Among the serological tests, the ELISA-k39 had the greatest discriminatory power for the serodiagnosis of CVL in both locations (Figure 6).
DISCUSSION
The large mining potential of Juruti in the west of the State of Para has attracted enterprises that, in spite of contributing to the economic development of the region, provoke rapid environmental transformations capable of influencing the epidemiology of visceral leishmaniasis and causing impacts on public health20,7. Strengthening of surveillance actions, including the detailed investigation of the risk factors, should provide the necessary knowledge for the creation of control strategies to be implemented by the municipal agents.
In this study, surveillance actions on visceral leishmaniasis (VL) were carried out throughout a period of 18 months, during a phase of bauxite prospecting in Juruti. The actions focused on its canine reservoir and on its vector. Epidemiologically distinct rural locations and denominated sentinel sites were selected: Santa Maria, with transmission of HVL and exposed to urban influences due to its peripheral location in relation to the municipal seat located just 12 km away, and Capiranga, located at 60 km from the urban area, where there were no reports of HVL. However, the latter is situated nearby the bauxite mine, where there are ongoing activities related to the extraction of that mineral.
Initially, the variations over time of the frequency of infection determined by the serology (ELISA) of the canine population in the locations under study were investigated. Then, the study sought to establish an infection/disease profile of the dogs in each location, aiming to support the discussion on potential control actions that focus on dogs as reservoirs.
Upon determining the prevalence of the dogs that were seropositive for visceral leishmaniasis at the beginning of the study, especially during the second biannual survey, both locations seemed to be exposed to the same risk (Figure 2A). Based on these results and on the occurrence of a new case of HVL in Santa Maria during the research period, the surveillance team in the Municipality decided to euthanize the seropositive dogs. The euthanasia was partially carried out only in Santa Maria. The third and fourth serological surveys revealed increasing levels of IgG in the dogs from Santa Maria, even during the intervention, while in Capiranga, where there was no euthanasia of dogs and no cases of HVL, the levels of IgG remained low or undetectable during the 18 months of monitoring (Figure 2B).
In areas of intense transmission, the levels of anti-Leishmania IgG antibodies in dogs are high or are increasing19, and are related to the parasitemia7,21, which have occurred with the dogs in Santa Maria. The low levels of IgG are similar to those described for the canine population of Capiranga, and suggest control actions of the canine infection (in this case, Capiranga would be a silent location). They could also be the consequence of nonspecific reactions in the serology, which may occur due to the high sensitivity of the ELISA test because the dogs are exposed to many other infectious diseases9,3,10 that induce B lymphocytes to produce IgG. This IgG can sometimes cross-react with the antigens of L. (L.) infantum chagasi1
Differences between the canine populations of the two locations would not have been revealed if the frequency rate of seropositive dogs had been the only factor considered in the second serological survey (Santa Maria: 40%, and Capiranga: 30%, as shown in figure 2A), for example. Therefore, surveillance actions for visceral leishmaniasis focused on the canine reservoir must consider the temporal aspects of the serological surveys, as well as the investigation of the levels of IgG, especially when entomological surveys are not feasible due to the lack of local technical support.
The intriguing low levels of IgG in the dogs from Capiranga, which showed a complete absence of reactivity in the last survey (Figure 2B), prompted an investigation of the profile of canine infection/disease in the samples of the two locations to compare the results. Dogs from both Santa Maria and Capiranga with similar ages (Table 2) were identical in terms of the clinical presentation of visceral leishmaniasis, considering the intensity of signs and the number of animals distributed among the asymptomatic, oligosymptomatic, and polysymptomatic categories. The frequency rate of infections, after parasitological test in Santa Maria (86%) was as high as in Capiranga (84%), and, with the use of kDNA-PCR (Figures 3 and 4), it reached 100% in dogs from both locations, which indicated that the enzootic disease reaches the entire canine population of the two areas.
