Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.1 n.4 Ananindeua dez. 2010
http://dx.doi.org/10.5123/S2176-62232010000400011
ORIGINAL ARTICLE
Characterization of antimicrobial resistance of samples of Shigella spp. isolated in Belém, Pará State, Brazil (1990-2000)
Flávia Corrêa BastosI; Edvaldo Carlos Brito LoureiroII
IInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil. Universidade Federal do Pará, Belém, Pará, Brasil
IIInstituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
We evaluated the antimicrobial resistance of Shigella spp. samples isolated from inhabitants of the City of Belém, Pará State, Brazil, aged between 6 months and 81 years, from 1990 to 2000. We analyzed 50 samples of Shigella spp. that had been identified in the Laboratory of Enterobacterial Infections at the Bacteriology and Mycology Section of the Instituto Evandro Chagas and stored in its bacteria bank. After the phenotypic characterization, 32 (64%) isolates were identified as S. flexneri and 18 (36%) as S. sonnei. Resistance to cephalothin, cefazolin, cefuroxime, cefuroxime axetil and tobramycin was observed in 100% of the samples. However, they presented 100% susceptibility to cefpodoxime, ceftriaxone, levofloxacin and norfloxacin. Of the samples, 2% showed resistance to ticarcillin/clavulanic acid, 8% to cefoxitin and 44% to ticarcillin. The results showed a marked resistance to various antibiotics in these enterobacteria that cause serious infections in humans.
Keywords: Diarrhea; Shigella; Drug Resistance, Microbial.
INTRODUCTION
Shigellosis is recognized by the World Health Organization (WHO) as a major global public health problem. This disease is caused by four species of the genus Shigella and is among the leading causes of diarrhea in children. Shigellosis occurs mainly in developing countries but is also responsible for diarrhea in developed countries. Moreover, its transmission mode is associated with poor sanitation and/or poor personal hygiene1,2.
It is estimated that Shigella is responsible for approximately 500,000 cases of diarrhea in the United States annually, causing about 6,231 cases of hospitalizations and 70 deaths. The genus Shigella was ranked third among foodborne pathogens in 2004, according to the Foodborne Diseases Active Surveillance Network (Foodnet) of the Centers for Disease Control and Prevention3.
In addition to several other studies performed in Brazil, Peirnano et al4 analyzed Shigella isolates from the period of 1999 to 2004 and reported the prevalence of S. flexneri (52.7%) over S. sonnei (4.2%). The highest rates of isolation of this enteric pathogen were observed in the southeastern region (39%), followed by the northeastern region (34%) and a slight occurrence in the Southern region (3%) of the country.
The uncontrolled use of antibiotics allows for the selection of resistant microorganisms, thereby reducing the effectiveness of the medications. The establishment of a permanent system of laboratory analysis that shows infectious processes and examines the level of bacterial resistance of samples isolated from inpatients and outpatients is an essential measure towards resistance control5.
The analysis of antibiotic resistance has been widely applied and combined with other methods that aim to characterize several pathogens, including the genus Shigella. Such characterization has been carried out in Brazil2 and other countries, such as Chile1, South Africa6, Bangladesh7 and Belgium8.
In Belém, Pará State, little information is known about the Shigella spp. resistance to antibiotics. In this study, we characterize the resistance of Shigella spp. samples isolated from patients with acute diarrhea between 1990 and 2000.
MATERIALS AND METHODS
This study analyzed a total of 50 samples (viables) of Shigella spp. isolated between 1990 and 2000. All of the samples were isolated from stool cultures from patients with clinically acute diarrhea, including males and females with ages ranging from 0 to 81 years.
The isolation of enteropathogens was performed by seeding the suspension of fecal material on selective media indicators (MacConkey agar - MC, Salmonella Shigella agar - SS) (Difco™) and in enrichment medium (Selenite Cystine Broth) (Difco™). The suspected colonies were then inoculated in screening medium (Triple sugar iron - TSI) (Difco™). Afterwards, the biochemical characterization was performed in an automated system (Vitek 2 Compact - bioMerieux™) using the GN Test card. The serological identification followed the recommendations of Kauffmann9 and Ewing10 using the antiserum (Bio-Rad Laboratories) relative to Shigella flexneri (Group A), Shigella boydii (Group B), Shigella dysenteriae (Group C) and Shigella sonnei (Group D).
To characterize the resistance of the Shigella spp. samples, the automated system Vitek 2 Compact - bioMerieux™and the AST-GN05 card were used.
