Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Pan-Amazônica de Saúde
versión impresa ISSN 2176-6215versión On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.1 Ananindeua mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100019
Isolated bacteria from hematophagous Culicidae (Diptera: Nematocera) in Belém, Pará State, Brazil*
Willy Cristiano Luz AlvesI; Inocêncio de Sousa GorayebII; Edvaldo Carlos Brito LoureiroIII
IUniversidade Federal do Pará,
Belém, Pará, Brasil
IIMuseu Paraense Emílio
Goeldi, Belém, Pará, Brasil
IIIInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
Bacteria are largely distributed in nature, especially when carried by a vector. They comprise large portions of the human and animal microbiota, and some cause diseases. The diptera of the family Culicidae are directly involved in the vectoring of epidemics of great interest for public health. However, the association between bacteria and Culicidae has been scarcely studied. In order to deepen the knowledge on this subject, we isolated and identified bacteria which have been transported in hematophagous Culicidae in the City of Belém, Pará State. The collection of 296 mosquitoes was carried out using a CDC light trap in eight collection localities, which presented different environmental characteristics within the metropolitan area of Belém. Some were identified to the species level (9) and others to the subgenus (4). It was possible to identify 17 species of bacteria; seven bacteria could only be identified up to their genus. Culex quinquefasciatus and Anopheles aquasalis were the most frequent Culicidae. The most frequent species of bacteria found in the samples were Gemella haemolysans and Enterobacter cloacae. The collection localities in the Terra-firme and Curió-utinga districts presented the largest diversity of species of Culicidae.
Keywords: Bacteria; Culicidae; Biological Transport; Amazonian Ecosystem.
INTRODUCTION
The order Diptera, which includes flies and mosquitoes, has close to 150 thousand species and is the fourth largest of the class Insecta. Its members occupy various niches in different aerial, aquatic, and terrestrial habitats. The order Diptera includes different families of medical interest in the suborder Nematocera (Culicidae, Ceratopogonidae, Simuliidae and Psychodidae). In Brazil, there are approximately 20,000 dipterous species in approximately 100 families listed in the Catalogue of the Neotropical Region42, which is still not complete. The Diptera in Brazil are still not well understood and are proposed to encompass about two to three times more species than currently recorded8.
There are about 3,600 species in the family Culicidae that have a worldwide distribution. These compose approximately 40 genera, with the neotropical species having the highest level of endemism, as 27% of these groups are restricted to this biogeographical region. Little is known about the Culicidae fauna of the Amazon. The last study on the distribution of mosquitoes that covered the entire Amazon region was carried out in 19619 and collected 218 species, 152 of which were in the State of Pará51. Mosquitoes comprise a vast group of insects, containing many genera and species. From the perspective of human health, the most important genera are Anopheles, Aedes and Culex.
There are few studies published worldwide on the relationship between bacteria and dipterous with even fewer on bacteria and Culicidae. Only in the last ten years has research on this relationship begun to emerge and gain importance in the scientific community. Bacteria have been identified in the digestive tract of various insect species that constitute the intestinal microbiota40. Studies performed with mosquitoes bred in insectariums in Mexico20 and Brazil24 showed the presence of Gram-negative bacteria in their intestines. Some bacteria are being used as biological pest control of adult insects and larvae in plantations13,49,5,50 while others are being tested as an alternative to controlling mosquito populations that cause epidemics43,30,14,3. Some other studies are evaluating the association between bacteria with mechanical vectors and the possibility of transmission by various insects31,41,11,37,44. In a 2007 study31, more than 20 bacteria species were isolated from horseflies and preserved, including those belonging to the Staphylococcus, Streptococcus and Serratia genera.
The process of city planning contributes to the distribution of dipterous species. Environments that are more heavily populated contain species with a greater ability to adapt to these sites, whereas areas with greater forestation and less human influence naturally select other dipterous species. The flies, therefore, are separated in niches within the same city. The bacteria that the flies carry are also varied and previous research does not elucidate the role of the bacteria in this problem. There are few studies on this subject in Brazil and further studies for the advancement of knowledge in this area are necessary. The main objective of this paper is to evaluate the entomological and bacteriological diversity associated with distinct urban areas in the city of Belém.
METHODOLOGY
AREAS OF STUDY
In the City of Belém, seven collection points were selected with different urbanizations characteristics. The eighth point was selected in an area near the banks of the Pará River estuary, located in Outeiro, an administrative district in Belém.
Central area of Belém
The inner city area of Belém, characterized by the existence of many houses and buildings and few areas of bare soil. It comprises many cemented and paved areas, and vegetation is restricted to gardens and ornamental flora of the city. The selected sampling points were located in the Cremação and Nazaré neighborhoods.
Pericenter of Belém
This area is characterized by many houses and few buildings, with areas of bare ground and little cement and asphalt. Most vegetation is in backyards and less ornamental vegetation exists here than in the downtown. The selected sampling points were located in the Curió-Utinga and Jurunas neighborhoods.
Outskirts of Belém
This is an area with few houses and no buildings where houses are separated by empty lots. There are soil lots without cement and asphalt, and abundant vegetation on uninhabited land and in backyards. Stretches of forests can still be found in this area. The selected sampling points were located in the neighborhoods of Tapanã, Terra Firme and Icoaraci.
Estuary Area
The floodplain of the Para River estuary is a distinct ecosystem with unique biotic and abiotic characteristics. We propose that hematophagous insects and their relations to bacteria influence the dynamic properties of this ecosystem. Collections were made in the estuary of the Pará River in Outeiro.
COLLECTION OF CULICIDAE
Insects were collected from May 2007 to April 2008, from 17 h until 22 h or until 6 h the next day. Samples were collected with appropriate techniques to minimize contamination of the traps and containers used for collecting Culicidae.
We used CDC light traps47, which are generally used for sampling of hematophagous insects (especially Culicidae, phlebotominae and ceratopogonidae). These traps attract insects to a small tungsten light source. When these small insects are close enough to the light, they are sucked into the trap by a small fan that is driven by a 12 V current. Using this method, the collected insects remain alive until they are removed from the apparatus.
