Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.1 no.1 Ananindeua Mar. 2010
http://dx.doi.org/10.5123/S2176-62232010000100024
Review of a tetravalent vaccine (RRT-TV) for prevention of gastroenteritis caused by rotavirus
Consuelo Silva de OliveiraI; Alexandre da Costa LinharesI; Nildo Alves BatistaII
IInstituto Evandro
Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIUniversidade
Federal de São Paulo, São Paulo, São Paulo,
Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
Original Title: Reanálise da vacina tetravalente (RRV-TV) no contexto da prevenção das gastrenterites por rotavírus. Translated by: American Journal Experts
ABSTRACT
Rotaviruses are considered the leading cause of severe gastroenteritis in children under five years of age, especially in developing countries. Vaccination in the first months is the most effective public health action for the control and prevention of infections by such agents. Despite the recent licensing of two vaccines for use in infants (Rotarix® and Rota Teq®), researchers continue to seek new alternatives for prevention and treatment. Herein, we provide a review of the Rhesus-Human Reassortant Rotavirus Tetravalent Vaccine (RRV-TV), with an emphasis on its clinical efficacy as regards clinical parameters, the most prevalent serotypes in the region, the occurrence of severe adverse events (e.g., intussusception), and selective protection in the most severe cases. The clinical and epidemiological data were obtained from medical records pertaining to 91 episodes of diarrhea among children in a previous investigation conducted in Belém, Pará State, Brazil. Clinical patterns and a scoring system commonly used in studies on the efficacy of RRV-TV were considered as indicators of severity. The most impressive results of this study, such as a significant protection (p < 0.05) by RRV-TV in five of the seven clinical conditions assessed, the cumulative efficacy rate of 100% against episodes with a clinical score of >14 related to serotype G2, a 75% efficacy rate against severe episodes, and the non-occurrence of intussusception, are discussed in the context of current knowledge on this issue.
Keywords: Rotavirus Vaccines; Gastroenteritis; Rotavirus Infections.
INTRODUCTION
Acute rotavirus gastroenteritis constitutes a serious health problem, especially in developing countries in Africa and Asia, where the disease is associated with approximately 6,000 deaths per year among children less than 5 years of age. In the United States, despite the low mortality rates (20 to 60 deaths per year), rotaviruses are responsible for 30% to 70% of hospitalizations due to gastroenteritis1.
Studies conducted by Bishop et al3 in Melbourne, Australia, were the first to demonstrate an association between acute diarrhea and rotavirus. After the pioneering findings by Linhares et al.18 in the Northern Region of Brazil, epidemiological studies were performed in other regions of the country. These studies confirmed the major role of rotaviruses in childhood mortality due to diarrhea, which accounts for 30% of the hospitalizations and 10% of diarrheal cases in the community22. Recent epidemiologic investigations conducted in Brazil have estimated that 120,513 hospitalizations and 2,475 deaths are associated with rotavirus7.
Rotavirus has great antigenic diversity with a multiplicity of serotypes; the G1 to G4 and G9 types are the most prevalent and are responsible for 95% of diarrheal episodes in children worldwide21. A recent review by Leite et al.12 on an impressive number of samples (2,691) from various regions of Brazil between 1982 and 2007 deserves to be highlighted. In this study, G1, G2, G3, G4, G5 and G9 were identified as the major circulating serotypes all over the country.
Infections by rotavirus exhibit a well-defined seasonal pattern, particularly in regions of temperate climate, where their prevalence is observed in the coldest months. In tropical areas, the seasonality is less marked, with a greater concentration of cases during the driest months of the year1.
Based on observations that improvement in sanitation conditions does not interfere in the high prevalence rates of diarrhea due to rotavirus in developed and developing countries and that increased hospitalization rates are observed in spite of the large-scale usage of oral re-hydration solutions, vaccination is considered the most effective measure of control and prevention of rotavirus disease.
The development of an efficacious and safe vaccine is a priority of the World Health Organization (WHO). This vaccine would primarily be administered to children up to 2 years of age who can become dehydrated and die when infected by rotavirus. Studies with candidate vaccines have evolved since the so-called Jennerian procedures, which utilize animal-derived strains of rotavirus, followed by second-generation vaccines and strategies based on genetic engineering techniques.