RESULTS
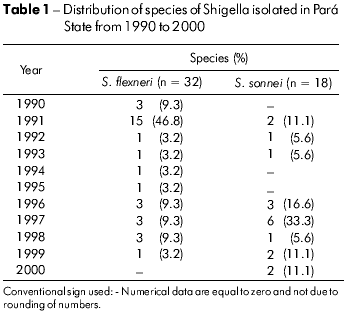
Among the 50 samples of Shigella that were isolated, 32 (64%) were identified as S. flexneri and 18 (36%) as S. sonnei, regardless of the isolation period and the age of the patients. The S. dysenteriae and S. boydii species were not detected in the samples. S. flexneri prevailed in 1991 (46.8%), and its occurrence was reduced in the following years. S. sonnei showed similar rates throughout the period of ten years (Table 1).
The resistance of Shigella spp. samples to the antibiotics tested is shown in table 2. All of the samples showed resistance to cephalothin, cefazolin, cefuroxime, cefuroxime axetil and tobramycin. However, 100% of the strains were also susceptible to cefpodoxime, ceftriaxone, levofloxacin and norfloxacin. All of the strains showed sensitivity to all of the antibiotics tested.
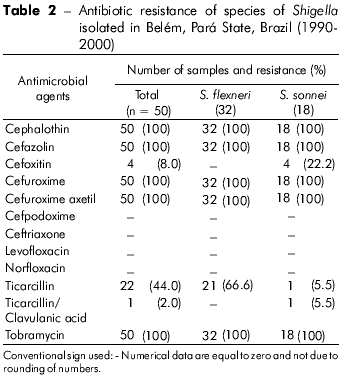
Considering all of the samples analyzed, 22 (44%) samples were resistant to ticarcillin: 21 were S. flexneri, and one was S. sonnei. With respect to the antibiotic ticarcillin/clavulanic acid, only one sample (2%; S. sonnei) was resistant, and only four (8%) samples (S. sonnei) showed resistance to cefoxitin.
DISCUSSION
Shigella spp. is one of the enterobacteria most commonly isolated from the enteric system all over Brazil11,12,13, including the northern region.
Regarding the epidemiologic studies of shigellosis in the northern region , it is important to evaluate the performance of antimicrobial susceptibility through permanent analyses in addition to considering the bacteriological aspects in the characterization of prevalent species14,15,16.
The analysis of the resistance of the Shigella spp. samples indicated that these enterobacteria are more difficult to treat with cephalothin, cefazolin, cefuroxime and cefuroxime axetil because all of the strains were resistant to these antibiotics. In contrast, the results showed promise for other options for treatment, such as cefpodoxime, ceftriaxone, levofloxacin and norfloxacin, because resistance to these antibiotics was not detected. Other treatment options were also suggested, such as using cefoxitin and ticarcillin because of low resistance (8% and 44%, respectively; Table 1).
The data shown in this study are consistent with data shown for other states of Brazil4,13,17,18 and other countries from Central America, North America, Africa, Middle East and India as described by Drews et al., 201019.
CONCLUSION
The results of this study have demonstrated that the continuous evaluation of antibiotic resistance of Shigella spp. samples is extremely important for determining the appropriate treatment for shigellosis, a disease that has great importance in public health.
ACKNOWLEDGEMENTS
We thank the following technicians from the Bacteriology and Mycology Section of the Instituto Evandro Chagas for their technical support: Madalena Lobato, Maria Odete Arouche, José Caetano Silva and Raimundo Nonato Araújo.
REFERENCES
1 Fullä N, Prado V, Duron C, Lagos R, Levine MM. Surveillance for antimicrobial resistance profiles among Shigella species isolated from a semirural community in the northern administrative area of Santiago, Chile. Am J Trop Med Hyg. 2005 Jun;72(6):851-4. [Link]
2 Penatti MP, Hollanda LM, Nakazato G, Campos TA, Lancellotti M, Angellini M, et al. Epidemiological characterization of resistance and PCR typing of Shigella flexneri and Shigella sonnei strains isolated from bacillary dysentery cases in Southeast Brazil. Braz J Med Biol Res. 2007 Nov;40(2):249-58. Doi: 10.1590/S0100-879X2006005000069 [Link]
3 Center for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System for Enteric Bacteria: human isolates final report 2004. Atlanta: Center for Disease Control and Prevention (US), Department of Health and Human Services; 2007. [Link]
4 Peirano G, Souza FS, Rodrigues DP. Shigella Study Group. Frequency of serovars and antimicrobial resistance in Shigella spp. from Brazil. Mem Inst Oswaldo Cruz. 2006 May;101(3):245-50. Doi: 10.1590/S0074-02762006000300003 [Link]
5 Niyogi SK. Increasing antimicrobial resistance-an emerging problem in the treatment of shigellosis. Clin Microbiol Infect. 2007 Oct;13(12):1141-3. DOI: 10.1111/j.1469-0691.2007.01829.x [Link]
6 Yah CS. Plasmid-encoded multidrug resistance: a case study of Salmonella and Shigella from enteric diarrhea sources among humans. Biol Res. 2010 May;43:141-8.