IDENTIFICATION OF THE CULICIDAE
After being collected, the mosquitoes were immediately taken to the Department of Arbovirology of the Instituto Evandro Chagas (IEC), Belém, Pará. With the help of specialists, we identified the specimens using identification keys cited by three classic articles on taxonomy of Culicidae: Foranttini19, Gorham et al21 and Faran and Finthicum17.
IDENTIFICATION OF BACTERIA
After the mosquitoes were identified, they were immediately treated for the detection of bacteria.
The mosquitoes were separated into groups (pools) according to the number of specimens that were collected from each species. We studied a total of 41 pools with three specimens and six pools with two specimens for a total of 129 Culicidae. In some cases, pools with two specimens were used because of insufficient samples collected from a particular species.
To create the pools, the mosquitoes were separated aseptically (near a Bunsen burner) using a biological safety cabinet. There was no direct handling of the collected Culicidae specimen in any stage of the research; specimens were placed in individual test tubes, which facilitated identification and reduced sources of contamination.
After the groups were identified and defined, a suspension of the mosquitoes was prepared by grinding them in a mortar with a sterile physiological solution. Next, an aliquot was withdrawn and inoculated in one of two culture media, the Tryptic Soy Broth (TSB) and sodium thioglycollate, at 37° C for 24 h.
An aliquot of the material contained in the tubes of TSB and thioglycollate where growth (turbidity) had been observed was thereafter plated onto blood agar in 5 to 10% CO2, Chapman agar, and MacConkey agar. After plating, these were then incubated at 37° C for 24 h.
The colonies grown on blood agar and Chapman agar were submitted to a bacterioscopy by the Gram stain method and the Gram-positive cocci and bacilli were identified28. Three to five colonies from MacConkey agar were plated onto a sorting TSI (triple sugar and iron) medium and the Gram-negative bacilli were thereafter identified15,25.
For the biochemical characterization, the ID 32 E, API 20 E, API 50 CH, API Staph, API Corine and API 20 Strep systems were also applied using the API Bio Mini apparatus (Mérieux, France). The quality control of the kits for biochemical determinations was performed using the standardized samples ATCC-25922 E. coli, ATCC-27853 P. aeruginosa and ATCC-25923 S. Aureus.
RESULTS
We collected a total of 296 hematophagous Culicidae throughout the study period, but not all specimens were used for the bacteriological study. Only 129 were divided into 41 pools of three specimens of the same species and three pools containing two specimens. Most of the Culicidae collected were identified at the species level, including Culex (Culex) coronator, Anopheles (Nyssorhynchus) triannulatus, Coquillettidia (Rhynchotaenia) venezuelensis, Mansonia (Mansonia) titillans, Culex (Culex) quinquefasciatus, Mansonia (Mansonia) titillans, Aedes (Stegomyia) aegypti, Anopheles (Nyssorhynchus) aquasalis and Psorophora (Janthinosoma) ferox. Other specimens, however, were identified only at the genus level, including Culex (Culex) spp., Phoniomyia spp. and Culex (Melanoconion) spp.
Out of all the mosquitoes collected, only Psorophora (Janthinosoma) ferox and Phoniomyia spp. showed no bacteria growth in the medium selected for identification. However, bacterial growth only appeared in specimens collected from Nazaré when comparing Anopheles (Nyssorhynchus) aquasalis specimen collected at the Nazaré and Outeiro points.
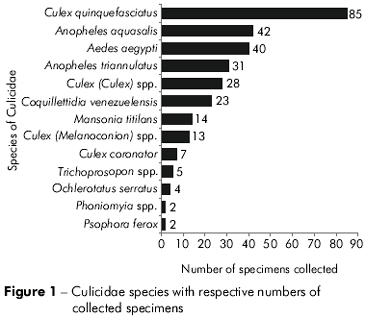
The C. quinquefasciatus species was the most frequently collected (85 samples), representing 28.7% of the total mosquitoes collected (Figure 1). We collected 42 specimens of A. aquasalis and 40 specimens of A. aegypti, which represented 14.1% and 13.5% of the mosquitoes, respectively. The Terra Firme sampling site had the highest number of Culicidae species, where it was possible to identify the following specimens at the species level: Coquillettidia venezuelensis, Ochlerotatus serratus and Psorophora ferox. The subgenera Culex (Culex) spp., Trichoprosopon (Trichoprosopon) spp., and the Phoniomyia spp genus were identified. The Curió-Utinga collection point also contained a large number of Culicidae; at this site, four specimens were identified at the species level and one at the subgenus level. In the Cremação neighborhood, only the Culex quinquefasciatus species was collected.
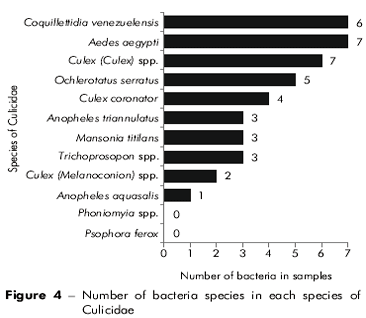
Out of the collected Culicidae, 17 species and seven genera of bacteria were identified. Among the identified bacteria, the following species were dominant: Gemella haemolysans, Enterobacter cloacae and Enterococcus faecalis (Figure 2), which represented 14.5%, 12.3% and 8.9% of the total identified bacteria, respectively. The Staphylococcus genus (negative in the coagulase test) was identified in 10% of the samples analyzed. Figure 3 illustrates the number of bacteria species in each of the mosquito collection points. Culex quinquefasciatus, Coquillettidia venezuelensis and A. aegypti were the Culicidae species that contained the greatest number of bacteria species. There was no bacterial growth in culture media with specimens of Psorophora ferox and Phoniomyia spp.
