Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.1 Ananindeua mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100022
Occurrence of toxic cyanobacterial bloom in the left margin of the Tapajós river, in the Municipality of Santarém (Pará State, Brazil)
Lena Líllian Canto de SáI; José Maria dos Santos VieiraII; Rosivaldo de Alcântara MendesIII; Samara Cristina Campelo PinheiroI; Elivam Rodrigues ValeI; Francisco Arimatéia dos Santos AlvesI; Iracina Maura de JesusIV; Elisabeth Conceição de Oliveira SantosV; Vanessa Bandeira da CostaI
ILaboratório de Microbiologia
Ambiental, Seção de Meio Ambiente, Instituto Evandro Chagas/SVS/MS,
Ananindeua, Pará, Brasil
IIFaculdade de Farmácia,
Instituto de Ciências da Saúde, Universidade Federal do Pará,
Belém, Pará, Brasil
IIILaboratório de Toxicologia,
Seção de Meio Ambiente, Instituto Evandro Chagas/SVS/MS, Ananindeua,
Pará, Brasil
IVSeção de Meio
Ambiente, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
VDiretoria, Instituto Evandro
Chagas/SVS/MS, Ananindeua, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
Original Title: Ocorrência de uma floração de cianobactérias tóxicas na margem direita do Rio Tapajós, no Município de Santarém (Pará, Brasil). Translated by: American Journal Experts
ABSTRACT
The presence of cyanobacterial blooms and their subproducts interferes directly in water quality and may cause negative effects, both aesthetically and to public health, due to the production of potentially toxic and carcinogenic compounds. The most common type of intoxication involving cyanobacteria is caused by microcystin-LR (hepatotoxin), which can cause severe damage to the liver. The objective of this study was to identify the genera that caused cyanobacterial blooms in the Tapajós River (Santarém, Pará, Brazil) in March 2007, as well as to execute acute toxicity bioassays in Swiss-webster mice. Sample collection was performed at five sampling points throughout the left margin of the Tapajós River, by horizontal dragging with the aid of a 20 μm plankton net. Samples of raw water (5,000 ml) were also collected in amber propylene bottles. Optical microscopy was applied to identify the organisms, and the determination of microcystin-LR was executed through ELISA and HPLC. The analyses showed that, at P01 and P02, there was an ecological imbalance in the phytoplanktonic community, characterized by an intense proliferation of the genera Anabaena and Microcystis. The concentrations of microcystin-LR reported in the raw water samples were below the maximum values permitted by Brazil's legislation for drinking water. However, it is important to note that the blooming observed in locu occupied around 10 cm of the water column surface and therefore presented cyanobacterial cells enough to cause rashes in people who swam or bathed in the rivers during this period.
Keywords: Cyanobacteria; Microcystins; Water Quality.
INTRODUCTION
Cyanobacteria are prokaryotic organisms capable of fixing carbon through photosynthesis. As part of the phytoplankton community, they are responsible for a large portion of the primary productivity and energy flow in aquatic ecosystems17. These microorganisms inhabit a wide range of environments (freshwater, brackish, marine and terrestrial)24 and are present in all aquatic biotopes (water/air interface, water column and sediment)14.
The presence of cyanobacteria in a body of water is associated with a group of environmental factors (concentration of nitrogen and phosphorus, high temperatures and availability of light) that, when altered, can cause blooming. This phenomenon is characterized by the intense growth of these microorganisms in the water10.
The occurrence of blooms has usually been attributed to the accelerated eutrophication process in aquatic environments, which is produced mainly by human activity (domestic and agro-industrial sewage). The presence of cyanobacterial blooms and their byproducts in rivers, lakes and reservoirs along the water supply line directly interferes with water quality. They may potentially add negative aesthetic and organoleptic features, such as undesirable colors, odors and tastes. They may also have a negative impact on public health due to the production of potentially toxic and carcinogenic compounds7.