7 Rahman M, Shoma S, Rashid H, El Arifeen S, Baqui AH, Siddique AK, et al. Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. J Health Popul Nutr. 2007 Jun;25(2):158-67. [Link]
8 Vrints M, Mairiaux E, Meervenne V, Collard JM, Bertrand S. Surveillance of antibiotic susceptibility patterns among Shigella sonnei strains isolated in Belgium during the 18-year period 1990 to 2007. J Clin Microbiol. 2009 May;47(5):1379-85.
9 Ka uffm ann F. E nterobacteria ceae. 2nd E d. Copenhagen: Munksgaard; 1954.
10 Ewing WH. Edward and Ewing's identification of Enterobacteriaceae. 4th ed. New York: Elsevier; 1986. 536 p.
11 Aranda KR, Fabbricotti SH, Fagundes-Neto U, Scaletsky IC. Single multiplex assay to identify simultaneously enteroaggregative, enterotoxigenic, enteroinvasive and Shiga toxin-producing Escherichia coli strains in Brazilian children. FEMS Microbiol Lett. 2007 Feb;267(2):145-50. DOI: 10.1111/j.1574-6968.2006.00580.x [Link]
12 Diniz-Santos DR, Santana JS, Barretto JR, Andrade MGM, Silva LR. Epidemiological and microbiological aspects of acute bacterial diarrhea in children from Salvador, Bahia, Brazil. Braz J Infect Dis. 2005 Jan;9(1):77-83. Doi: 10.1590/S1413-86702005000100013 [Link]
13 Loureiro CBL, Souza CO, Sousa EV, Santos DV, Rocha DCC, Ramos FLP, et al. Detecção de bactérias enteropatogênicas e enteroparasitas em pacientes com diarréia aguda em Juruti, Pará, Brasil. Rev Pan-Amaz Saude. 2010 jan-mar;1(1):143-8. Doi: 10.5123/S2176-62232010000100020 [Link ]
14 Orlandi PP, Magalhães GF, Matos NB, Silva T, Penatti M, Nogueira PA, et al. Etiology of diarrheal infections in children of Porto Velho (Rondonia, Western Amazon region, Brazil). Braz J Med Biol Res. 2006 Apr;39(4):507-17. Doi: 10.1590/S0100-879X2006000400011 [Link]
15 Maroja RC, Lowery WD. Estudos sobre diarréias agudas II. Frequência de Shigella e Salmonella nos casos de diarréias agudas em Santarém-Pará. Rev Fund SESP 1956 dez;8(2):585-9. [Link]
16 Linhares AC, Monção HC, Gabbay YB, Araújo VL, Serruya AC, Loureiro ECB. Acute diarrhoea associated with rotavirus among children living in Belém, Brazil. Trans R Soc Trop Med Hyg. 1983;77(3):384-90. [Link]
17 Oplustil CP, Nunes R, Mendes C, Resistnet group. Multicenter evaluation of resistance patterns of Klebsiella pneumoniae, Escherichia coli, Salmonella spp and Shigella spp isolated from clinical specimens in Brazil: resistnet surveillance program. Braz J Infect Dis. 2001 Feb;5(1):8-12. Doi: 10.1590/S1413-86702001000100002 [Link ]
18 Silva T, Nogueira PA, Magalhães GF, Grava AF, Silva LHP, Orlandi PP. Characterization of Shigella spp. by antimicrobial resistance and PCR detection of ipa genes in an infantile population from Porto Velho (Western Amazon region), Brazil. Mem Inst Oswaldo Cruz. 2008 Nov;103(7):731-3. Doi: 10.1590/S0074-02762008000700017 [Link]
19 Drews SJ, Lau C, Andersen M, Ferrato C, Simmonds K, Stafford L, et al. Laboratory based surveillance of travel-related Shigella sonnei and Shigella flexneri in Alberta from 2002 to 2007. Global Health. 2010 Nov;6:20. Doi:10.1186/1744-8603-6-20 [Link]
 Correspondência / Correspondence / Correspondencia:
Correspondência / Correspondence / Correspondencia:
Flávia Corrêa Bastos
Instituto Evandro Chagas,
Seção de Bacteriologia e Micologia,
Rodovia BR 316, km 7, s/no, Levilândia
CEP:67030-000
Ananindeua-Pará-Brasil
Tel.: + 55 (91) 3214-2122
E-mail:flavia_bastos@hotmail.com
Recebido em / Received / Recibido en: 18/4/2011
Aceito em / Accepted / Aceito en: 16/5/2011











 texto em
texto em 