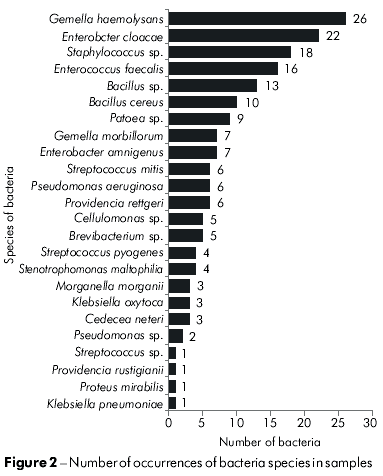
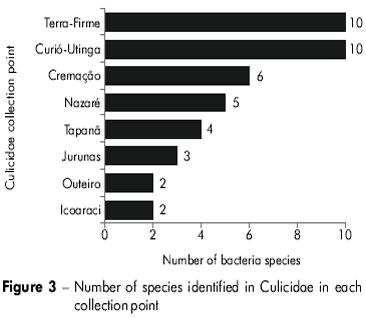
Table 1 presents the obtained results relating the isolated bacteria to the Culicidae species and showing the frequency of bacteria in each Culicidae species to the points where they were collected. It was observed that E. cloacae was found in at least 6 species of Culicidae while G. haemolysans and Staphylococcus sp. were present in at least four species. B. cereus and Phatoes sp. were found in three species and the other bacteria species were found in only one or two species of Culicidae. E. cloacae also occurred in the greatest number of collection points (five districts). G. haemolysans, Phantoea sp. and Staphylococcus sp. occurred in three districts, and the other bacteria species appeared in one to two neighborhoods. It was also observed that some bacteria species exhibited an elevated frequency in certain Culicidae species: G. haemolysans in C. (C.) quinquefasciatus (7.82% of all isolated and identified strains); Staphylococcus sp. and Culex (Culex) spp. (6.7%), E. faecalis in C. (C.) quinquefasciatus (6.14%), B. Cereus in Culex (Culex) spp. (5.59%); G. haemolysans in A. (N.) aquasalis (5.02%); Bacillus sp. in Coquillettidia (R.) venezuelensis (4.47%). All others had rates below 4%.
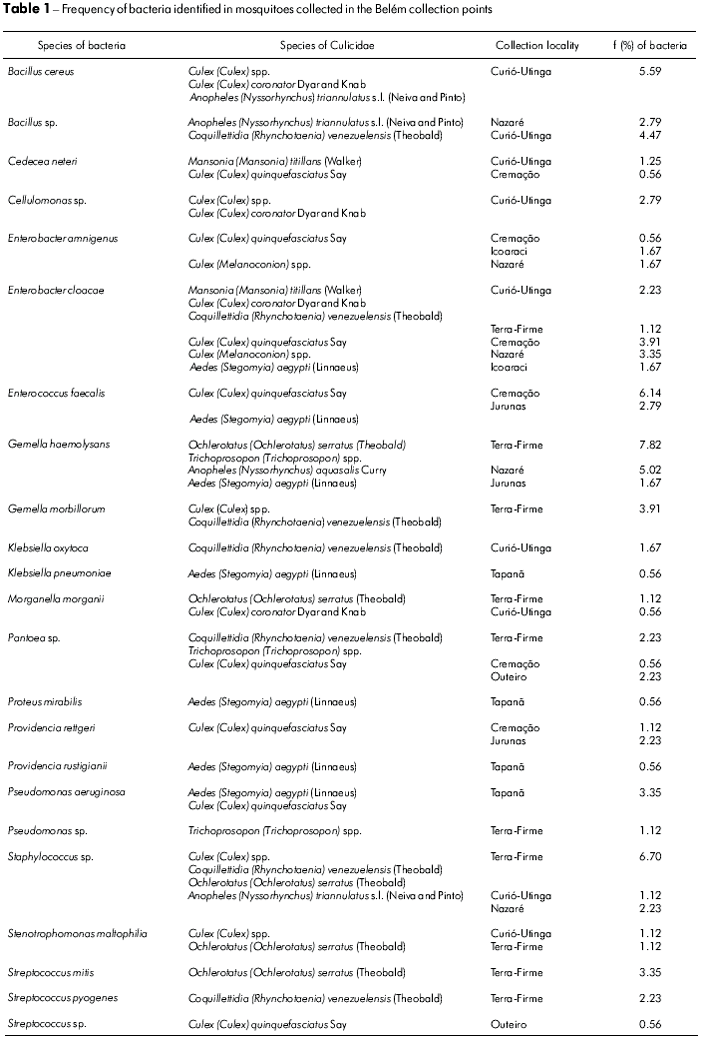
Figure 3 illustrates the number of bacteria species at each Culicidae collection point. The Curio-Utinga and Terra-Firme sampling points had the greatest number of bacteria species. The most frequently occurring bacteria in these points were Bacillus cereus (5.59% of all strains identified) and Gemella haemolysans (7.82% of all strains identified). Moreover, the Outeiro and Icoaraci collection points had only two bacteria species, with the Pantoea sp. (2.23% of all strains identified) dominant in Outeiro and E. cloacae (1.67% of total strains identified) in Icoaraci.
Figure 4 shows the number of bacteria species found in each Culicidae species. Culex quinquefasciatus, Coquillettidia venezuelensis and A. aegypti were Culicidae species that contained the greatest number of bacteria species. There was no bacterial growth in culture media with Psorophora ferox and Phoniomyia spp. specimens.
DISCUSSION
The Culicidae are closely associated with human activity, which provides these species with artificial oviposition sites, and allows the maintenance of their populations. Urban centers favor denser, more disperse populations of mosquitoes, since the organized social space influences the interaction between the vectors, infectious agents, and humans. Among the species of collected mosquitoes, C. quinquefasciatus and A. aegypti were the species with the greatest ability to colonize these urban areas.
C. quinquefasciatus were collected in five of the eight defined collection points, confirming that it is a species with a strong ability to disperse. In a study performed in 1 99145, females in this species were initially marked and were recaptured 1 km from the release point. This factor is essential for determining the potential spread of this species, which can also be confirmed by the bacteria identified in the samples because the Enterobacter amnigenus species was isolated in districts located far from each other like Cremação and Icoaraci, and Pantoea sp. was isolated in samples from Cremação and Outeiro. These points are distant and have distinct environmental characteristics, but they presented the same vector and bacterial microbiota.