Among the various vaccine strategies studied, the Rhesus-human Reassortant Rotavirus Tetravalent Vaccine (RRV-TV), the first vaccine against the rotavirus that was evaluated in Brazil, more precisely in Belém, Pará, during the 1990s, is emphasized14. This vaccine involves the genetic reassortment between four attenuated strains from simian and human origins (4x104 pfu/dose) and was analyzed by various clinical studies conducted in developed countries11 and in South America (Peru, Brazil and Venezuela). The results demonstrated the vaccine efficacy in relation to severe episodes, reaching protection rates comparable to other studies in which the vaccine was used at higher concentrations10,24. The analysis of results obtained from various clinical trials led to the licensure of the first rotavirus vaccine in the USA (Rotashield®) in July 1998, with a recommendation of three doses at 2, 4 and 6 months of age5. However, 9 months later, and after 1 million doses had been given to approximately 500 thousand children, the vaccine was withdrawn from the immunization program due to an extensive evaluation of notified cases of intussusception that were associated with the vaccine6. Subsequent analyses of data confirmed this relationship, with an estimated risk between 1/10,000 and 1/32,000 and a greater incidence between the 3rd and the 14th day after the first dose in children older than 3 months of age; thus, a clear association was observed in this age group20. It is important to note that the clinical trials of this vaccine were discontinued in the rest of the world.
Currently, it is believed that there was an overestimation of the risk of intussusception in the USA because 35% of children received the first doses at later ages than indicated in the current studies25. Therefore, it is concluded that in developing countries the benefits obtained from this vaccine could outweigh a possible risk of intussusception. Thus, in the attempt to elucidate questions that remain ambiguous, retrospective analyses of the accumulated experiences with the tetravalent vaccine are warranted. These analyses might help in the global attempt to accelerate the introduction of new vaccines into immunization programs, particularly in countries like India, Indonesia and China, where one-third of childhood deaths are attributable to rotavirus9.
MATERIALS AND METHODS
The current study focused on a re-analysis of the tetravalent vaccine RRV-TV produced by Wyeth-Ayerst Research laboratories (Philadelphia, Pennsylvania, USA) based on 91 diarrheal episodes that occurred in children participating in a previous investigation conducted in Belém, Pará, Brazil. This investigation was approved by the Medical Ethics Committee of the Instituto Evandro Chagas, Para's Regional Council of Medicine, Pará State Health Secretariat, Brazil's Ministry of Health and the WHO Ethics Commission, Geneva, Switzerland.
The aforementioned investigation was carried out in the city of Belém, which is located in the north of Brazil, Eastern Amazon. The study was conducted over two years and was characterized as prospective, randomized, and double-blinded. Either vaccine or placebo was administered at a ratio of 1:1 to 540 children. The vaccination scheme consisted of three doses: the first vaccine dose was administered in the first month of life, and was followed by two other doses in the third and fifth months. A total of 540 children received the first dose, 513 received the second, and 499 children (92%) received all three doses of the vaccination scheme. Among those with full-course vaccination, 466 (94%) were monitored until the end of the study. The observed differences in the number of children relate to strict compliance with the study protocol based on pre-defined limits for age groups of each of the three doses approved for the vaccination schema.
The vaccine efficacy rate was calculated by considering the episodes of diarrhea due to rotavirus recorded from two weeks after administration of the third dose to two years after vaccine administration.
The clinical parameters analyzed were obtained from the medical records utilized in the surveillance of the diarrheal episodes. In this investigation, one week after the first vaccination dose, each child was followed up on a twice-a-week basis until the end of the study to determine the incidence of the diarrheal episodes. Upon detection of a case of diarrhea, the patient received daily visits until the end of the episode. The clinical criteria to assess severity included: a) diarrhea - three or more liquid or semi-liquid stools in a 24 h period (definition adopted by WHO for studies of rotavirus vaccine); b) presence of vomiting; c) fever - rectal temperature ≥ 38o C; d) signals of dehydration (criteria established by WHO); e) maximum number of liquid or semi-liquid stools ≥ 6 in 24 h; f) average number of liquid or semi-liquid stools ≥ 6 in 24 h; and g) visits to hospital or health units. Along with these parameters, a clinical scoring system was also utilized in the efficacy analysis of RRV-TV8, which is commonly applied to assess protection against clinical severity. This clinical scoring system quantifies the severity of symptoms, which may reach an overall maximum value of 20 points (Table 1). Episodes with clinical scores ranging from 0 to 8, 9 to 14 and above 14 were defined as light, moderate to severe and very severe, respectively.