There are several records of poisoning deaths in cattle, horses, pigs, sheep, dogs, fish and invertebrates caused by the ingestion of/contact with these toxic blooms16. Another recorded example of the action of these toxins was the death of 60 hemodialysis patients in Caruaru, Pernambuco because of the presence of hepatotoxins in the water34.
According to Falconer16, the toxins produced by cyanobacteria can be divided into neurotoxins, dermatotoxins and hepatotoxins, according to their toxic effects on mammals. The species that have been identified as producing hepatotoxins are included in the genera Microcystis, Anabaena, Nodularia, Oscillatoria, Nostoc and Cylindrospermopsis]0. The species Microcystis aeruginosa is thought to have the widest distribution in Brazil, and Anabaena is the genus with the highest number of potentially toxic species, according to Carmichael25. The most common type of intoxication involving cyanobacteria is caused by hepatotoxins, mainly microcystins (-LR, -LL and -YA), which can cause severe liver damage23.
To better understand the problems related to cyanobacterial blooms and water quality in general, our aims in this study were to identify the genera that caused a cyanobacterial bloom along the right shore of the Tapajós River (Santarém, Pará, Brazil), to verify that microcystins were produced by this bloom and to determine the toxicity of these cyanobacteria using toxicity tests in mice.
MATERIALS AND METHODS
DESCRIPTION OF THE STUDY AREA AND SAMPLING
Samples were collected on March 21, 2007, at five sites along the right shore of the Tapajós River in Santarém, Pará, Brazil. The sampling sites were: P01 - Arariá beach (54°44l10.28" S, 2o24'33.20" W); P02 - Carapanari beach (54°44'29.66" S, 2°24'55.01'' W); P03 - Ponta de Pedra inlet (54°53'43.71'' S, 2°26'04.78'' W); P04 - in front of the entrance to the Sururu River (54°51'02.36'' S, 2°17'06.24" W), which is influenced by the Amazon River at this time of the year; and P05 - the river bed (54°58'44.59'' S, 2°20'20.29'' W), an area influenced by the Arapiuns River (Figure 1).
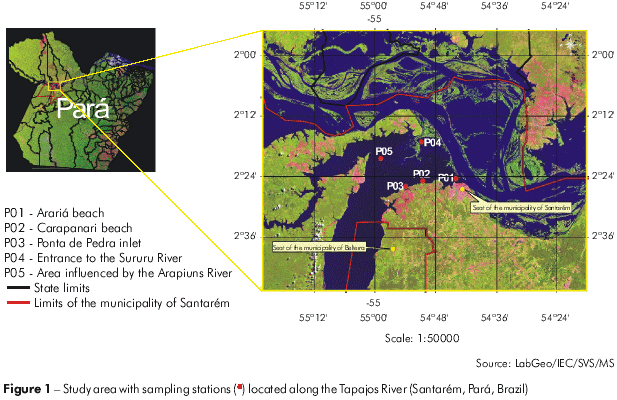
METHODS
Sample collection
The samples used for qualitative study of the cyanobacteria were collected by horizontal hauls on the surface of the water using a plankton net with a 20-um mesh size. Approximately 2,000 L of water were filtered. A 100-mL aliquot was fixed in formalin, and a 250-mL aliquot was refrigerated. We also collected untreated surface water using a 5,000-mL amber-type polypropylene bottle. These samples were stored in a polystyrene box with recyclable ice packs until analysis.
Species identification
In the laboratory, the samples were analyzed by observing temporary slides under a binocular microscope. The species identification and nomenclature were carried out according to specialized literature2'3'18'15.
Determination of microcystins in the water using an ELISA technique
In the laboratory, a 100-mL aliquot of untreated water was sonicated to promote cell lysis and the release of toxins into the water and then filtered using a Millipore AP20 filter system. A 20-/A aliquot of the filtrate was analyzed using the methods described by An and Carmichael1 with an EnviroLogix Inc. EP-022 kit, according to the manufacturer's instructions. The kit detects microcystin-LR using polyclonal antibodies. All the analyses were performed in duplicate, and the average of the results was considered the sample concentration.