Among Culicidae, the C. quinquefasciatus species was the most abundant (85 specimens) and contained 8 different bacteria samples, with Enterococcus faecalis most frequently occurring (13 occurrences). Adult females of this species tend to feed on human blood10, which allows their development in urban environments. In addition to being a nuisance to the communities in close proximity to their nests48. C. quinquefasciatus is a vector of several pathogens to humans19. Thus this species is characterized as being of great importance to public health, justifying its control policies in infested areas.
A. aquasalis is identified as an anthropophilic, zoophilic or, occasionally, eclectic vector. It is a major coastal malaria vector in several locations in Brazil and the Americas, and has shown highly varying feeding habits18. Forty two samples were collected during two collections in the neighborhood of Nazaré. Gemella haemolysans bacteria was the sole species identified in the samples.
The A. aegypti species was the third most frequently collected (40 specimen) during the study period. This species was encountered in the Jurunas and Tapanã neighborhoods and also in the administrative district of Icoaraci. In the samples, six different species were identified, including, Enterococcus faecalis and Pseudomonas aeruginosa. The Aedes aegypti mosquito is potentially the most critical health problem in Brazil today, considering its presence in all states and its transmission of the virus that causes dengue fever, hemorrhagic dengue fever, and yellow fever6.
Coquillettidia venezuelensis, which was collected in the neighborhoods of Curió-Utinga and Terra-Firme, presented a large variety of bacterial samples (8), but has a decreased transmission potential because of its required forested habitat. According to Guimarães23, in areas of the Serra do Mar in São Paulo, the Coquillettidia species require certain environmental factors for their life cycle, such as high rainfall and the presence of aquatic plants for their development. The work of Guimarães23 confirms the results of this study, because the neighborhoods where Coquillettidia were collected had the ideal characteristics for their life cycle. Although this species possesses a wide variety of bacteria, it can only cause problems for people living in these ideal conditions.
Among the bacteria, Gemella haemolysans was the most frequently identified (14.51% of all identified strains). This species was present in samples of A. aegypti, A. aquasalis, Trichoproson spp. and O. serratus. This bacterium can normally be found in oral cavities, provoking gingivitis, or even meningitis, bronchitis and pneumonia34,16. Bacteria of the Gemella fgenus were the main finding in the Moreira38 study, when isolating ants from hospitals in Rio de Janeiro.
The Enterobacter cloacae species occurred 22 times in 6 Culicidae samples and represented 12.28% of all strains identified. The only sample collection point where these bacteria were not found was in the neighborhood of Jurunas. This species is usually present in water, sewage, soil, and plants and is also part of the commensal enteric microbiota. It is thought not to cause diarrhea and is also associated with a variety of opportunistic infections that affect the urinary tract, respiratory tract, wounds and septicemia28. This species was also found in a study developed with cockroaches in Goiânia44. Gouveia et al22 found a significant prevalence of E. cloacae in distinct populations of Lutzomyia, which is similar to the present study's findings for Culicidae.
Bacteria of the coagulase-negative Staphylococcus genus are widely distributed in the environment and are part of the nasal microbiota, but can also be found in the oral cavity33. All strains of staphylococci of the Culicidae tested negative for coagulase, excluding the possibility of Staphylococcus aureus, which is one of the most important of the genus because it is involved in several pathologies from food poisoning33 to septicemia16. The results of a recent study developed by Costa11 revealed that bacteria of the genus Staphylococcus, that tested negative for coagulase, were the main finding of the bacteriological research on ants in hospitals in Minas Gerais, illustrating the species' ability to be transported by mechanical vectors.
Enterococcus faecalis was isolated from C. quinquefasciatus in the collection points of Cremação and Jurunas and from A. aegypti in the Jurunas collection point. This species represented 8.93% of all isolated bacterial colonies and was most frequent in samples of C. quinquefasciatus from the Cremação collection point (6.14%). Enterococci are Gram-positive cocci that are usually found in pairs and short chains. They can be found in soil, food, water, animals, birds and insects. The main human reservoir of Enterococci is the gastrointestinal tract, but it can also be found less frequently in the oral cavity, gall bladder, vagina and male urethra53. In recent years, several studies were commissioned because Enterococci have become significant agents of human diseases primarily due to its resistance to antimicrobial agents38.
Bacillus cereus and Bacillus sp. represented 5.59% and 7.26% of all bacteria strains identified, respectively. B. cereus was isolated from C. coronator, C. (Culex) spp. and A. triannulatus captured at the Curió-Utinga collection point. Bacillus sp. was isolated from A. triannulatus at the Nazaré sampling site and C. venezuelensis was isolated from sampling in the Curió-Utinga neighborhood. B. cereus is a Gram-positive bacterium found in soil. However, due to the resistance of its spores, the bacteria can be isolated from a variety of points and is widely distributed in nature. According to Mendes et al35 its main anthropologic implication is in food contamination because it may cause deterioration of food in stock and diarrhea when it is consumed39. However, Bacillus sp. is usually associated with contamination of milk. According to Vittoril et al52, thermal processing of milk is not able to destroy these bacteria.
Pantoea sp. was isolated from C. venezuelensis, T. (Trichoprosopon) spp. and C. quinquefasciatus collected at the Terra-Firme, Cremação and Outeiro collection points. The frequency of this species was 5.02% of all strains identified. Pantoea sp. are short Gram-negative bacilli and are usually isolated from plant surfaces, seeds, soil and water. They are opportunistic pathogens and therefore may be present in wounds, blood and human urine26.
Gemella morbillorum was isolated from C. (Culex) spp. and Coquillettidia venezuelensis at the Terra-Firme collection point at a frequency of 3.91% of all strains identified. It is a commensal bacterium of the oropharynx, upper respiratory, urogenital and gastrointestinal tracts, although they rarely cause infections in humans. However, a growing number of infections in different locations have been reported16,25,29. Brain abscesses caused by this bacterium are extremely rare with only four cases described in scientific literature29.