A total of 131 diarrheal episodes due to rotavirus were recorded during the study; however, only 91 were considered because these were episodes that began 15 days after the third dose. For the diagnostic definition of these cases, fecal specimens and, less often, rectal swabs were obtained as soon as a diarrheal episode was detected. Samples were subjected to a routine ELISA method for the detection of rotavirus (or its antigens), utilizing a Dakopatts kit (Copenhagen, Denmark), which is recommended by the WHO.
RESULTS
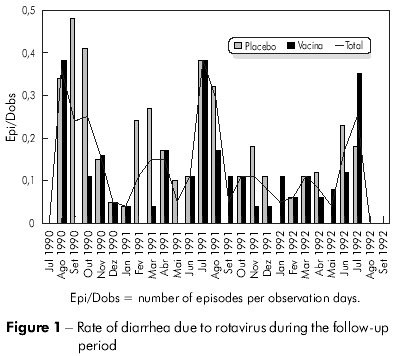
The incidence analysis (number of episodes /observation days) of the 91 diarrheal episodes caused by rotavirus in the 3 years of follow-up revealed significantly higher rates in the months of June through September (p = 0.007) when compared to the other months in the same study period. This high incidence period corresponds to the driest months of the year (Figure 1).
With regard to the efficacy of RRV-TV related to clinical severity, a protective effect was observed for almost all parameters except fever, against which vaccination did not offer protection (Table 2).
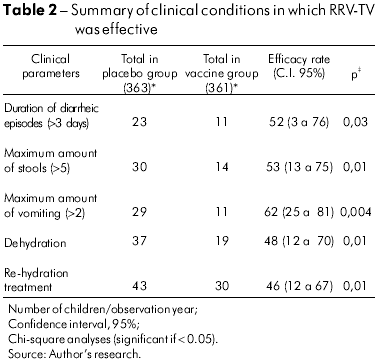
During the two years of this study, the relative vaccine efficacy in relation to the maximum number of stools greater than five was significant in all episodes: 53% (p = 0.01%). With regard to the duration of vomiting, full protection was observed (1 00%, p = 0.03) in the second year of follow-up, but only when G2 serotype-related diarrheal cases were considered.
During the two years of follow-up, significant RRV-TV efficacy was observed with regard to dehydration in all diarrheal episodes, similarly to what had been noted concerning the need for re-hydration. The protective efficacy rates were 48% (p = 0.01) and 46% (p = 0.01).
The efficacy analysis of RRV-TV in all of the diarrheal episodes, according to clinical severity scores (Table 3), showed that this vaccine did not protect against episodes graded between 0 and 8 and induced moderate protection against all cases with clinical scores between 9 and 14. In relation to the very severe cases (score > 14), the RRV-TV conferred significant levels of protection (75%, p = 0.02).
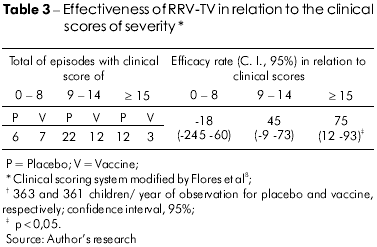
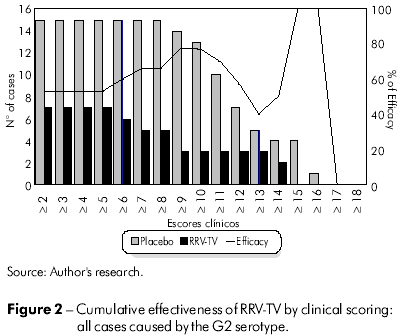
Figure 2 exhibits the vaccine efficacy against all cases associated with the G2 serotype. However, it was observed that the curve does not exhibit interval variations for clinical scores from ≥ 2 to ≥ 5 (53%), with a rise from ≥ 6 until ≥ 10 (60% to 77%, respectively), and there was a lower level of protection, of 40%, against episodes graded ≥ 13. For scores ≥14 through ≥ 16, the efficacy curve starts rising again, reaching a maximum level of 100% against cases of greatest severity.