Determination of microcystins in the water using HPLC
The determination of microcystins in the water samples was performed by extracting 2L of a water sample using solid-phase extraction (SPE) followed by High Performance Liquid Chromatography (HPLC) analysis. The SPE extractions were performed using the methodology proposed by Tsuji et al28 that consisted of: activating a C18-ODS cartridge with 20 mL of methanol and 20 mL of deionized water; adding, under a vacuum, 2 L of a water sample to the activated C18-ODS cartridge; adding 20 mL of deionized water to "clean-up" the activated C18-ODS cartridge; adding 20 mL of methanol to the activated C18-ODS cartridge to elute the toxin; completely drying the methanol fraction, which contains the toxin, in a rotating evaporator; resuspending the sample in 1 mL of methanol; and filtering the solution through a 0.45-J/m nylon filter. For the chromatographic analysis of the samples, we used the following conditions for HPLC (Varian): C-18 reverse phase column, ODS, 5-/Jm, 250 mm x 4 mm (Varian); acetonitrile mobile phase: 20 mM ammonium acetate, pH 5.0 (28:72 v/v), flow - 1.0 mL/min; photodiode detection array (PDA) at 238 mm; injection volume 20 /A; time of analysis of 30 minutes; and Microcystin-LR pattern from M. aeruginosa (Sigma M-2912).
Determination of microcystins in lyophilized cells using HPLC
After lyophilizing 250 mL of the sample collected by horizontal hauls using a plankton net (20/um), the lyophilized sample was extracted with butanol:methanol: double deionized water (1:4:15, v/v) in a ratio of 20 mL for each 100 mg of sample, according to the methodology described by Krishnamurthy et al19 and modified by Domingos et al13. The material obtained was agitated for one hour, and the fragmented cells were removed by centrifugation at 3,000 g for ten minutes. The procedure was repeated twice to ensure the total extraction of microcystins. The extraction supernatants were combined, evaporated at 400°C to 30% of their initial volume and passed through an octadecylsilane cartridge (C18) (Bond-Elut Varian). Afterwards, the supernatants were sequentially washed in 20 mL of deionized water and 20 mL of 100% methanol to elute the toxins. The fraction eluted with methanol was collected, evaporated until dry and diluted in 1 mL of 50% methanol. The sample was then filtered using a 0.45-um nylon filter, and the toxins were identified by HPLC using the same chromatographic conditions as for the water samples.
Bioassays of acute toxicity in mice
The lyophilized material was dissolved in a sterile saline solution and sonicated for five cycles of 30 s at 100 W to release the toxin22. Toxicity was determined by intraperitoneal injection of lyophilized cell extract diluted in saline solution, at concentrations of 1,200, 1,000 and 100 mg/Kg, into Swiss-Webster male mice weighing between 20-25 g. The median lethal dose (LD50) was determined using five mice for each dose. To obtain the expected concentration we injected 0.1 mL/10 g of mouse body weight of solution into each mouse. The signs of intoxication, survival time and post-mortem examination of the liver confirmed acute toxicity and were used to determine the LD505,6,26,20. For each concentration tested, two control animals were inoculated with the saline solution used to dilute the extract.
RESULTS
While collecting samples at the P01 and P02 sites, we observed a clear ecological imbalance in the phytoplankton community, characterized by the appearance of a bloom (Figures 2A and B). The analysis of the samples collected at these sites confirmed the presence of the genera Anabaena (Figures 2C and D) and Microcystis (Figures 2E e F). The genus Anabaena comprises species with coiled trichomes and a worldwide distribution that are commonly found in different bodies of water, especially in eutrophic environments. Microcystis is composed of microorganisms with dense mucilage that are recognized for frequently forming surface blooms.