Pseudomonas aeruginosa was isolated from A. aegypti and C. quinquefasciatus from the Tapanã collection point with a frequency of 3.35%. This species is Gram-negative and is an extremely versatile bacteria that can be found in various environments, especially soil, water, plants and animals, and may cause opportunistic infections. In humans, P. aeruginosa causes infections in immunocompromised individuals, such as AIDS and cancer patients, burn victims and those with cystic fibrosis1. P. aeruginosa is also commonly found in nosocomial infections since it is able to adhere to different materials, allowing it to contaminate catheters, ventilators, prosthetics and contact lenses. Because of the high resistance to antibiotics and the great amount of virulence factors, infections caused by this bacterium are difficult to control4.
Providencia rettgeri and Providencia rustigianii Providencia rettgeri and Providencia rustigianii together showed a frequency of 3.91% of the all strains identified. They were isolated from C. quinquefasciatus captured in the Cremação and Jurunas collection points, respectively, and from A. aegypti that were captured at the collection point of Tapanã. The Providencia genus is currently composed of five species P. alcalifaciens, P. stuartii, P. rettgeri, P. rustigianii and P. heimbachae, of which the first four are recognized as human pathogens. These species are commonly associated with urinary tract infections in healthy communities and in patients with catheters. They may cause various opportunistic infections in hospitalized patients with burns, skin lesions, surgical wounds and septicemia2.
The genus Streptococcus contains many species of Gram-positive cocci, facultative, commensal and pathogenic anaerobes that colonize the skin and mucous membranes of the respiratory tract, genitourinary and alimentary canals of humans and other mammals32. In this work, Streptococcus mitis and Streptococcus pyogenes were isolated and identified by species; a third strain could not be categorized to the species level. Streptococcus mitis was isolated from Ochlerotatus serratus collected from Terra-Firme at a frequency of 3.35% of the total of strains identified. This is a dominant species in mucous membranes and on tongues of humans32. S. pyogenes had a frequency of 2.23% and was isolated from C. venezuelensis captured at the Terra-Firme collection point. This species is also known as Group A beta-hemolytic streptococcus (GABHS). It is the main representative of the beta-hemolytic streptococci, which has shown a strong ability to adapt to a human host over time, acting as an important etiologic agent in a number of clinical manifestations, predominately in the oropharynx32, as well as non-suppurative sequelae, which are characterized by rheumatic fever and glomerulonephritis.
Stenotrophomonas maltophilia was isolated from Culex spp. collected at the Curió-Utinga collection point and from Ochlerotatus serratus collected at Terra-Firme. It occurred at a frequency of 2.24% of all strains identified. It is a bacterium in the form of Gram-negative bacillus that can be found in a wide variety of environments and geographical regions, occupying different ecological niches and multiple sources of water. Other sources of isolation include soil, debris, raw milk, frozen fish, eggs and animal carcasses46. In hospital environments, this species has been isolated from tap water, sinks, respirators, suction catheters, blood pressure monitors, dialysis equipment and, occasionally, the hands of health care professionals12. Currently, S. maltophilia is considered an emerging pathogen because it occupies an important role in the setting of nosocomial infections, accounting for high morbidity because of its intrinsic resistance to most available antibiotics12.
Morganella morganii was isolated from O. serratus and C. coronator that were collected at sampling sites of Terra Firme and Curió-Utinga, respectively. It presented a frequency of less than 2% of all isolated strains. This species is Gram-negative and occurs naturally in the soil and feces of animals and humans. Recent studies of Kara José et al27 described M. morganii as a major contaminant of ophthalmic solutions, which can cause eye inflammation.
The species Klebsiella oxytoca and Klebsiella pneumoniae were isolated from C. venezuelensis and A. aegypti, respectively. Both had less than 2% frequency in Culicidae analyzed. However, these species are important because they cause severe infections and are resistant to several antibiotics. K. pneumoniae is a gram-negative bacillus of the family Enterobacteriaceae that is found in the upper respiratory, gastrointestinal and urinary tracts and can cause lobar pneumonia, urinary tract infection and septicemia. Several studies have tested the sensitivity of K. pneumoniae strains to antibiotics. Menezes et al36 found that the drug Meropenem is a good choice for treating infections caused by this bacterium. Since K. oxytoca is more opportunistic, it may worsen conditions and cause bacteremia after invasive procedures are performed7.
Cedecea neteri occurred in less than 2% of all identified strains, and was isolated from Mansonia titillans from the Curió-Utinga sampling point and Culex quinquefasciatus from the Cremação collection point. Enterobacteria of the genus Cedecea are characterized as short bacilli possessing biochemical reactions similar to those of the genus Serratia. Described in 1981, they have not had their pathogenic relevance well defined yet. The genus Cedecea includes the species C. davisae, C. neteri and C. lapagei, along with two species that are not yet named. This species has been isolated from humans in about 50% of the cases of respiratory tract infection. There are few reports of bacteremia in humans caused by C. neter given that the genus Cedecea is a rare opportunistic agent39.
Proteus mirabilis represented less than 1% of the all strains identified and was isolated from A. aegypti captured at the Tapanã collection point. Although few strains were isolated in this study, P. mirabilis is one of the most clinically significant species as it accounts for 10% of uncomplicated urinary tract infections and is the fifth most common pathogen responsible for urinary tract infections in hospitals. This species may also cause infections of wounds and sepsis in hospitalized patients.
CONCLUSION
The results of this study evidence an important relationship between Culicidae and bacteria by which a diverse and dynamic natural reservoir is maintained for the colonization of humans and animals. Moreover, this relationship reveals the importance of ecological and epidemiological studies involving bacteria and their vectors.
In the past ten years, there has been an increase in scientific production on relationships between insects and bacteria. This work is still being carried out, and continued efforts are important for the advancement of knowledge and eventual consolidation of an emerging line of research.