DISCUSSION
Rotaviruses are widespread worldwide. In the current study, a profile similar to the one described for tropical regions was identified. However, there was a difference when compared to previous observations that recorded the occurrence of rotavirus episodes throughout the year22. It should be pointed out that such a profile can vary over time, according to recent analyses conducted by the CDC. These investigations correlated the seasonal pattern of infections with the large-scale usage of vaccines, which is Brazil's current strategy after the recent introduction of the monovalent vaccine ( Rotarix®) into the Programa Nacional de Imunizações - National Immunization Program (PNI]4.
Regarding its efficacy against the prevalent serotypes, the current findings demonstrate that the protection conferred by the vaccine should be considered in light of the prevalent circulating serotypes in the region. In particular, retrospective studies conducted from 1980 to 1992 by Linhares et al17 in the urban populations of this region revealed a greater prevalence of serotype 1, primarily during the first year of life, followed by serotype 2. In our study, variations in the efficacy curve of RRV-TV were observed in relation to all cases associated with the G2 serotype, which might reflect periods of greater and lesser circulation of this serotype in the region. This variation also demonstrated the significant protection of the vaccine in episodes with clinical scores ≥ 14, which are defined as the most severe. Nevertheless, the low absolute numbers that are related to the highest scores can correspond to apparently more relevant efficacy rates.
It should be noted that in this analysis it was not possible to show protection against the serotypes that are considered emergent (for example: G9], which constitute a major challenge in the currently licensed vaccines. Because of the broad temporal and geographic variation of the serotypes, the emergence of new serotypes of epidemiological relevance, and the possible co-circulation of different serotypes in the same region, the routine monitoring of the circulating serotypes is strongly recommended, especially in post-licensing studies21.
The risk of intussusception (IS] associated with the Rotashield vaccine has provoked ample discussion and detailed revision of the recorded cases. Preliminary results have identified the age at the start of vaccination as the risk factor for the occurrence of IS2. The experience with Rotashield in the USA followed the recommendation of the first dose at 2 months of age followed by doses at 4 and 6 months of age. However, subsequent re-evaluations have demonstrated that 61% of the children initiated the vaccination at 3 months of age or older and that 80% of IS cases associated with the vaccine occurred in this age group, in which there is a greater incidence of this condition in the population in general2. Thus, the recommendation that, in the clinical trials with vaccines, the vaccination scheme be reduced to two doses with the first administered in the neonatal period seems to be warranted. Moreover, proposed early administration of first vaccine dose may provide additional benefits with regard to possibly milder adverse events, such as fever, commonly associated with the tetravalent vaccine28.
There is a great deal of evidence indicating that a suitable vaccine against rotavirus must ensure protection against the most severe diarrheal cases, which are responsible for high rates of childhood mortality throughout the world. In this study, significant protection by the vaccine was observed in five out of seven clinical conditions that indicate severity, such as: the duration of diarrhea, the maximum number of stools and vomiting, dehydration and the need for rehydration. Similar clinical conditions have been identified in previous studies, such as those conducted in our region by Linhares et al.13, in which vomiting and the number of liquid stools and dehydration associated with the rotavirus-related diarrheal episodes showed greater severity in comparison to other etiologies. These protection rates were comparable to those obtained in studies conducted in other regions of the world with more concentrated formulations of RRV-TV10,24. Analyses of signals and symptoms grouped together, based on the clinical scores, demonstrated the selective protection of the vaccine, and particular protection against episodes classified as very severe (75%, p = 0.02), which is in agreement with the main objective of rotavirus vaccination strategies15. These findings could be confirmed in more recent studies that led to the recent licensing of RotaTeq® and Rotarix® vaccines1929.