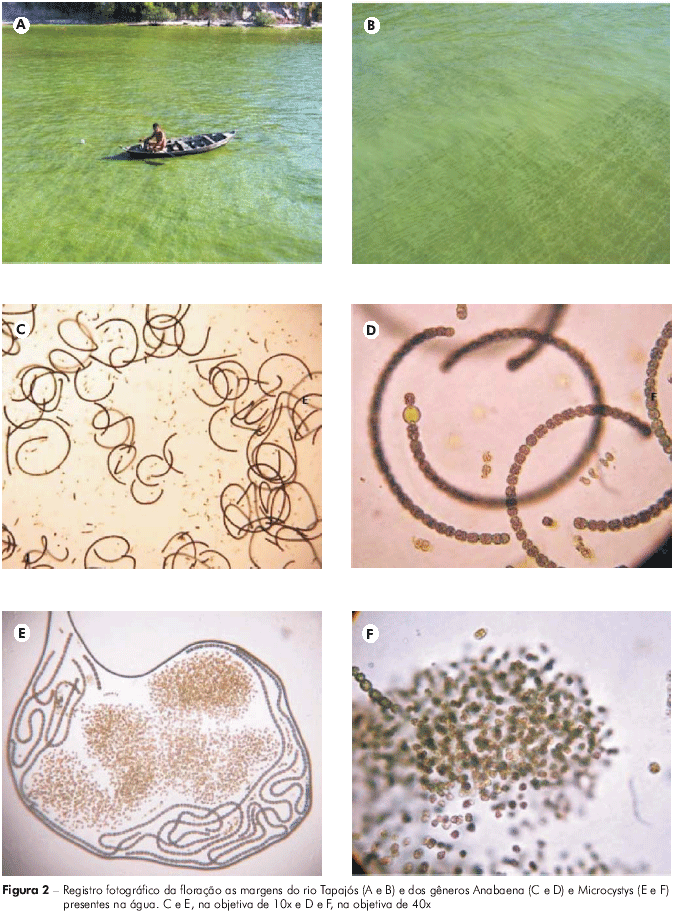
Table 1 presents the results of the analyses performed using direct microscopy and the microcystin quantification by ELISA and HPLC of concentrated (lyophilized) and untreated water samples.
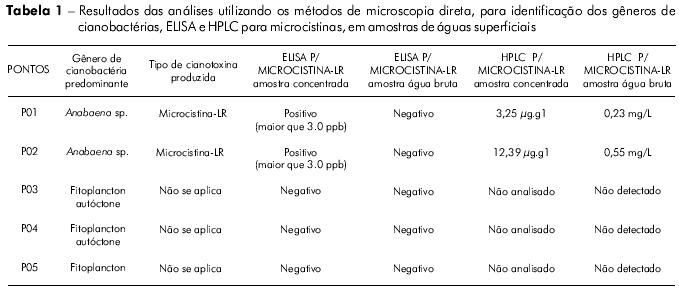
The ELISA for detecting microcystins in the untreated water samples was negative for all sites. The only concentrated (lyophilized) samples that were positive at concentrations above 3 ppb were those from the P01 and P02 sites. The microcystin-LR analysis using HPLC recorded cyanotoxin concentrations of 3.25 μg.L-1 for P01 and 12.39 μg.L-1 for P02 in untreated water samples, and 0.23 μg.L-1 for P01 and 0.55 μg.L-1 for P02 in concentrated and lyophilized water samples (Table 1).
In the mouse toxicity tests, there were no deaths during or after the seven days of observation at any of the concentrations tested, although the animals showed limited mobility and signals of abdominal contractions within minutes of the application. Thus, these data suggest that the observed blooms did not have sufficient concentrations of toxins to induce acute toxicity that would cause immediate harm to human health.