This is the first study developed in South America that researches the transport of bacteria in insects of the Culicidae family, and thus serves as a basis for further research on the relationship between these two living beings that present medical and veterinary significance.
ACKNOWLEDGMENTS
To Nazaré Segura and Hamilton Monteiro of the lEC's Arbovirology Section for their technical assistance in the identification of mosquitoes.
To José Caetano and Maria Odete of the lEC's Bacteriology Section for their support in the identification of bacteria.
To Rosimeire Trindade and Smith Santos of the Museu Paraense Emîlio Goeldi for their contribution to the collection of mosquitoes.
To the Museu Paraense Emîlio Goeldi and the lEC for the infrastructure and technical support for preparation in the various stages of this research.
REFERENCES
1 Ali NJ, Kessel D, Miller RF. Bronchopulmonary infection with Pseudomonas aeruginosa in patients infected with human immunodeficiency virus. Genitourin Med. 1995 Apr;71(2):73-7. DOI:10.1136/sti.71.2.73 [ Links ]
2 Almeida MTG, Bertelli ECP, Rossit ARB, Bertollo EMG, Martinez MB. Infecções hospitalares por Stenotrophomonas maltophilia: aspectos clínico-epidemiológicos, microbiológicos e de resistência antimicrobiana. Arq Cienc Saude [Internet]. 2005 jul-set [citado 2009 jan 13];12(3):141-5. Disponível em: http://www.cienciasdasaude.famerp.br/racs_ol/vol-12-3/04%20-%20ID129.pdf. [ Links ]
3 Alves LFA, Alves SB, Lopes J, Lopes RB. Avaliação de estirpes e de uma nova formulação granulada de Bacillus sphaericus Neide para o controle de mosquitos. Neotrop Entomol. 2006 jul-ago;35(4):493-9. DOI:10.1590/S1519-566X2006000400011 [ Links ]
4 Arruda EAG. Infecção hospitalar por Pseudomonas aeruginosa multi-resistente: análise epidemiológica no HC-FMUSP. Rev Soc Bras Med Trop. 1998 set-out;31(5):503-4. DOI:10.1590/S0037-86821998000500017 [ Links ]
5 Bobrowski VL, Fiuza LM, Pasquali G, Bodanese-Zanettini MH. Genes de Bacillus thuringiensis: uma estratégia para conferir resistência a insetos em plantas. Cienc Rural [Internet]. 2003 set-out [citado 2009 fev 20];33(5):843-50. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782003000500008&lng=en&nrm=iso&tlng=pt. DOI:10.1590/S0103-84782003000500008 [ Links ]
6 Braga IA, Valle D. Aedes aegypti: inseticidas, mecanismos de ação e resistência. Epidemiol Serv Saude [Internet]. 2007 dez [citado 2009 fev 20];16(4):279-93. Disponível em: http://scielo.iec.pa.gov.br/scielo.php?script=sci_abstract&pid=S1679-49742007000400006&lng=pt &nrm=iso&tlng=pt. [ Links ]
7 Campos GMR, Herani Filho B, Pereira CAP, Machado AMO, Baretta MCC. Bacteremia após a colangiopancreatografia retrógrada endoscópica, com e sem procedimento terapêutico: freqüência, fatores associados e significado clínico. Rev Assoc Med Bras [Internet]. 1997 out-dez [citado 2009 jan 21];43(4):326-34. Disponível em: http://www.scielo.br/scielo.php?pid=S0104-42301997000400009 &script=sci_arttext. DOI:10.1590/S0104-42301997000400009 [ Links ]
8 Carvalho CJB, Couri MS, Toma R, Rafael JA, Harada AY, Bonatto SR, et al. Principais coleções brasileiras de Diptera: histórico e situação atual. In: Costa C, Vanin SA, Lobo JM, Melic A. Proyecto de Red Iberoamericana de Biogeografía y Entomología Sistemática (PrIBES). Zaragoza: Sociedade Entomológica Aragonesa; 2002. Vol. 2, p. 37-52. [ Links ]
9 Cerqueira NL. Distribuição geográfica dos mosquitos da Amazônia (Diptera: Culicidae: Culicinae). Rev Bras Entomol. 1961;10:111-68.
10 Consoli RAGB, Lourenço-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: FIOCRUZ; 1994. 225 p. DOI:10.1590/S0102-311X1995000100027 [ Links ]
11 Costa SB, Pelli A, Carvalho G, Oliveira AG, Silva PR, Teixeira MM, et al. Formigas como vetores mecânicos de microorganismos no Hospital Escola da Universidade Federal do Triângulo Mineiro. Rev Soc Bras Med Trop. 2006 nov-dez;39(6):527-9. DOI:10.1590/S0037-86822006000600003 [ Links ]
12 Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998 Jan;11(1):57-80. [ Links ]
13 Dequech STB, Fiuza LM, Silva RFP, Zumba ARC. Histopatologia de lagartas de Spodoptera frugiperda (Lep., Noctuidae) infectadas por Bacillus thuringiensis aizawai e com ovos de Campoletis flavicincta (Hym., Ichneumonidae). Cienc Rural. 2007 jan-fev;37(1):273-6. DOI:10.1590/S0103-84782007000100045 [ Links ]
14 Dimopoulos G, Richman A, Müller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci U S A. 1997 Oct;94(21):11508-13. [ Links ]
15 Edwards PR, Ewing WH. Identification of Enterobacteriaceae. 4th ed. New York: Elsevier Science Publishing; 1986. 362 p.
16 Eisenhut M, Jones C, Hughes D, Herrington S, Kokai G. Acute renal failure associated with Gemella haemolysans pneumonia. Pediatr Nephrol. 2004 Apr;19(4):448-50. DOI:10.1007/s00467-003-1344-5 [ Links ]
17 Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq Syst. 1981;13:1-81.