The clinical protection conferred by the tetravalent vaccine in our study was similar to that yielded in other clinical trials conducted in Latin America and Finland and was confirmed in two post-licensing studies of Rotashield®, which reported 100% protection in cases of higher severity when using the three-dose scheme23,26. The potential protection of the vaccine, if introduced on a large scale, was clearly evidenced in the study conducted by Tate et al27 in the USA, based on a retrospective analysis involving a cohort of children vaccinated with Rotashield®, from 1999 to 2000. The vaccine was shown to be highly efficacious in the prevention of hospitalizations and emergency room visits due to all-cause gastroenteritis, thus strengthening previous estimates of the likely impact of a large-scale vaccination program.
Although the vaccine is no longer recommended for routine use, the findings with RRV-TV established the basis for the subsequent strategies that led to the current worldwide optimistic scenario with the oral licensed vaccines30.
CONCLUSION
The results obtained in our analysis confirm the significant protection of RRV-TV against the most severe diarrheal episodes and the most prevalent serotypes at the time this study was conducted.
The findings of the present re-analysis add to the data from studies of RRV-TV conducted in Latin America and the United States and constitute the basis for new recommendations toward the development of clinical trials of the new generation vaccines. This information will also aid in the global effort to expedite the introduction of new vaccines against rotavirus in the immunization programs of countries that have the greatest rates of rotavirus-related infant mortality, with an emphasis on India, Indonesia and China.
ACKNOWLEDGEMENTS
We would like to thank Mrs. Maria José Mateus (Evandro Chagas Institute Library) for her services in accessing scientific articles.
REFERENCES
1 Bernstein DI. Rotavirus overview. Pediatr Infect Dis J. 2009 Mar;28(3 Suppl):S50-3. [ Links ]
2 Bines J. Rotavirus vaccines and intussusception risk. Curr Opin Gastroenterol. 2005 Jan;21(1):20-5. [ Links ]
3 Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute, nonbacterial gastroenteritis. Lancet. 1973 Dec;2(7841):1281-3.
4 Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2009 Feb;58(2):1-26. [ Links ]
5 Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1999 Mar;48(2):1-20. [ Links ]
6 Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999 Nov;48(43):1007. [ Links ]
7 Constenla DO, Linhares AC, Rheingans RD, Antil LR, Waldman EA, Silva LJ. Economic impact of a rotavirus vaccine in Brazil. J Health Popul Nutr. 2008 Dec;26(4):388-96. [ Links ]
8 Flores J, Perez-Schael I, Gonzalez M, Garcia D, Perez M, Daoud N, et al. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr;1(8538):882-4. [ Links ]
9 Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, Steele AD. Rotavirus vaccines: targeting the developing world. J Infect Dis. 2005 Sep;192 Suppl 1:S160-6. DOI: 10.1086/431504 [ Links ]
10 Joensuu J, Koskenniemi E, Pang XL, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997 Oct;350(9086):1205-9. [ Links ]
11 Kapikian AZ, Chanock RM. Rotaviruses. In: Fields BN, Knipe DM, editors. Fields virology. 3rd ed. Philadelphia: Raven Publishers; 1996. Vol. 2, p. 1657-708.
