Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.1 no.3 Ananindeua Sept. 2010
http://dx.doi.org/10.5123/S2176-62232010000300008
ORIGINAL ARTICLE
Viral hepatitis, helminthiasis and protozoan disease in neotropical primates raised in captivity: potentially zoonotic affections with fecal-oral transmission
Washington Luiz Assunção PereiraI; Katiany Rocha GaloII; Klena Sarges Marruaz da SilvaIII; Manoel do Carmo Pereira SoaresIII; Max Moreira AlvesIII
IInstituto da Saúde e Produção
Animal, Universidade Federal Rural da Amazônia, Belém, Pará, Brasil
IIDivisão de Vigilância Sanitária da Prefeitura Municipal
de Vigia de Nazaré, Vigia de Nazaré, Pará, Brasil
IIIInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
Brazilian environmental legislation does not allow non-human primates to be raised in captivity. However, this remains a common practice in the Amazon region, and the close proximity of animals and humans facilitates the transmission of zoonotic diseases. The goal of the present study was to evaluate the presence of zoonotic agents in household-raised non-human primates. We analyzed animals donated or apprehended by Brazil's Environmental Police Battalion and/or the Instituto Brasileiro de Meio Ambiente e Recursos Naturais Renováveis in Pará State, Brazil, and sent to the Centro Nacional de Primatas. Blood samples taken from 25 animals during the quarantine period were subjected to serum and antibody tests for viral hepatitis (types A, B and E) at the Instituto Evandro Chagas. Parasitological analysis of fecal material was performed on 29 animals using direct examination and the Willis and Hoffman methods. None of the animals tested positive for anti-hepatitis B or anti-hepatitis E virus antibodies, but 12% were positive for total anti-hepatitis A antibodies. In addition, parasitological studies showed that 48.2% of the animals had parasites with zoonotic potential. Strongyloides stercoralis was observed in 17.2%, but this parasite was associated with Giardia lamblia in only 3.4% of the samples. Giardia lamblia and Entamoeba histolytica were detected in 3.4% and 10.3% of the samples, respectively. All of the pathogens described in this study are transmitted through the fecal-oral route. Therefore, we concluded that non-human primates should not be raised in captivity, and this practice should be addressed as an important public health concern.
Keywords: Zoonoses; Intestinal Diseases, Parasitic; Hepatitis Viruses; Primates.
INTRODUCTION
The rise and spread of zoonoses is an important public health and safety issue, especially when close contact occurs between humans and animals. Non-human primates in particular expose humans to a high risk of disease transmission due to their close taxonomic position and region of origin1.
The likelihood of disease transmission by these animals is primarily determined by the degree of physical proximity to humans and by the handling of organic material when animals are in captivity. In northern Brazil, it is a common regional and cultural practice to raise primates as pets2. The environments in which non-human primates are raised often lack minimum sanitary conditions, which facilitates the spread of zoonotic diseases as humans come into contact with body fluids and excrement produced by the animals3.
Non-human primates can transmit many zoonotic agents3, including protozoa such as Toxoplasma gondii, Giardia lamblia and Entamoeba histolytica, intestinal worms, heartworms, hookworms, strongyles (which spreads via the feces of its host) and Leishmania (a mosquito-borne disease).
Other groups of etiologic agents, such as viruses, can also cause zoonotic disease, some of which are extremely serious and dangerous, such as rabies and viral hepatitis. Hepatitis A and E can be transmitted to humans by nonhuman primates, primarily through the fecal-oral route. Animals carrying the virus and raised at home can contaminate the environment, food and, consequently, people4. Another virus of great importance due to its lethality and transmissibility by non-human primates is Herpesvirus simiae. This virus can be transmitted by indirect contact through saliva and aerosols, with a higher risk of transmission in cases of animal bites5.
The aim of this study was to evaluate the sanitary conditions of non-human primates raised in captivity in Pará state, Brazil, by testing the animals for zoonotic diseases, with a particular focus on viral hepatitis and intestinal parasites that can be transmitted to humans.
MATERIALS AND METHODS
We analyzed 29 non-human primates that were donated or apprehended by the Environmental Police and/or the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA/PA) in households located in the State of Pará. Animals were allocated to the National Primates Center (CENP/IEC/SVS/MS), where they remained in quarantine before selection for the study.
During the quarantine period, blood samples for serological analysis were collected by femoral vein puncture, and fecal samples were collected for general parasitological analysis. Blood cell count and tuberculin tests were performed according to established protocols.