DISCUSSION
In Brazil, until the mid-1990s, the relationship between the degradation of water supplies and public health was restricted to water contaminated with the causative agents of waterborne diseases, especially several species of bacteria, protozoa, worms and some viruses. Only after the tragic 1996 deaths of about 60 patients with chronic renal failure who underwent hemodialysis at a clinic in the city of Caruaru, Pernambuco, did authorities realize that another important but often disregarded factor could be responsible for human death via water ingestion: biologically produced toxins, which may be present in the water supply25. After this event, blooms of toxic cyanobacteria were recognized as a public health problem and maximum allowable limits of these toxins in the water supply21 and in multi-use water12 were established.
The toxic effects of cyanotoxins have received increasing attention from researchers around the world. Microcystins with hepatocarcinogenic effects in mice are the cyanotoxins most frequently found in bodies of water worldwide4. In Brazil, the occurrence of toxic cyanobacteria in reservoirs of water intended for human consumption has been observed in several states5. In Pará, we have been following significant events of toxic cyanobacterial blooms since 1999, indicating the alarming nature of this issue. In 1999, during a cyanobacteria monitoring study near the Utinga dam, which supplies the city of Belém, Pará, toxic strains of Radiocystis fernandoi and Microcystis viridis were found along with the presence of microcystins in untreated water from the dam31,29,32,30. During a bloom of Cylindrospermopsis raciborskii in the Iriri and Xingu Rivers (Altamira, Pará), there was a large fish die-off, and saxitoxins were found in the water33.
In the present study, the concentrations of microcystin-LR found in the untreated water samples from the Tapajós River are below the maximum allowable levels under the Brazilian legislation for water consumption21, which discards, at the moment, the possibility of acute poisoning to humans. However, it is important to emphasize that a cumulative effect of these heptapeptides on the body may lead to future health problems.
All genera of cyanobacteria have dermatotoxins (LPS) in their cell walls. These toxins may cause irritation upon contact with the skin27 as well as eye irritation, conjunctivitis, hives, nasal obstruction and asthma8.
Cases of contact dermatitis in humans associated with recreational water use have been reported9. Thus, we cannot discard the possibility of eventual skin irritation in persons, especially children, the immunosuppressed and the elderly, who frequent the Arariá and Carapanari resorts, which are among the most visited in the municipality of Santarém, Pará.
The confirmation that the bloom observed on the right shore of the Tapajós River comprised two genera of cyanobacteria (Anabaena and Microcystis) that potentially produce cyanotoxins calls attention to the need for environmental monitoring in this region to determine the causes and/or origins of these occurrences.
CONCLUSION
Considering its importance for public health and to improve our understanding of the local biodiversity and the natural and anthropogenic processes that may be related to these events, which have been occurring year after year in the Tapajós River, it is necessary to monitor the cyanobacteria in these river waters. This necessity arises because, during the study period, we found blooms of two genera of cyanobacteria {Anabaena and Microcystis) that produce toxins and also detected microcystin-LR in the untreated water. Over the years, these blooms may become increasingly abundant and cause health risks to the local population who use this water for consumption, fishing and recreation.
ACKNOWLEDGMENTS
The authors would like to acknowledge Raimundo Pio Girard for his support in collecting material and the Brazilian Navy {Santarém Station) for their support during the fieldwork.
REFERENCES
1 An J, Carmichael WW. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon. 1994 Dec;32(12):1495-507. [ Links ]
2 Anagnostidis K, Komárek J. Modern approach to the classification system of cyanophytes. 1 - Introduction. Arch Hydrobiol. 1985;38-39(Suppl 71):291-302.
3 Anagnostidis K, Komárek J. Modern approach to the classification system of cyanophytes. 3 - Oscillatoriales. Arch Hydrobiol. 1988;50-53(Suppl 80):327-472.