18 Flores-Mendoza C, Cunha RA, Rocha DS, Lourenço-de-Oliveira R. Determinação das fontes alimentares de Anopheles aquasalis (Diptera: Culicidae) no Estado do Rio de Janeiro, Brasil, pelo teste de precipitina. Rev Saude Publica. 1996 abr;30(2):129-34. DOI:10.1590/S0034-89101996000200003 [ Links ]
19 Forattini OP. Culicidologia médica: identificação, biologia, epidemiologia. São Paulo: EDUSP; 2002. Vol. 2, 860 p. [ Links ]
20 Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol. 2003 May;40(3):371-4. [ Links ]
21 Gorham JR, Stojanovich CJ, Scott HG. Clave ilustrada para los mosquitos anofelinos de Sudamerica Oriental. Atlanta: Public Health Service; 1967. 64 p.
22 Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SMP. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). Neotrop Entomol. 2008 Sep-Oct;37(5):597-601. DOI:10.1590/S1519-566X2008000500016 [ Links ]
23 Guimarães AE, Gentile C, Lopes CM, Mello RP. Ecology of Mosquitoes (Diptera: Culicidae) in areas of Serra do Mar State Park, State of São Paulo, Brazil. II - Habitat distribution. Mem Inst Oswaldo Cruz [Internet]. 2000 Jan-Feb [citado 2009 mar 6];95(1):17-28. Disponível em: http://www.scielo.br/scielo.php?pid=S0074- 02762000000100002script=sci_art text. DOI:10.1590/S0074-02762000000100002 [ Links ]
24 Gusmão DS, Santos AV, Marini DC, Russo ES, Peixoto AMD, Bacci Júnior M, et al. First isolation of microorganisms from the gut diverticulum of Aedes aegypti (Diptera: Culicidae): new perspectives for an insect-bacteria association. Mem Inst Oswaldo Cruz. 2007 Dec;102(8):919-24. DOI:10.1590/S0074-02762007000800005 [ Links ]
25 Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore: Williams & Wilkins; 1994. 787 p.
26 Hörner R, Liscano MGH, Maraschin MM, Salla A, Meneghetti B, Dal Forno NL, et al. Suscetibilidade antimicrobiana entre amostras de Enterococcus isoladas no Hospital Universitário de Santa Maria. J Bras Patol Med Lab. 2005 dez;41(6):391-5. DOI:10.1590/S1676-24442005000600004 [ Links ]
27 Kara José AC, Castelo Branco B, Ohkawara LE, Yu MCZ, Lima ALH. Uso ocular de água boricada: condições de manuseio e ocorrência de contaminação. Arq Bras Oftalmol. 2007 mar-abr;70(2):201-7. DOI:10.1590/S0004-27492007000200004 [ Links ]
28 Koneman EW, Allen SD, Janda WM, Schreckenberger PC. Diagnóstico microbiológico. 5. ed. Rio de Janeiro: MEDSI; 2001. 1465 p.
29 Lopes A, Providencia R, Pais RP, Frade MJ, Chaddad Neto F, Oliveira E. Cerebellar abscess by Gemella morbillorum in a patient with inter-atrial communication. Arq Neuropsiquiatr. 2007 Dec;65(4 A):1022-5. DOI: 10.1590/S0004-282X2007000600022 [ Links ]
30 Luz C, Sebba GJ, Silva NR, Silva HHG, Monerat R. Prospecção de bactérias entomopatogênicas em solos de cerrado para controle biológico de mosquitos. Inf Epidemiol Sus. [Internet]. 2001 [citado 2009 out 17];10 Suppl 1:49-50. Disponível em: http://scielo.iec.pa.gov.br/scielo.php?script=sci_artt ext&pid=S0104-16732001000500015&lng =pt&nrm=iso. [ Links ]
31 Luz-Alves WC, Gorayeb IS, Silva JCL, Loureiro ECB. Bactérias transportadas em mutucas (Diptera: Tabanidae) no nordeste do Pará, Brasil. Bol Mus Para Emilio Goeldi Cienc Nat. 2007 abr-jul;2(3):11-20. [ Links ]
32 Maciel A, Aca IS, Lopes ACS, Malagueño E, Sekiguchi T, Andrade GP. Portadores assintomáticos de infecções por Streptococcus pyogenes em duas escolas públicas na cidade do Recife, Pernambuco. Rev Bras Saude Mater Infant. 2003 jun;3(2):175-80. DOI:10.1590/S1519-38292003000200007 [ Links ]
33 Martins CAP, Koga-Ito CY, Jorge AOC. Presence of Staphylococcus spp. and Candida spp. in the human oral cavity. Braz J Microbiol. 2002 Jul-Set;33(3):236-40. DOI:10.1590/S1517-83822002000300009 [ Links ]
34 May T, Amiel C, Lion C, Weber M, Gerard A, Canton P. Meningitis due to Gemella haemolysans. Eur J Clin Microbiol Infect Dis. 1993 Aug;12(8):644-5.
35 Mendes RA, Azeredo RMC, Coelho AIM, Oliveira SS, Coelho MSL. Contaminação ambiental por Bacillus cereus em unidade de alimentação e nutrição. Rev Nutr. 2004 abr-jun;17(2):255-61. DOI:10.1590/S1415-52732004000200012 [ Links ]
36 Menezes EA, Nascimento KM, Soares KP, Amorim LN, Lima Neto JG, Cunha FA. Avaliação da atividade in vitro do meropenem contra cepas de Klebsiella pneumoniae produtoras de betalactamases de espectro expandido isoladas na cidade de Fortaleza, Ceará. Rev Soc Bras Med Trop. 2007 maio-jun;40(3):349-50. DOI:10.1590/S0037-86822007000300021 [ Links ]
37 Moreira DDO, Morais V, Vieira-da-Motta O, Campos-Farinha AEC, Tonhasca Junior A. Ants as carriers of antibiotic-resistant bacteria in hospitals. Neotrop Entomol. 2005 Nov-Dec;34(6):999-1006. DOI:10.1590/S1519-566X2005000600017 [ Links ]
38 Moreira M, Medeiros ACC, Pignatari SB, Wey SB, Cardo DM. Efeito da infecção hospitalar da corrente sanguínea por Staphylococcus aureus resistente à oxacilina sobre a letalidade e o tempo de hospitalização. Rev Ass Med Bras. 1998;44(4):263-8. [ Links ]
39 Murray PR, Pfaller MA, Kobayashi GS, Rosenthal KS. Microbiologia médica. 4. ed. Rio de Janeiro: Guanabara Koogan; 2004. 766 p.