12 Leite JPG, Carvalho-Costa FA, Linhares AC. Group A rotavirus genotypes and the ongoing Brazilian experience - A Review. Mem Inst Oswaldo Cruz. 2008 Dec;103(8):745-53. DOI: 10.1590/S0074-02762008000800001 [ Links ]
13 Linhares AC, Gabbay YB, Freitas RB, Travassos da Rosa ES, Mascarenhas JD, Loureiro EC. Longitudinal study of rotavirus infection among children from Belém, Brazil. Epidemiol Infect. 1989 Feb;102(1):129-45. [ Links ]
14 Linhares AC, Gabbay YB, Mascarenhas JDP, Freitas RB, Oliveira CS, Bellesi N, et al. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belém, Brazil. Bull World Health Organ. 1996;74(5):491-500. [ Links ]
15 Linhares AC, Lanata CF, Hausdorff WP, Gabbay YB, Black RE. Reappraisal of the Peruvian and Brazilian lower-titer tetravalent rhesus-human reassortant rotavirus vaccine efficacy trials: analysis by severity of diarrhea. Pediatr Infect Dis J. 1999 Nov;18(11):1001-6. [ Links ]
16 Linhares AC, Monção HC, Gabbay YB, Araújo VL, Serruya AC, Loureiro ECB. Acute diarrhoea associated with rotavirus among children living in Belém, Brazil. Trans R Soc Trop Med Hyg. 1983;77(3):384-90. [ Links ]
17 Linhares AC, Moura JM, Gabbay YB, Mendes PS, Mascarenhas JD, Azevedo RC. Rotavirus serotypes and electrophoretypes among children attending three pediatric hospitals in Belém, Brazil. J Trop Pediatr. 1993 Jun;39(3):137-41. [ Links ]
18 Linhares AC, Pinheiro FP, Schmetz C, Muller G, Peters D. Duovírus (rotavírus) em Belém do Pará, Brasil (nota prévia). Rev Inst Med Trop Sao Paulo. 1977 jul-ago;19(4):278-9.
19 Linhares AC, Velázquez RR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, doubleblind, placebo-controlled phase III study. Lancet. 2008 Apr;371(9619):1181-9. [ Links ]
20 Murphy BR, Morens DM, Simonsen L, Chanock RM, La Montagne JR, Kapikian AZ. Reappraisal of the association of intussusception with the licensed live rotavirus vaccine challenges initial conclusions. J Infect Dis. 2003 Apr;187(8):1301-8. [ Links ]
21 O'Ryan M. The ever-changing landscape of rotavirus serotypes. Pediatr Infect Dis J. 2009 Mar;28(3 Suppl):S60-2. [ Links ]
22 Pereira HG, Linhares AC, Candeias JAN, Glass RI. National laboratory surveillance of viral agents of gastroenteritis in Brazil. Bull Pan Am Health Organ. 1993;27(3):224-33. [ Links ]
23 Pérez Mato S, Perrin K, Scardino D, Bégué RE. Evaluation of rotavirus vaccine effectiveness in a pediatric group practice. Am J Epidemiol. 2002 Dec;156(11):1049-55. [ Links ]
24 Pérez-Schael I, Guntiñas MJ, Pérez M, Pagone V, Rojas AM, González R, et al. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997 Oct;337(17):1181-7. [ Links ]
25 Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005 Sep;192 Suppl 1:S36-43. [ Links ]
26 Staat MA, Cortese MM, Bresee JS, Bégué RE, Vitek C, Rhodes P, et al. Rhesus rotavirus vaccine effectiveness and factors associated with receipt of vaccine. Pediatr Infect Dis J. 2006 Nov;25(11):1013-8. [ Links ]
27 Tate JE, Curns AT, Cortese MM, Weintraub ES, Hambidge S, Zangwill KM, et al. Burden of acute gastroenteritis hospitalizations and emergency department visits in US children that is potentially preventable by rotavirus vaccination: a probe study using the now-withdrawn RotaShield vaccine. Pediatrics. 2009 Mar;123(3):744-9. [ Links ]
28 Vesikari T, Karvonen A, Forrest BD, Hoshino Y, Chanock RM, Kapikian AZ. Neonatal administration of rhesus rotavirus tetravalent vaccine. Pediatr Infect Dis J. 2006 Feb;25(2):118-22. [ Links ]
29 Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006 Jan;354(1):23-33. [ Links ]
30 World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007 Aug;82(32):285-95.
 Correspondência/Correspondence/Correspondencia:
Correspondência/Correspondence/Correspondencia:
Consuelo Silva de Oliveira
Instituto Evandro Chagas, Seção de Virologia
Rodovia BR316, km 7, s/no, Levilândia
CEP: 67030-000 Ananindeua-Pará-Brasil
E-mail:consuelooliveira@iec.pa.gov.br
Recebido em/Received/Recibido en: 31/07/2009
Aceito em/Accepted/Aceito en: 24/09/2009











 text in
text in 