4 Azevedo SMFO, Carmichael WW, Jockimsen EM, Rinehart KL, Lau S, Shaw GR, et al. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology. 2002 Dec;181-182:441-6. DOI:10.1016/S0300-483X(02)00491-2 [ Links ]
5 Azevedo SMFO, Evans WR, Carmichael WW, Namikoshi M. First report of microcystins from a Brazilian isolate of the cyanobacterium Microcystis aeruginosa. J Appl Phycology. 1994;6:261-5. DOI: 10.1007/BF02181936 [ Links ]
6 Baker PD, Humpage AR. Toxicity associated with commonly ocurring cyanobacteria in surface waters of the Murray-Darling Basin, Australia. Aust J Mar Freshwater Res. 1994;45:773-86. DOI:10.1071/MF9940773 [ Links ]
7 Bernardo L. Algas e suas influências na qualidade das águas e nas tecnologias de tratamento. Rio de Janeiro: Associação Brasileira de Engenharia Sanitária e Ambiental; 1995. 127 p.
8 Calijuri MC, Alves MSA, Santos ACA. Cianobactérias e cianotoxinas em águas continentais. São Carlos: Rima; 2006. 118 p.
9 Carmichael WW. Freshwater blue-green algae (Cyanobacteria) toxins - A review. In: Carmichael WW, editor. The water environment Algal Toxins and Health. New York: Plenum Press; 1981. p. 1-13.
10 Carmichael WW. The toxins of cyanobacteria. Sci Am. 1994 Jan;270(1):78-86.
11 Chorus I, Bartram J. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. New York: E & FN Spon; 1999. 416 p. [ Links ]
12 Conselho Nacional de Meio Ambiente (BR). Resolução no 357, de 17 de março de 2005. Diário Oficial da União; 17 mar. 2005. [ Links ]
13 Domingos P, Rubim TK, Molica RJR, Azevedo SMFO, Carmichael WW. First report of microcystin production by picoplanctonik cyanobacteria isolated froma northeast Brazilian drinking water supply. Environ Toxicol. 1999;14(1):31-5.
14 Esteves FA. Fundamentos de limnologia. 2. ed. Rio de Janeiro: Interciência; 1998. 602 p.
15 Ettl H, Gartner G, Heynig H, Mollenhauer D. Subwasserflora von mitteleuropa. In: Komárek J, Anagnostidis K, editors. Cyanoprokaryota. Berlin: Verlag; 1999. 548 p. Teil Chroococcales.
16 Falconer IR. An overview of problem caused by toxic blue-green algae (Cyanobacteria) in drinking and recreational water. Environ Toxicol. 1999;14:5-12. [ Links ]
17 Ferrão-Filho AS, Molica R, Azevedo SM. Ecologia, ecofisiologia e toxicologia de cianobactérias. Oecol Bras. 2009;13(2):225-8. [ Links ]
18 Komárek J, Anagnostidis K. Modern approach to the classification system of cyanophytes. 4 - Nostocales. Arch Hydrobiol. 1989;56(Suppl 83):291-302.
19 Krishnamurthy T, Carmichael WW, Sarvier EW. Toxic peptides freshwater cyanobacteria (blue-green algae). I. Isolation, purification and characterization of peptides from Microcystis aeruginosa and Anabaena flos-aquae. Toxicon. 1986;24(9):865-73. DOI: 10.1016/0041-0101(86)90087-5 [ Links ]
20 Magalhães VF, Soares RM, Azevedo SMFO. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon. 2001 Jul;39(7):1077-85. DOI: 10.1016/S0041-0101(00)00251-8 [ Links ]
21 Ministério da Saúde (BR). Portaria no 518, de 25 de março de 2004. Diário Oficial da União; 26 mar. 2004. [ Links ]
22 Negri AP, Jones GJ. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussel Alathyria condola. Toxicon. 1995 May;33(5):667-78. [ Links ]
23 Nishiwaki-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, et al. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin Oncol. 1992;118(6):420-4. [ Links ]
24 Sant'Anna CL, Azevedo MTP, Agujaro LF, Carvalho LR, Souza RCR. Manual ilustrado para identificação e contagem de cianobactérias planctônicas de águas continentais brasileiras. Rio de Janeiro: Interciência; 2006. 58 p.