40 Oliveira SMP, Morais BA, Gonçalves CA, Giordano-Dias CM, Vilela ML, Brazil RP, et al. Microbiota do trato digestivo de fêmeas de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae) provenientes de colônia alimentadas com sangue e com sangue e sacarose. Cad Saude Publica [Internet]. 2001 [citado 2009 mar 15];17(1):229-32. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext & p i d = S 0 1 0 2 - 3 1 1 X 2 0 0 1 0 0 0 1 0 0 0 2 4 &lng=en&nrm=iso&tlng=pt. DOI:10.1590/S0102-311X2001000100024 [ Links ]
41 Oliveira VC, D’Almeida JM, Abalem IV, Mandarino JR, Solari CA. Enterobactérias associadas a adultos de Musca domestica (Linnaeus, 1758) (Diptera: Muscidae) e Chrysomya megacephala (Fabricius, 1754) (Diptera: Calliphoridae) no Jardim Zoológico, Rio de Janeiro. Arq Bras Med Vet Zootec. 2006 ago;58(4):556-61. DOI:10.1590/S0102-09352006000400017 [ Links ]
42 Papavero N, editor. A catalogue of Diptera of the Americas South of United States. São Paulo: USP; 1967.
43 Polanczyk RA, Garcia MO, Alves SB. Potencial de Bacillus thuringiensis israelensis Berliner no controle de Aedes aegypti. Rev Saúde Publica. 2003;37(6):813-6. DOI:10.1590/S0034-89102003000600020 [ Links ]
44 Prado MA, Pimenta FC, Hayashid M, Souza PR, Pereira MS, Gir E. Enterobactérias isoladas de baratas (Periplaneta americana) capturadas em um hospital brasileiro. Rev Panam Salud Publica. 2002 feb;11(2):93-8. DOI:10.1590/S1020-49892002000200005 [ Links ]
45 Reisen WK, Milby MM, Meyer RP, Pfutner AR, Spoehel J, Hazelriqq JE, et al. Mark-release-recapture studies with Culex mosquitoes (Diptera: Culicidae) in southern California. J Med Entomol. 1991 May;28(3):357-71.
46 Segabinazi SD. Presença de bactérias da família Enterobacteriaceae nas superfícies externa e interna de Alphitobius diaperinus (panzer) oriundos de granjas avícolas dos estados do Rio Grande do Sul e Santa Catarina [dissertação]. Santa Maria (RS): Universidade Federal de Santa Maria; 2004. 105 p. [ Links ]
47 Sudia WD, Chamberlain RW. Battery operated light trap and improved model. Mosq News. 1962;22(2):126-9.
48 Taipes-Lagos CB, Natal D. Abundância de culicídeos em área metropolitana preservada e suas implicações epidemiológicas. Rev Saude Publica. 2003;37(3):275-9. DOI:10.1590/S0034-89102003000300002 [ Links ]
49 Teixeira MLF, Franco AA. Susceptibilidade de larvas de Cerotoma arcuata Olivier (Coleoptera: Chrysomelidae) a Beauveria bassiana (Bals.). Vuillemin, Metarhizium anisopliae (Metsch.) Sorokin e Bacillus thuringiensis Berliner. Cienc Rural [Internet]. 2007 jan-fev [citado 2009 jun 3];37(1):19-25. Disponível em: http://www.scielo.br/scielo.php? script=sci_arttext&pid=S0103-84782007000100 004 lng=en&nrm=iso&tlng=pt. DOI:10.1590/S0103-84782007000100004 [ Links ]
50 Valicente FH, Barreto MR, Vasconcelos MJV, Figueiredo JEF, Paiva E. Identificação através de PCR dos genes CryI de cepas de Bacillus thuringiensis Berliner eficientes contra a lagarta do cartucho, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). An Soc Entomol Bras [Internet]. 2000 mar [citado jun 19];29(1):147-53. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext &pid=S0301-80592000000100018&lng=en& nrm=iso&tlng=pt. DOI:10.1590/S0301-80592000000100018 [ Links ]
51 Vieira PCB. Métodos de coletas de mosquitos (Diptera: Culicidae) alternativos ao de atração humana direta [dissertação]. Belém: Universidade Federal do Pará, Programa de Pós-graduação em Zoologia; 2007. 71 p.
52 Vittoril J, Schocken-Iturrino RP, Poiatti ML, Pigatto CP, Chioda TP, Ribeiro CAM. Qualidade microbiológica de leite UHT caprino: pesquisa de bactérias dos gêneros Staphylococcus, Bacillus e Clostridium. Cienc Rural. 2008 maio-jun;38(3):761-5. DOI: 10.1590/S0103-84782008000300026 [ Links ]
53 Xavier CAC, Oporto CFO, Silva MP, Silveira IA, Abrantes MR. Prevalência de Staphylococcus aureus em manipuladores de alimentos das creches municipais da cidade do Natal/RN. Rev Bras Anal Clin. 2007;39(3):165-8. [ Links ]
 Correspondência
/ Correspondence / Correspondencia
Correspondência
/ Correspondence / Correspondencia
Willy Cristiano Luz Alves
Rua Waterloo Prudente nº 96,
Setor: Jardim Umuarama
CEP: 68552-210
Redenção - Pará - Brasil
E-mail:willycristiano@gmail.com
Recebido em / Received / Recibido en: 31/7/2009
Aceito em / Accepted / Aceito en: 25/9/2009
*Parte da dissertação ao curso de "Mestrado em Saúde, Sociedade e Endemias da Amazônia", Universidade Federal do Pará, Brasil.











 texto en
texto en 