The occurrence of HVL in a specific area depends, basically, on the existence of two factors, an infected vector and a susceptible human host11. Even though canine infection (domestic reservoir) rates in both locations are proportionally the same, the high frequency rate of Lutzomyia longipalpis in Santa Maria, but not in Capiranga (Table 1), differentiated the two locations in terms of the risk of transmission of visceral leishmaniasis to humans. The habit of making nocturnal fires in Capiranga, incorporated by natives from the local indigenous culture, certainly contributed to inhibit the presence of sandflies around their homes, and, consequently, the transmission of L. (L.) infantum chagasi to humans. This hypothesis should be investigated with a study on the knowledge, attitudes, and practices (KAP) of the inhabitants of these locations in relation to visceral leishmaniasis and its prevention.
Although a parasitological test guarantees absolute confirmation of the infection, in practice it is necessary to work with tests that are not highly sensitive or specific. For the same sample, the ELISA revealed a much lower frequency rate of positives than the real number of infected dogs, with variations in the sensitivity in relation to the antigens utilized (Figure 3). Despite the higher sensitivity of the crude promastigote lysate of L. (L.) infantum chagasi in detecting high levels of IgG in symptomatic dogs (Figure 5D), it did not have the same performance in both areas for asymptomatically infected dogs. The two recombinant antigens (Hsp83 and k39) were the most sensitive under these circumstances, while ELISA-k39 had the greatest d iscrim in atory power (Figure 6) for detecti n g asymptomatically infected dogs both in Santa Maria and in Capiranga (Figures 5A and 5C).
For this type of analysis, the use of dogs that are similar to those that will be tested serologically during surveillance is advisable, just as it is important that the choice of the gold standard test, nonexistent in the case of CVL, be appropriate8. Therefore, it was necessary to establish a gold standard for calculating the indices of performance of the different antigens, which was based on the association of the two most traditional tests, the parasitological test and the ELISA-lysate test. This fact and the unusual nature of the sample in CA (84% of the infected dogs) influenced the estimation of specificity and other related indices, such as the negative prediction value, which was extremely low in relation to the small number of negative patterns (Table 3). These indices still need to be investigated in dogs from locations with low rates of infection by L. (L.) infantum chagasi because although the ELISA test is the method of choice for epidemiological research11, it is important to consider variations in the performance associated with the burden of the disease (or intensity of transmission) in a certain location. Sensitivity and specificity of the ELISA test are also significantly reduced during the latent period of canine infection7. Therefore, under different conditions (or in different canine populations), sensitivity could be even lower than that described in this study.
The lack of official records in the past five years or of reports from inhabitants, on the occurrence of HVL in Capiranga (where dog euthanasia was not performed), associated with the risk factors described in this study, confirm the need for surveillance of VL in the other locations within the bauxite mining environment. The surveillance actions should be founded upon the local epidemiological conditions in order to be effective.
In Capiranga, entomological surveillance is preferential to dog euthanasia because the potential of these animals to infect sandflies, with subsequent transmission to humans, is currently limited. The findings in Santa Maria, however, indicate an imminent risk of urbanization of VL, and rigorous measures should be immediately taken to avoid its expansion, including environmental education, monitoring of emerging vectors for chemical control, euthanasia of polysymptomatic dogs, and the search for new foci in the vicinity, in rural and urban locations.
Some actions that could be developed for the chemical control of the vector population are the spraying of residences with insecticide and the use of dog collars or mosquito nets, both containing deltamethrin5. Recently, in an unpublished study in Latin America, it was demonstrated that washing dogs with this pyrethroid has a residual effect (entomological effectiveness) of 5.2 months, similar to that observed with the use of collars. The advantage is the low cost (US$ 0.06-0.10) of the former in comparison with the latter (US$ 10-15)6.
In spite of the possible interventions, either already available or still under study, any attempt to chemically control the vector depends on previous entomological studies16 which Amazonian municipalities are commonly unable to perform. However, the effective control of VL by means of elimination of dogs requires the removal of a large proportion of dogs infected with the vector from the location7. This is generally not feasible, especially when the entire canine population is found to be infected, as demonstrated in this study.