Fecal analysis of 29 animals from different species was performed in the parasitology laboratory at CENP/IEC/SVS/MS. The animals analyzed belonged to the following species: Cebus apella (n = 20), Saguinus niger (n = 2), Callicebus moloch (n = 1), Saimiri sciureus (n = 4), Saguinus fuscicolis (n = 1) and Cebus nigrivittatus (n = 1). Fecal analyses were performed using the Willis and Hoffman methods and by direct examination. Briefly, direct examination was performed by placing a small amount of feces homogenized with a mixture of Lugol's solution and saline on a microscope slide, which was observed using an optical microscope to detect the presence of protozoa (especially trophozoites).
To determine the seroprevalence of hepatitis B (HBV), hepatitis A (HAV) and hepatitis E (HEV) antibodies, 25 samples of blood serum were analyzed. The collected sera were stored at -20o C and thawed at the time of processing. Serological testing was performed using enzyme immunoassays for markers of HBV (HBsAg and anti-HBc), HAV (anti-HAV) and HEV (anti-HEV). Positive serum samples were subjected to quantitative tests for antigens and/or antibodies. These analyses were performed in the laboratory of the Section of Hepatology (SAHEP) of the Evandro Chagas Institute (IEC).
RESULTS
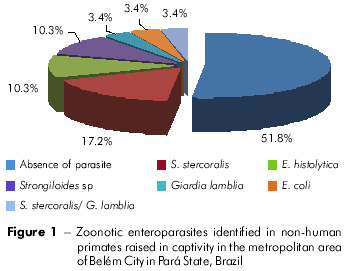
Of the 29 stool samples analyzed, 48.2% tested positive for parasites with zoonotic potential. The helminth Strongyloides stercoralis was found in 17.2% of samples and was associated with the parasite Giardia lamblia in 3.4% of samples (Figure 1). It is noteworthy that 100% of Saimiri scuireus stool samples contained this helminth, which was found in only 4.5% of Cebus apella samples. In addition, 10.3% of samples tested positive for Entamoeba histolytica and 3.4% contained Entamoeba coli. Viral hepatitis analysis showed that 12% of samples were positive for specific anti-HAV antibodies, but with no IgM and IgG specificity.
DISCUSSION
In this study, we found that Strongyloides stercoralis (17.2% positive samples) can be hosted by a variety of species of non-human primates. The presence of Strongyloides in monkeys deserves special attention not only due to the danger of natural infection of other animals, but also because the parasite has the potential to infect humans6. Strongyloidiasis, which is caused by an intestinal nematode, occurs asymptomatically in most infected individuals. However, it is considered of great medical importance because it results in hyperinfection and dissemination in immunodeficient patients7,8,9. Strongyloides hyperinfestation was previously reported in marmosets10, causing severe damage mainly to the lungs and liver, with chronic inflammation, fibrosis and widespread bleeding, a progression very similar to what is observed in humans6. In addition, Strongyloidiasis can lead to death, especially in malnourished animals.
Giardia lamblia was identified in only 3.4% of analyzed samples and showed no host specificity. Because it can parasitize both humans and animals, it is considered to be a potential zoonotic agent. Infected non-human primates are usually asymptomatic and are important sources of infection11,12. Entamoeba histolytica, which was the most common pathogenic parasite in the samples analyzed in this study (10.3%), is responsible for many deaths in Neotropical primates. However, Old World apes are asymptomatic carriers of Entamoeba infection2. Therefore, natural infection of Neotropical monkeys presents more severe pathogenic effects compared to those observed in Old World monkeys13.
Most of the infections with intestinal parasites in the studied animals were asymptomatic, which highlights the need for prophylactic deworming of animals in captivity because they may be able to transmit the disease to both other animals and humans.
Hepatitis A infection is usually asymptomatic in non-human primates and children; however, when clinical manifestations are present, they are nonspecific and range from mild disease symptoms to death. The disease spreads primarily via the fecal-oral route or by ingestion of the virus from contaminated food or objects. The virus replicates in the liver and is eliminated in the feces14,4.
The results obtained in this study for viral hepatitis show the importance of this zoonosis and confirm that nonhuman primates are hosts of the Hepatitis A virus. Raising these animals at home favors human infection because monkeys do not have hygienic habits like humans and often contaminate their hands and body with their own stool. In these conditions, direct and intimate interactions between monkeys and humans facilitate the transmission of this virus.
CONCLUSION
We have shown that non-human primates host of a variety of parasites with zoonotic potential, including Giardia lamblia, Entamoeba histolytica and Strongyloides stercoralis. These results justify the need for better health surveillance to curb household captivity and improve human health by reducing exposure to zoonotic agents. Because zoonoses can be transmitted from animals to humans and vice versa, humans exposed to direct contact with animals in household environments are at high risk for viral diseases such as hepatitis A.