25 Sant'anna CL, Azevedo MTP. Contribution to the knowledge of potentially toxic cyanobacteria from Brazil. Nova Hedwigia. 2000;71:359-85.
26 Sedmack B, Kosi G. Microcystins in Slovene freshwaters (Central Europe) First report. Nat Toxins. 1997;5(2):64-73. [ Links ]
27 Sivonen K, Jones G. Cianobacterial toxins. In: Chorus I, Bartram J, editors. Toxin Cyanobacteria in water: A guide to their Public Health Consequences, Monitoring and Management. Londres: E & FN Spon; 1999. p. 42-111.
28 Tsuji K, Naito S, Kondo F, Watanabe M, Suzuki S, Nakazawa H, et al. A clean-up method for analysis of trace amounts of microcystins in lake water. Toxicon. 1994;32(10):1251-9. [ Links ]
29 Vieira JMS, Azevedo MTP, Azevedo SMO, Honda RY, Correa B. Microcystin production by Radiocystis fernandoi (Chroococcales, Cyanobacteria) isolated from a drinking water reservoir in the city of Belém, PA, Brazilian Amazonic region. Toxicon. 2003 Dec;42(7):709-13. [ Links ]
30 Vieira JMS, Azevedo MTP, Azevedo SMO, Honda RY, Corrêa B. Toxic cyanobacteria and microcystin concentrations in a public water supply reservoir in the Brazilian Amazonia region. Toxicon. 2005 Jun;45(7):901-9. [ Links ]
31 Vieira JMS, Azevedo MTP, Honda RY, Azevedo SMFO, Correa B. Produção de microcistinas por Radiocystis fernandoi (Cyanobacteria/Chroococcales) isolada da represa de abastecimento da cidade de Belém-PA [resumos]. In: 8o Congresso Brasileiro de Limnologia, 2001, João Pessoa, PB: Sociedade Brasileira de Limnologia; 2001. p. 217.
32 Vieira JMS, Sá LLC, Santos ECO, Lima MO, Bernardo RR, Azevedo SMFO. Ocorrência de Cylindrospermopsis raciberskii e saxitoxinas durante uma mortandade de peixes nos rios Iriri e Xingu, Altamira, Pará, Brasil [resumos]. In: 22o Congresso Brasileiro de Microbiologia, 2003, Florianópolis, SC: Sociedade Brasileira de Microbiologia; 2003.
33 Vieira JMS, Vieira ABR. Floração de Microcystis sp (Cianobacteria) em uma praia de rio da Região Amazônica do Brasil [resumos]. In: 22o Congresso Brasileiro de Microbiologia, 2003, Florianópolis, SC: Sociedade Brasileira de Microbiologia; 2003.
34 Yuan M, Carmichael WW, Hilborn ED. Microcystin analysis in human sera and liver from human fatalities in Caruaru, Brasil 1996. Toxicon. 2006 Nov;48(6):627-40. DOI: 1016/j.toxicom.2006.07.031 [ Links ]
 Correspondência
/ Correspondence / Correspondencia:
Correspondência
/ Correspondence / Correspondencia:
Lena Líllian Canto Sá
Instituto Evandro Chagas,
Seção de Meio Ambiente
Rodovia BR 316, km 7, s/no,
Bairro: Levilândia
CEP:67030-000
Ananindeua-Pará-Brasil
E-mail:lenasa@iec.pa.gov.br
Recebido em / Received / Recibido en: 31/7/2009
Aceito em / Accepted / Aceito en: 25/9/2009











 texto em
texto em 
 Curriculum ScienTI
Curriculum ScienTI