The best way to control CVL and, consequently, to prevent the human disease would be the development of an effective vaccine for dogs, which is still not available. The research associated with the development of this vaccine has indicated promising candidate formulations17, and the advances in the understanding of the mechanisms by which parasites of the Leishmania genus infect and evade the immune response of mammal hosts are opening research fronts in search of new vaccines against the disease12.
Because of the unavailability of a vaccine for dogs, even if the ideal diagnostic method for the detection of canine infection were to be found, it would not be effective without the solutions to the local problems faced by the majority of Amazonian municipalities. These problems include deficiencies in management, the scarcity of human and financial resources, and the frequent conflicts between the health authorities and the local population related to the current practice of dog euthanasia.
The results of this study reveal the occurrence and extent of enzootic CVL in Capiranga, and, consequently, the possibility of emergence of HVL not only in this location, but also in other silent locations similar to Capiranga, which are exposed to the direct impact of large environmental transformations related to mining activities. The risk of expansion of the number of HVL cases in transmission areas exposed to increasing urban influences was also shown (Santa Maria and similar locations). The results orient HVL surveillance, prevention, and monitoring actions in Juruti, in rural locations, in areas surrounding the mining camps and in peri-urban areas.
ACKNOWLEDGMENTS
We thank the communities of Santa Maria and Capiranga, for the always warm reception and support for the research, and Dr. Ana Márcia Souza da Cunha Oliveira and the other professionals of the Secretaria Municipal de Saúde de Juruti (Municipal Secretariat of Health of Juruti) for their support and participation. We also thank the technical teams of the Instituto Evandro Chagas, especially those of the Laboratories of Immunology and Epidemiology (Parasitology Section) and of Geoprocessing. We are grateful to the technicians Rosângela Barros and Rita Félix, the students Eduardo Mota and Luís Dickson and Dr. Nelson Veiga for their invaluable technical support. Finally, we thank Dr. Nazaré Souza of the Universidade Federal Rural da Amazônia, the mining company Alcoa Alumínio S/A, the Secretariat of Science, Technology, and Strategic Inputs, and Brazil's National Council for Scientific and Technological Development (CNPq) for the financial support.
REFERENCES
1 Alves WA, Bevilacqua PD. Quality of diagnosis of canine visceral leishmaniasis in epidemiological surveys: an epidemic in Belo Horizonte, Minas Gerais, Brazil, 1993-1997. Cad Saude Publica. 2004 Jan-Feb;20(1):259-65. [ Links ]
2 Celeste BJ, Angel SO, Castro LG, Gidlund M, Goto H. Leishmania infantum heat shock protein 83 for the serodiagnosis of tegumentary leishmaniasis. Braz J Med Biol Res. 2004 Nov;37(11):1591-3.
3 Colpo CB, Monteiro SG, Stainki DR, Colpo ETB, Henriques GB. Natural infection by Trypanosoma cruzi in dogs. Cienc Rural. 2005 May-Jun;35(3):717-19.
4 Costa CHN, Vieira JBF. Mudanças no controle da leishmaniose visceral no Brasil. Rev Soc Bras Med Trop. 2001 mar-abr;34(2):223-228. DOI: 10.1590/S0037-86822001000200013 [ Links ]
5 Courtenay O, Gillingwater K, Gomes PAF, Garcez LM, Davies CR. Deltamethrin-impregnated bednets reduce human landing rates of sandfly vector Lutzomyia longipalpis in Amazon households. Med Vet Entomol. 2007 Jun;21(2):168-76. DOI: 10.1111/j.1365-2915.2007.00678 [ Links ]
6 Courtenay O, Kovacic V, Gomes PA, Garcez LM, Quinnell RJ. A long-lasting topical deltamethrin treatment to protect dogs against visceral leishmaniasis. Med Vet Entomol. 2009 Sep;23(3):245-56. [ Links ]
7 Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis. 2002;186(9):1314-20. DOI: 10.1086/344312 [ Links ]