In summary, non-human primates are reservoirs for a variety of infectious agents that present severe implications for public health. However, despite the existence of Brazilian legislation prohibiting the raising of wild animals in household captivity, the number of apprehended nonhuman primates in captivity remains high, reflecting the public's ignorance about the risk of zoonotic transmission.
ACKNOWLEDGEMENTS
National Primate Center (CENP/IEC/SVS/MS) and the Scholarship Program for Undergraduate Research - PIBIC/CNPq/UFRA.
REFERENCES
1 Organização Mundial de Saúde Animal. Código Sanitário de Animais Terrestres. Zoonoses transmissíveis por primatas não humanos. São Paulo: OIE; 2008.
2 Ribeiro ASS, Palha MDC, Tourinho MM, Whiteman CW, Silva ASL. Utilização dos recursos naturais por comunidades humanas do Parque Ecoturístico do Guamá, Belém, Pará. Acta Amazônica. 2007;37:235-40. [ Links ]
3 Diniz LSM. Primatas em cativeiro: manejo e problemas veterinários. São Paulo: ícone; 1997. 196 p.
4 Souza Junior JC. Perfil sanitário de bugios ruivos, Alouatta guariba clamitans (Cabrera, 1940) (Primates: Atelidae): um estudo com animais recepcionados e mantidos em perímetro urbano no município de Indaial, Santa Catarina - Brasil [dissertação]. Florianopólis (SC): Universidade Federal de Santa Catarina, Programa de Pós-Graduação em Saúde Pública; 2007.
5 Florence G, Bretigny F. Herpes B: importante zoonose transmitida pelos macacos. A Hora vet. 1998;17.
6 Fiennes R. Pathology of Simian primates. New York: Karger; 1972.
7 Neves DP. Parasitologia Humana. 9. ed. Rio de Janeiro: Ateneu; 1997.
8 Harcourt-Webster JN, Scaraville F, Darwish AH. Strongyloides stercoralis hyperinfection in an HIV positive patient. J Clin Path. 1991 Apr;44(4):346-8. Doi:10.1136/jcp.44.4.346 [ Links ]
9 Sudré AP, Macedo HW, Peralta RHS, Peralta JM. Diagnóstico da estrongiloidíase humana: importância e técnicas. Rev de Patol Trop. 2006;35(3):173-84. [ Links ]
10 Pereira WLA, Cardoso AMC, Benigno RNM, Almeida VT. Hiperinfestação por Strongyloides sp. em calitriquídeos mantidos em cativeiro. In: 13o Congresso Brasileiro de Patologia Veterinária; 2007; Campo Grande. Campo Grande: Universidade de São Paulo; 2007.
11 Falchi RLR. Contaminação por protozoários potencialmente patogênicos ao homem na água de diferentes pontos da Laguna dos Patos, Rio Grande, RS [dissertação]. Pelotas (RS): Universidade Federal de Pelotas, Programa de Pós-Graduação em Parasitologia; 2006. [ Links ]
12 Silva RG, Andrade MCR, Gonçalves MAB. Infecção por Entamoeba histolytica em primatas não humanos mantidos em cativeiro. Rev Bras Med Vet. 2000;22:37-41.
13 Harper JS, Genta RM, Gam A, London WT, Neva FA. Experimental Disseminated Strongyloidiasis in Erythrocebus patas. Am J Trop Med and Hyg. 1984 May;33:431-43. [ Links ]
14 Assis SB, Valente JG, Fontes CJF, Gaspar AMC, Souto FJD. Prevalência de marcadores do vírus da hepatite B em crianças de 3 a 9 anos em um município da Amazônia brasileira. Rev Panam Salud Publica/Pan Am J Public Health. 2004;15(1):26-34. [ Links ]
15 Stezer AP, Picinatti A, Gaspar AMC. Soroprevalência de anticorpos anti-hepatite A em primatas neotropicais mantidos em cativeiro. Anais do 6o Congresso e 11o Encontro da Associação Brasileira de Veterinários de Animais Selvagens; 2001; Guarapari. Guarapari: Associação Brasileira de Veterinários de Animais Selvagens; 2001. p. 6.
 Correspondência
/ Correspondence/ Correspondencia:
Correspondência
/ Correspondence/ Correspondencia:
Washington Luiz Assunção Pereira
Instituto da Saúde e Produção Animal,
Universidade Federal
Rural da Amazônia
Caixa postal: 917
CEP: 66077-530
Belém-Pará-Brasil
Tel./Fax: (91)3210-5137
E-mail:wkarton@terra.com.br
Recebido em / Received / Recibido en: 11/6/2010/
Aceito
em / Accepted / Aceito en: 9/8/2010











 text in
text in 