8 Fletcher RH, Fletcher SW. Epidemiologia clínica: elementos essenciais. Porto Alegre: Artmed; 2006. 288 p.
9 Garcez LM, Souza NF, Mota EF, Dickson LAJ, Abreu WU, Cavalcanti VF, et al. Focos de dirofilariose canina na Ilha do Marajó: um fator de risco para a saúde humana. Rev Soc Bras Med Trop. 2006 jul-ago;39(4):333-6. [ Links ]
10 Glaser B, Gothe R. Imported arthropod-borne parasites and parasitic arthropods in dogs. Species spectrum and epidemiologic analysis of the cases diagnosed in 1995/96. Tierarztl Prax Ausg K Klientiere Heimtiere. 1998 Feb;26(1):40-6. [ Links ]
11 Gontijo CMF, Melo MN. Leishmaniose visceral no Brasil: quadro atual, desafios e perspectivas. Rev Bras Epidemiol. 2004 set;7(3):338-49. [ Links ]
12 Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res. 2006 Mar;123(3):423-38. [ Links ]
13 Lachaud L, Marchergui-Hammami S, Chabbert E, Dereure J, Dedet JP, Bastien PJ. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol. 2002 Jan;40(1):210-5. DOI: 10.1128/JCM.40.1.210-215.2002 [ Links ]
14 Lainson R, Shaw JJ, Lins ZC. Leishmaniasis in Brazil. IV. The fox Cerdocyon thous (L) as a reservoir of Leishmania donovani in Pará state. Brazil. Trans R Soc Trop Med Hyg. 1969;63(6):741-5.
15 Lainson R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters WE, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. London: Academic Press; 1987. p. 2-118. [ Links ]
16 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância e controle da leishmaniose visceral. Brasília; 2006. 120 p. [ Links ]
17 Nogueira FS, Moreira MAB, Borja-Cabrera GP, Santos FN, Menz I, Parra LE, et al. Leishmune® vaccine blocks the transmission of canine visceral leishmaniasis: absence of Leishmania parasites in blood, skin and lymph nodes of vaccinated exposed dogs. Vaccine. 2005 Sep;23(40):4805-10. DOI:10.1016/j.vaccine.2005.05.011 [ Links ]
18 Quinnell RJ, Courtenay O, Davidson S, Garcez L, Lambson B, Ramos P, et al. Detection of Leishmania infantum by PCR, serology and cellular immune response in a cohort study of Brazilian dogs. Parasitology. 2001 Mar;122(Pt3):253-61. [ Links ]
19 Quinnell RJ, Courtenay O, Garcez LM, Kaye PM, Shaw MA, Dye C, et al. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2003 Feb;91(3-4):161-8. DOI:10.1016/S0165-2427(02)00311-2 [ Links ]
20 Werneck GL, Pereira TJCF, Farias GC, Silva FO, Chaves FC, Gouvêa MV, et al. Avaliação da efetividade das estratégias de controle da leishmaniose visceral na cidade de Teresina, Estado do Piauí, Brasil: resultados do inquérito inicial 2004. Epidemiol Serv Saúde. 2008 abr-jun;17(2):87-96. [ Links ]
21 Xiong G, Jin C, Cheng X, Su Z. Deltamethrin bath of domestic dog in the prevention of sandfly bite. End Dis Bull. 1994;9:32-34.
22 Yong DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sandflies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Memoirs of the American Entomological Institute, 54, Florida: Associated Publishers; 1994. 881p.
23 Zijlstra EE, Daifalla NS, Kager PA, Khalil EAG, El-Hassan AM, Reed SG, et al. rk39 enzyme-linked Immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998 Sep;5(5):717-20. [ Links ]
 Correspondência/Correspondence/Correspondencia:
Correspondência/Correspondence/Correspondencia:
Lourdes Maria Garcez
Instituto Evandro Chagas
Seção de Parasitologia, Laboratório de Imunologia e Epidemiologia
Rodovia BR 316, km 7, s/no, Bairro: Levilândia
CEP: 67030-000 Ananindeua-Pará-Brasil
E-mail: lourdesgarcez@iec.pa.gov.br
Recebido em/Received/Recibido en: 31/7/2009
Aceito em/Accepted/Aceito en: 21/9/2009











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI
